Abstract
Poxviral infection was identified in a crimson rosella presented to the Australian Wildlife Health Centre (Victoria) in 2002, and from a second crimson rosella in 2008. Both cases were characterized by proliferative lesions on non-feathered skin. Routine histopathology identified intra-lesional epidermal changes consistent with those caused by poxvirus. Electron microscopy confirmed the presence of poxvirus in inclusions in the first case, and genetic analysis of DNA extracted from both cases found an identical viral genome that differs from all other known poxviruses. We conclude that this infection in crimson rosellas is caused by a previously unrecognized avian poxvirus endemic to this region of Australia, and with low virulence.
Introduction
Avian poxviruses are known to affect a wide diversity of species, including both passerines and psittacines (van Riper & Forrester, Citation2007). Although some cross-species infections are known to occur particularly for passerines, in general poxviruses show considerable host specificity (Ladds, Citation2009). Pox infections of domesticated and production birds are very well documented, but there are relatively few reports of pox infections involving parrots (Bolte et al., Citation1999).
Poxviruses that infect psittacines have been considered exotic to Australia (Macwhirter, Citation2000). Psittacine poxvirus disease has been described elsewhere in Amazon parrots (Amazona spp.), African grey parrots (Psittacus erithacus), eastern rosellas (Platycercus eximius), blue bonnet parrots (Psephotus haematogaster), lories, lovebirds, lorikeets, macaws, pionus and quaker parrots (Myiopsitta monachus) (Kraft & Teufel, Citation1971; Graham, Citation1978; Gaskin, Citation1989; Terregino et al., Citation1999; van Riper & Forrester, Citation2007). Several years ago the discovery of fatal poxviral disease in two introduced rosellas in New Zealand prompted drastic action by the relevant authorities fearing spread from these birds to endangered indigenous psittacine species, and all birds on the affected property were destroyed (Gartrell, Citation2003; Gartrell et al., Citation2003).
In 2002, we investigated a proliferative skin disease in a wild-caught crimson rosella (Platycercus elegans), and confirmed that the lesions were associated with poxviral infection. No other affected birds were recognized in local wild parrot populations. In 2008, we investigated a similar disease in another local, wild crimson rosella and confirmed this was also associated with poxviral infection. DNA analysis from both of these cases indicates that the same, previously uncharacterized poxvirus was responsible for both these infections. There have been no additional cases identified since the case in 2008. We conclude that there is a poxvirus that sporadically causes classical pox lesions in crimson rosellas in southern Victoria. The lack of disease in other native psittacines suggests that the virus might only be pathogenic to this specific psittacine species. This virus seems to be of low virulence for other native Australian parrots and is of no threat to other avian species.
Materials and Methods
Case A
In 2002, a wild crimson rosella was submitted to the Australian Wildlife Health Centre (AWHC) at Healesville Sanctuary in Victoria with hyperkeratotic growths on the right eyelid and smaller growths on both feet (). A biopsy was taken from one of the lesions on the feet, fixed and processed routinely for histopathology.
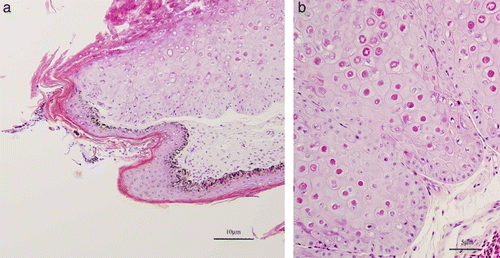
Figure 2. (Colour online) 2a: Proliferative skin lesion with hyperkeratosis. Biopsy from the foot of Case A. Haematoxylin and eosin stained section. 2b: Higher power version of (2a) illustrating numerous large intracytoplasmic eosinophilic inclusions within hypertrophied epidermal cells.
Additional samples were subsequently collected, and unfixed material in saline was submitted for viral culture in embryonated chicken eggs and chicken fibroblast cell culture. Material was also fixed in phosphate-buffered glutaraldehyde and processed for transmission electron microscopy.
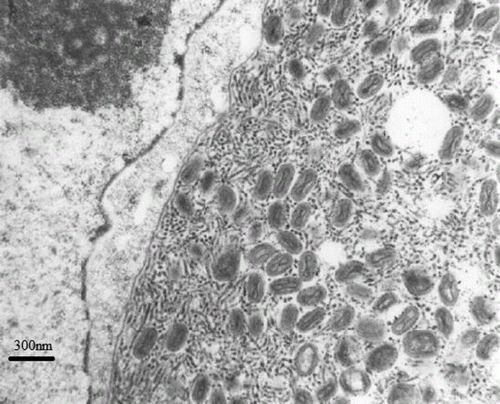
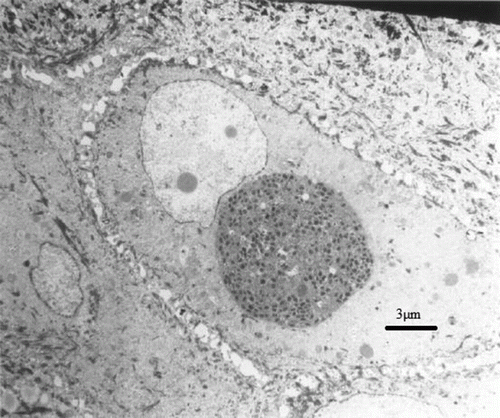
Figure 3. Intracytoplasmic viral inclusion within an epidermal cell. The inclusion consists of mature virions as well as filaments. Transmission electron microscopy, uranyl acetate–lead citrate.
From unfixed frozen toe tissue, DNA was extracted by 5 µl proteinase K and 500 µl solubilizing solution consisting of 1 M Tris–HCl at pH 8.0 to 8.6, 0.5 M ethylenediamine tetraacetic acid in NaOH, 5 M NaCl and 20% sodium dodecyl sulphate.
After overnight incubation at 65°C, 200 µl NaCl (5 M) was added and mixed by inversion. The preparation was centrifuged for 10 min, the supernatant transferred to a new tube, and centrifugation steps repeated until the supernatant was clear. An equal amount of isopropanol was added to the supernatant, and mixed by inversion until a DNA precipitate became visible. The precipitate was retrieved by high-speed centrifugation; the pellet was washed with 70% ethanol and dried. After drying, the pellet was resuspended in Tris-EDTA buffer (8.0 to 8.3).
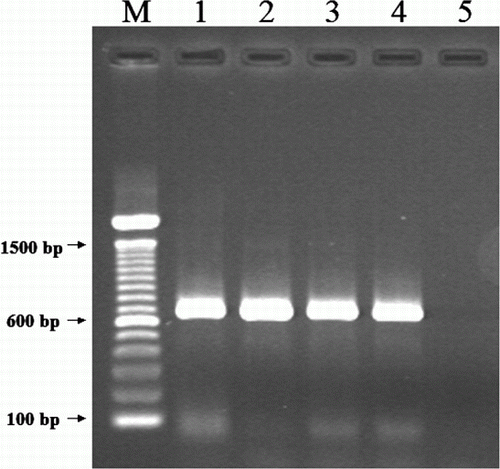
The extracted DNA was tested by a polymerase chain reaction (PCR) assay. A pair of primers were designed based on the sequences of the conserved core protein 4b gene of fowl poxvirus (HP444) (GenBank accession number M25781). The sequences of primers were APV-4b2F (5′-CAGCAGGTGCTAAACAACAA-3′) and APV-4b2R (5′-CAGCAGGTGCTAAACAACAA-3′). The PCR was carried out in a 50 µl volume containing 10 mM Tris–HCl, 50 mM KCl, 1.5 mM MgCl2, 2.5 U Taq DNA polymerase (Roche Applied Science, Castle Hill, NSW, Australia), 0.2 mM each of dATP, dCTP, dGTP and dTTP, 0.5 µM of each primer, 5% glycerol, and 5 µl DNA template. Thermal cycling was performed in a Mastercycler Gradient (Eppendof, Hamburg, Germany). The reaction conditions were: 95°C for 2 min, 35 cycles at 95°C for 30 sec, 51°C for 1 min and 72°C for 1 min, followed by a final elongation at 72°C for 5 min. The PCR products were analysed using gel electrophoresis on 1.5% agarose gels and visualized on an ultraviolet light transilluminator. Gel electrophoresis analysis demonstrated a PCR product at expected size (approximately 630 base pairs), in comparison with reference avian poxviruses ().
Figure 5. Gel electrophoresis of a PCR for detection of avian poxvirus core protein 4b gene. M, 100 base pairs (bp) DNA ladder (Invitrogen, Mulgrave, Victoria, Australia); lane 1, avian poxvirus isolate from crimson rosella; lane 2, canary poxvirus; lane 3, sparrow poxvirus; lane 4, fowl poxvirus; lane 5, negative control.
For sequence analysis, the PCR products were purified using a QIAGEN PCR Clean-up Kit (QIAGEN Pty Ltd, Hilden, Germany), according to the manufacturer's instructions. The purified PCR products were sequenced using the MagaBACE DNA Analysis System and dye terminators (Waikato DNA Sequencing Facility, University of Waikato, Hamilton, New Zealand). The sequences were analysed using blastn (http://blast.ncbi.nlm.nih.gov/Blast.cgi) for a similarity search at GenBank.
The sequences of this poxvirus and those from Case B have been submitted to GenBank (accession numbers JX97153 and JX971537).
Case B
In 2008 a wild crimson rosella from Sherbrooke Forest, approximately 40 km east of Melbourne, Victoria, was submitted to the AWHC with similar lesions to those described for the bird 6 years previously. There had been no reports of poxvirus affecting crimson rosellas or other closely related species in the intervening period. In this case, poxvirus was confirmed by the characteristic histopathology of skin lesions and samples of fixed tissue used for DNA extraction.
Results
Case A
Histopathology revealed a hyperplastic and vacuolated epidermis, with superficial crusting, necrosis and inflammation. Many enlarged epidermal cells contained prominent eosinophilic intracytoplasmic inclusions (Figure 2). A diagnosis of hyperplastic dermatitis caused by poxvirus was made. Attempts to isolate virus from chick embryo chorioallantoic membranes and cell culture were unsuccessful.
Transmission electron microscopy revealed large mature virions and filaments within cytoplasmic inclusions, typical of those described for poxviruses ( and ).
Sequence analysis showed that the poxvirus from the crimson rosella is different from all known avian poxviruses available at GenBank, and poxviruses of several other species. The sequence has only 73 to 75% identity to those of poxviruses from other avian species, including canary poxviruses, fowl poxviruses and penguin poxviruses. This poxvirus is also far less closely related to any mammalian isolates.
Case B
Sequences from this sample yielded an identical unique poxviral sequence profile to that found in Case A.
Discussion
Poxviral disease in psittacine species is currently considered exotic to Australian birds, and, given appropriate concerns regarding the potential for spread to endangered species, we thoroughly investigated the first case. By the time these investigations had concluded, several months had elapsed and we were confident that this disease had not become rampant amongst the local psittacine bird species because of the lack of admissions to the AWHC. While there is only passive monitoring of wildlife diseases by the AWHC, it is implausible that this strain of poxvirus would go unnoticed if virulent and subsequently only one additional case has been identified over a 6-year period, with none since 2008. We concluded that this unique poxvirus is probably of low virulence for crimson rosellas and we have no evidence to indicate that it is pathogenic for any other species of native parrot that co-habits this environment, including eastern rosellas, cockatoos and galahs (Eolophus roseicapilla). The pathogenic effects of this virus in these two cases could have been influenced by individual factors, such as immunosuppression, and so forth. A pathogenicity study would be required to elucidate this.
In a previous study, the poxviruses from different avian species were classified into three genetic clades (Jarmin et al., Citation2006), representing a fowl poxvirus group, a canary poxvirus group and a psittacine poxvirus-like group. This classification was based on partial sequences of core protein 4b gene amplified by a pair of primers initially described by Huw Lee & Hwa Lee (Citation1997). This set of primers has been widely applied in PCR for detection and characterization of avipoxviruses. Thus far, most sequences of different avipoxviruses available are generated from this set of primers. We also conducted PCR using this set of primers for the detection of poxvirus from crimson rosellas in both cases, but failed to detect the viruses, indicating sequence heterology of the virus. In the present study, a new set of primers targeting different regions of the core protein 4b gene were applied, which successfully detected the poxvirus from the crimson rosellas. Sequence analysis confirmed significant sequence variations between poxvirus detected from the crimson rosellas and those from other avian species (Boulanger et al., Citation1998; Tulman et al., Citation2004; Carulei et al., Citation2009). The poxvirus detected from this study represents a new genetic clade of avipoxviruses.
Additionally, in this study, attempts were made to isolate the poxvirus from both crimson rosellas using both embryonated chicken eggs and chicken fibroblast cell cultures, without success. This could have been due to a lack of viable virus in the samples, or poor sampling and/or transportation before laboratory testing, as the two systems are widely applied for the isolation of poxvirus from other avian species. On the contrary, it is also possible that isolation of this novel virus would require prolonged passaging with different conditions using these two common isolation systems.
Although case reports of pox in psittacine species have described severe disease leading to mortality (Gerlach, Citation1994; Gartrell et al., Citation2003), the condition described in caged African grey parrots from Saudi Arabia (Tarello, Citation2004) was principally a skin disease typically affecting the head and feet, and is therefore clinically similar to the disease we describe for crimson rosellas. While we are unable to exclude the possibility that the disease described occurred through sporadic introduction into southern Australia, it seems most likely that this virus is a well-adapted strain endemic to crimson rosellas in southern Australia with low virulence and high species specificity, since we are unaware of similar disease expression in other local native species of parrots.
Acknowledgements
The authors thank Liliana Tartarchuk for assistance with electron microscopy, Gribbles Veterinary Pathology for sample processing for light microscopy, and Dr Mark Williamson for attempts to culture the virus from samples sent to the Victorian Institute of Animal Science, Attwood.
References
- Bolte , A.L. , Meurer , J. and Kaleta , E.F. 1999 . Avian host spectrum of avipoxviruses . Avian Pathology , 28 : 415 – 432 . doi: 10.1080/03079459994434
- Boulanger , D. , Green , P. , Smith , T. , Czerny , C.P. and Skinner , M.A. 1998 . The 131-amino-acid repeat region of the essential 39-kilodalton core protein of fowlpox virus FP9, equivalent to vaccinia virus A4L protein, is nonessential and highly immunogenic . Journal of Virology , 72 : 170 – 179 .
- Carulei , O. , Douglass , N. and Williamson , A.L. 2009 . Phylogenetic analysis of three genes of Penguinpox virus corresponding to Vaccinia virus G8R (VLTF-1), A3L (P4b) and H3L reveals that it is most closely related to Turkeypox virus, Ostrichpox virus and Pigeonpox virus . Virology Journal , 6 : 52 doi: 10.1186/1743-422X-6-52
- Gartrell , B. 2003 . Psittacine poxvirus outbreak in New Zealand . In Association of Avian Veterinarians Australian Committee Annual Conference 19 – 23 Manly , NSW, Australia .
- Gartrell , B. , Stone , M. , King , C. and Wang , J. 2003 . An outbreak of disease caused by psittacinepoxvirus in rosellas . Surveillance , 30 : 11 – 13 .
- Gaskin , J. 1989 . Psittacine viral diseases: a perspective . Journal of Zoo and Wildlife Medicine , 20 : 249 – 264 .
- Gerlach , H. 1994 . “ Viruses ” . In Avian Medicine: Principles and Application , Edited by: Ritchie , B.W. , Harrison , G.J. and Harrison , L.R. 862 – 948 . Florida : Wingers .
- Graham , C.L.G. 1978 . Poxvirus infection in a spectacled Amazon parrot (Amazona albifrons) . Avian Diseases , 22 : 340 – 343 . doi: 10.2307/1589547
- Huw Lee , L. and Hwa Lee , K. 1997 . Application of the polymerase chain reaction for the diagnosis of fowl poxvirus infection . Journal of Virological Methods , 63 : 113 – 119 . doi: 10.1016/S0166-0934(96)02119-2
- Jarmin , S. , Manvell , R. , Gough , R.E. , Laidlaw , S.M. and Skinner , M.A. 2006 . Avipoxvirus phylogenetics: identification of a PCR length polymorphism that discriminates between the two major clades . Journal of General Virology , 87 : 2191 – 201 . doi: 10.1099/vir.0.81738-0
- Kraft , V. and Teufel , P. 1971 . Nachweis eines Pockenvirus bei Zwergpapageien (Agapornis personata und Agapornis roseicollis). A pox-like disease in lovebirds (Agapornis personata and Agapornis roseicollis) . Berliner Und Münchener Tierärztliche Wochenschrift , 84 : 83 – 87 .
- Ladds , P. 2009 . Viral diseases in birds . In Pathology of Australian Native Wildlife 29 – 52 . Collingwood : CSIRO .
- Macwhirter , P. 2000 . Viral diseases of concern . In Birds 2000 171 – 186 . Sydney : Proceedings 334 of the Post Graduate Foundation in Veterinary Science .
- Tarello , W. 2004 . Avian pox in psittacine birds from Saudi Arabia . Revue de Medecine Veterinaire , 155 : 483 – 485 .
- Terregino , C. , Catelli , E. , Delogu , M. , Capua , I. and Tonelli , A. 1999 . Poxvirus infection in a blue bonnet parrot . The Veterinary Record , 145 : 264
- Tulman , E.R. , Afonso , C.L. , Lu , Z. , Zsak , L. , Kutish , G.F. and Rock , D.L. 2004 . The genome of canarypox virus . Journal of Virology , 78 : 353 – 366 . doi: 10.1128/JVI.78.1.353-366.2004
- van Riper , C. and Forrester , D.J. 2007 . “ Avian pox ” . In Infectious Diseases of Wild Birds , Edited by: Thomas , N.J. , Hunter , D.B. and Atkinson , C.T. 131 – 176 . Ames : Blackwell .