Abstract
Outbreaks of respiratory disease were investigated in reared pheasants (Phasianus colchicus) aged approximately 18 to 32 weeks, released into the semi-wild on four shooting estates in southern England. The clinical signs in the affected birds included swelling of the face and eyes, loss of condition, gasping respirations and coughing. The gross pathology findings included sinusitis, airsacculitis, pleural oedema and lung lesions. The histopathological findings in the affected lungs were characterized by a granulomatous pneumonia. Ornithobacterium rhinotracheale (ORT) was isolated from respiratory tract tissues, and 16S rRNA gene sequencing on three isolates revealed two distinct genotypes, one previously associated with some electrophoretic type (ET) 1 strains and the other a novel genotype that clustered among sequences previously associated with ET 3, ET 4, ET 5 and ET 6 isolates. The localization of ORT within the lung tissue was demonstrated by fluorescent in-situ hybridization in the bronchial exudate of three cases, although not within the granulomatous lesions themselves. In each case, ORT was identified as part of a complex of other respiratory agents including avian paramyxovirus type 2, avian coronavirus, Mycoplasma gallisepticum, Mycoplasma synoviae and other Mycoplasma species, Escherichia coli, Pasteurella multocida, other Pasteurellaceae and Syngamus trachea, suggesting synergism with other agents. Exposure to other intercurrent factors, including adverse weather conditions and internal parasitism, may also have exacerbated the severity of disease.
Introduction
Respiratory disease is a significant cause of ill-health and mortality in captive-reared pheasants (Phasianus colchicus) in Great Britain, especially following their release into a semi-wild existence from late summer onwards. Several infectious agents have been implicated in the aetiology, either singly or in combination with other agents or other factors. Mycoplasma gallisepticum has been identified as one of the most important infectious agents in respiratory disease, as a primary cause of infectious sinusitis and conjunctivitis (Keymer, Citation1961; Bradbury et al., Citation2001b; Forrester et al., Citation2011), but is not usually implicated in disease of the lungs and air sacs in pheasants (Welchman et al., Citation2002; Bencina et al., Citation2003). Other infectious agents including avian metapneumovirus (aMPV) and coronaviruses related to infectious bronchitis virus of chickens have also been associated with both upper and lower respiratory tract disease in pheasants, either in their own right or concurrently with other infectious agents such as M. gallisepticum and Pasteurella multocida (Cavanagh et al., Citation2002; Welchman et al., Citation2002). In contrast to M. gallisepticum, an aetiological role for Mycoplasma synoviae has not been confirmed in respiratory disease in pheasants (Bradbury et al., Citation2001a, Citationb).
Ornithobacterium rhinotracheale (ORT) is a Gram-negative pleomorphic bacterium that was isolated by Charlton et al. (Citation1993) from domestic turkeys and chickens with respiratory disease, and named by Vandamme et al. (Citation1994). ORT has subsequently been isolated from several bird species and is most commonly associated with respiratory disease in domestic fowl and turkeys, and also with joint infections, osteomyelitis and encephalitis (Chin et al., Citation2008). ORT has been shown to have little significant effect as a primary respiratory pathogen in its own right. However, respiratory tract lesions are more severe in concurrent infections with other respiratory pathogens, when the pathogenic effects of ORT are particularly characterized by the development of a fibrinopurulent and necrotic pneumonia and airsacculitis (De Rosa et al., Citation1996; van Empel & Hafez, Citation1999; Marien et al., Citation2005; Chin et al., Citation2008). The isolation of the organism from an unspecified pheasant sample was reported by Charlton et al. (Citation1993), and it was detected in pheasants with respiratory disease by Welchman et al. (Citation2002), but the authors are unaware of detailed descriptions of disease associated with ORT in pheasants. It has also been reported in cases of otitis and cranial osteomyelitis in red-legged partridges (Alectoris rufa) (Moreno et al., Citation2009).
Since the 1990s, outbreaks of respiratory disease in pheasants have been recorded on several shooting estates in southern England. In some of these outbreaks the presenting signs have been sinusitis and facial swelling typical of M. gallisepticum infection, but in other outbreaks the clinical findings have included gasping respirations and in some cases a distinctive “200 yard cough” audible from a distance of 200 to 400 yards (183 to 366 m). Affected birds failed to respond to appropriate treatment for “gapes” (Syngamus trachea infection; syngamosis) (Dalton et al., Citation2002). The outbreaks have occurred in the autumn and winter months. In addition to sinusitis, pneumonia and airsacculitis have been consistent findings in the affected birds. In the present study we report the findings in a series of four field outbreaks of respiratory disease in which ORT was identified in pheasants with pathological changes in the lungs and air sacs. The clinical presentation is described together with the pathological findings and the results of investigation into ORT and other potential aetiological agents detected.
Materials and Methods
Affected flocks
Outbreaks of respiratory disease were investigated in pheasants from four shooting estates in southern England between November 2009 and December 2010. Three of the estates were situated within approximately 8 km of each other, and one shared a common boundary with the other two. The fourth estate was separated from the others by approximately 19 km. In each case the birds had been released into a semi-wild state following the conventional practice of captive-rearing and releasing pheasants in Great Britain. Each estate had released in excess of 10,000 pheasant poults. There was not known to be a common source of poults or eggs and there was no intentional exchange of birds between estates, but it is possible some birds may have strayed across the boundaries between neighbouring estates. A representative selection of pheasants affected with respiratory disease was submitted for diagnostic investigation to the Veterinary Laboratories Agency Regional Laboratory at Winchester. A brief clinical history was obtained from the gamekeeper for each case and included loss of condition or wasting, swollen eyes and sinuses, gasping and difficulty in breathing, lethargy and coughing. In Case B238-11 (investigated in November 2009), five live pheasants aged approximately 18 to 20 weeks were examined from an estate where the birds were reported to be losing condition and coughing. The birds had been bred and hatched from the estate's own eggs and were vaccinated with a commercial aMPV (turkey rhinotracheitis [TRT]) vaccine at approximately 7 weeks of age. In Case B293-2 (February 2010), six live adult pheasants aged approximately 32 weeks were examined from an estate where loss of condition and difficulty in breathing had been reported in pheasants since November. The birds had been imported as chicks. The vaccination history was not available. In Case B267-11 (November 2010), one live and five dead pheasants were examined aged approximately 23 weeks. Loss of condition, swollen eyes and rising mortality were reported. The birds had been purchased as young poults and were vaccinated with an inactivated M. gallisepticum vaccine and a live TRT vaccine at 6 weeks of age. In Case B210-12 (December 2010), six live birds were examined aged approximately 27 weeks. Swelling of the eyes had been seen in August and had responded to antimicrobial treatment, but had reappeared in October 2010 accompanied by coughing, open-mouthed breathing and loss of condition. The birds had been purchased as young poults and were vaccinated with a commercial live TRT vaccine at 7 weeks of age.
Gross pathology, histopathology and microscopy
Following clinical examination the live birds were humanely euthanized and diagnostic post-mortem examinations were carried out. Samples of lung with grossly visible lesions were collected for histopathology from two birds in each of Cases B238-11 (Birds 3 and 5), B293-2 (Birds 4 and 6) and B210-12 (Birds 3 and 4), and one bird from Case B267-11 (Bird 6). Samples of abnormal air sac were collected from the two birds from each of Cases B293-2 and B210-12. The samples were fixed in 10% buffered formol saline, embedded in paraffin wax and 5 µm serial sections of the tissues were cut and stained with haematoxylin and eosin, and in some cases Gram, Ziehl Neelsen or Periodic acid–Schiff stains. Similarly, serial sections were cut for fluorescent in-situ hybridization. Wet preparations of intestinal smears were examined microscopically for evidence of intestinal parasites.
Fluorescent in-situ hybridization
A novel, specific oligonucleotide probe, 5′-ATCACTCCCCGAAAACGA-3′, targeting nucleotides 826 to 843 (Escherichia coli numbering) in 16S rRNA of ORT was designed using the function Probe Design in the software ARB (a software environment for sequence data: http://www.arb-home.de/; Technische Universität München, Munich, Germany). In addition, a probe for Domain Bacterium (Eub 338) was included (Amann et al., Citation1990). The oligonucleotide probes were 5′ labelled with fluorescein isothiocyanate or the isothiocyanate derivative Cy3 (Eurofins MWG Operon, Ebersberg, Germany). According to Ribosomal Database Project Release 10 (http://rdp.cme.msu.edu/) the novel probe had at least two mismatches to all other bacteria. Testing the specificity of the novel probe was furthermore done in formalin-fixed tissue samples each containing one of three different reference strains: ORT (CCUG 23171 T; positive control tissue), P. multocida subsp. septica (HIM746-6T), and E. coli (9910297-2STM [O138:F18]).
Before hybridization the sections were deparaffinated in xylene and transferred to ethanol. The hybridization was carried out at 45°C with 40 µl hybridization buffer (100 mM Tris [pH 7.2], 0.9 M NaCl, 0.1% sodium dodecyl sulphate) and 200 ng of each probe for 16 h in a Sequenza slide rack (Thermo Shandon, Runcorn, UK). The sections were then washed three times in prewarmed (45°C) hybridization buffer for 15 min and subsequently three times in prewarmed (45°C) washing solution (100 mM Tris [pH 7.2], 0.9 M NaCl). The sections were rinsed in water, air dried, and mounted in Vectashield (Vector Laboratories Inc., Burlingame, California, USA) for epifluorescence microscopy.
An Axioimager M1 epifluorescence microscope equipped for epifluorescence with a 120-W HBO lamp and filter sets 43 and 38 were used to visualize Cy3 and fluorescein, respectively. Images were obtained using an AxioCam MRm version 3 FireWiremonocrome camera and AxioVision software, version 4.5 (Carl Zeiss, Oberkochen, Germany).
Bacterial isolation and identification
Samples of lung and thickened air sac from the selected birds as described previously were cultured on 5% sheep blood agar (Columbia base CM331; Oxoid, Basingstoke, UK) and MacConkey agar (CM7; Oxoid) and incubated at 37°C for 72 h under 7.5% carbon dioxide. Additionally, in some cases, cultures on Sabouraud's agar (CM41; Oxoid) were also undertaken. A limited number of cultures were also set up on infra-orbital sinus swabs and on samples of the liver and spleen from the affected birds. Preliminary identification of organisms followed standard phenotypic procedures including those described for ORT (Chin et al., Citation2008), and biochemical confirmation of suspect isolates of ORT was undertaken using the API 20NE system (bioMérieux, Marcy-l'Etoile). The identity of three isolates of ORT was further confirmed by 16S rRNA gene sequence analysis. The virtually complete 16S rRNA gene locus was amplified by polymerase chain reaction (PCR) using primer pair 5′-AGTTTGATCCTGGCTCAG-3′ and 5′-ACGGCTACCTTGTTACGACTT-3′ and sequenced using a series of internal primers. Phylogenetic comparison of 16S rRNA sequences with previously described sequences (Amonsin et al., Citation1997) was carried out with the MEGA4 package (Tamura et al., Citation2007) using the neighbour-joining approach following CLUSTAL alignment of sequences trimmed to a 1370 base pair consensus contig. Sequences were submitted to Genbank and assigned the accession numbers HF548213 (isolate B238-11), HF548214 (isolate B293-2A) and HF548215 (isolate B293-2B).
Sinus and tracheal swabs were collected from the birds and pooled together to inoculate an Eaton's broth (Nicholas & Baker, Citation1998). The samples were examined for Mycoplasma species by 16S rDNA PCR and denaturing gel gradient electrophoresis (McAuliffe et al., Citation2003, Citation2005).
Serology
Blood samples were collected from the birds submitted alive, immediately after euthanasia, for rapid serum agglutination testing for M. gallisepticum as described in OIE (Citation2008a) (Cases B238-11 and B293-2) or immunoblot testing for M. gallisepticum and M. synoviae (Cases B210-12 and B267-11). The immunoblot test for M. gallisepticum used a whole cell antigen, whilst for M. synoviae a 0.05 M (pH 9.6) carbonate–bicarbonate sonicated lysate washing was used as the antigen. Then 12% sodium dodecyl sulphate-polyacrylamide gel electrophoresis was used to electrophoretically separate the antigens before they were electrophoretically transferred to a nitrocellulose membrane using a semi-dry method. The membranes were cut into strips for immunoblotting. Samples and controls were diluted 1 in 50 in phosphate-buffered saline (pH 7.2; PBS) containing 0.1% (w/v) skimmed milk powder and 0.1% (w/v) chicken egg albumin and the strips were incubated for 2 h at 37°C with agitation, before washing three times with PBS with 0.1% Tween and once with PBS. These were then incubated for 1 h at 37°C with an optimized concentration of rabbit–anti-chicken/turkey IgG–horseradish peroxidase conjugate (Invitrogen, Paisley, UK). The washing steps were repeated before developing the membrane in 4-chloro-1-naphthol solution (Pierce/Thermo Fisher, Cramlington, UK) at room temperature. The reaction was stopped by washing in excess deionized water within 15 min. For the M. gallisepticum test to be interpreted as positive at least four of six critical proteins must be present in the positive control and test samples with approximate molecular weights of 100, 80, 62, 53, 43 and 36 kDA, whereas for M. synoviae at least three of four critical proteins must be present in the positive control and test samples with approximate molecular weights of 85, 81, 45 and 35/37 kDa. The negative controls must not have these specific proteins.
Sera from two submissions underwent a blocking enzyme-linked immunosorbent assay (ELISA) test for aMPV antibodies (Svanovir APV-Ab ELISA; Svanova Biotech AB, Uppsala, Sweden) as described by the manufacturer. Sera from Case B238-11 underwent haemagglutination inhibition testing for avian paramyxovirus-2 (APMV-2) antibodies (OIE, Citation2008b).
Virology
Pooled trachea and pooled caecal tonsil samples were taken from the pheasants at post-mortem examination and a 20% w/v suspension of the tissues was prepared in an antibiotic solution and inoculated into embryonated specific pathogen free fowls' eggs for attempted virus isolation (Gough et al., Citation1988). Sequencing and phylogenetic analysis of the haemagglutinin-neuraminidase gene was carried out on four APMV-2 isolates; the prototype strain PMV-2/chicken/Yucaipa-California/56, PMV-2/chicken/Scotland/7702/06, PMV-2/pheasant/Denmark/68408/03 and the isolated virus designated PMV-2/pheasant/England/38221/09. In brief, viral RNA was extracted from allantoic fluid followed by reverse transcription (RT)-PCR and amplicon purification for sequencing as described by Mahmood et al. (Citation2010). Testing for aMPV was undertaken on oropharyngeal swabs from Cases B210-12 and B267-11 using real-time RT-PCR to detect and differentiate aMPV subtypes A, B and C. The test comprised an initial automated nucleic acid extraction step using a Roche Magna Pure LC extraction robot (Roche-Diagnostics, Lewes, UK) followed by three separate one-step, real-time RT-PCR TaqMan® reactions on a Stratagene MX3000P platform (Stratagene, La Jolla, California, USA).
Results
Clinical examination, gross pathology and microscopy
The clinical findings assessed in the live birds following submission to the laboratory varied between different birds, but included open-mouthed breathing and increased respiratory effort (dyspnoea), conjunctival discharge, swelling of the infra-orbital sinuses on one or both sides, audible respiratory râles, lethargy and occasional coughing, although the latter was difficult to assess clinically in the laboratory. The pathological findings also varied between individuals, but the findings in the respiratory tract included a mucoid or caseous sinusitis, increased amounts of mucus in the trachea, gapeworms (S. trachea) in one bird from Case B238-11, thickening of the abdominal air sacs sometimes accompanied by white caseous deposits over the air sac membranes, pleural oedema and scattered pale grey lesions approximately 3 to 4 mm in diameter in the lung tissue. The birds from Case B210-12 showed no clinical or gross pathological evidence of sinusitis. The body condition of the majority of the birds was assessed as fair or poor. In all cases, intestinal parasitism with Heterakis and Capillaria species nematodes was demonstrated by examination of intestinal smears.
Histopathology
Histologically the lesions in the lungs were characterized by a granulomatous pneumonia centred on the airways (bronchi, bronchioles and surrounding parabronchi). The granulomas were of varying sizes and comprised central necrotic lesions containing occasional bacterial colonies surrounded by degenerate cells and multinucleate cells. There was a degree of plasma cell, lymphocyte and granulocyte infiltration in the surrounding lung parenchyma, but elsewhere the lung parenchyma was relatively unaffected with only occasional foci of plasma cells, lymphocytes and macrophages and perivascular inflammatory cells. The bronchial epithelium was hyperplastic with mucus gland hyperplasia. Gram-stained sections showed Gram-negatively staining bacteria in some of the granulomas. No fungal hyphae were seen in Periodic acid–Schiff stained sections and no acid-fast organisms in Ziehl Neelsen stained sections of the lesions. In the lung of Bird 3 from Case B210-12, several smaller granulomas contained structures resembling nematode eggs and probable transverse sections through parasites, surrounded by multinucleate cells at the edge of the lesions. In another lung (Bird 6, Case B293-02), early lymphoid follicle formation was present. In the air sacs there was thickening with hyperplasia of the epithelium (areas of which had a cystic appearance), mucous gland hyperplasia and congestion. There was plasma cell, lymphocyte, macrophage and occasional granulocyte infiltration of the connective tissue and occasional clusters of multinucleate cells. Fibrin-like material was present in air sac tissue and on the surface of the lung of Bird 6 from Case B293-2.
Fluorescent in-situ hybridization
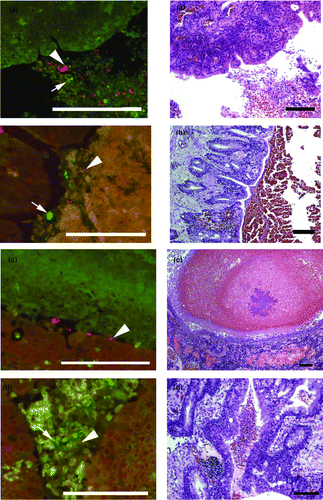
The novel oligonucleotide probe for ORT gave a clear signal when tested on the positive control tissue only. Applied on the case tissues, ORT organisms were visualized as a bright, red fluorescing signal in sections of lung from Cases B293-2, B267-11 and B210-12, but none were seen in Case B238-11. Where present, the organisms were located in inflammatory exudate within the bronchi (). In each case they were part of a mixed bacterial flora, with other bacterial species showing as green as the in-situ hybridization was performed as a double hybridization for both ORT (red) and bacteria in general (Domain Bacterium) (green).
Figure 1. Parallel FISH (left side) and haematoxylin and eosin (H & E; right side) stained images of lung tissue from affected pheasants. The FISH images show ORT in red (arrowhead) and other bacteria in green (arrow) in the lumen of the bronchi (1a to 1d) with cellular debris. 1a: Case B210-12 Bird 4. 1b: Case B293-2 Bird 4. 1c: Case B293-2 Bird 6. 1d: Case B293-2 Bird 4. The H & E images of the same tissues show cellular debris and red blood cells in the lumen and in (1c) inflammatory cell infiltration of the lung parenchyma adjacent to the bronchus. Bar = 100 µm.
Bacterial isolation and identification
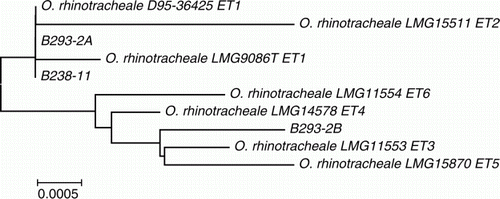
Various bacteria were isolated on microaerophilic culture from the tissue samples, and the following results refer to the birds with pathological changes in the lungs and on which histopathology was carried out. ORT was isolated in mixed growth with E. coli and Avibacterium gallinarum from the lung tissue of Case B238-11 Bird 3; in mixed growth with E. coli from the lung of Case B210-12 Birds 3 and 4; and from the sinus of Case B267-11 Bird 6, but P. multocida was the predominant organism isolated from the lung, air sac and sinuses of this bird. ORT was also isolated in mixed growth with an unidentified Pasteurella-like organism from the lung and air sac of Case B293-2 Birds 4 and 6 and in addition from the lung of Bird 1, which showed no grossly visible lung granulomas. ORT was not isolated from spleen or liver tissues from any bird tested. Most isolates identified phenotypically as ORT gave a profile of 0020004 on the API 20NE system. The identity of ORT from Case B238-11 Bird 3 and Case B293-2 Birds 1 and 4 was confirmed by PCR amplification and sequencing of the16S rRNA gene. This showed that Case B238-11 and one of the Case B293-2 isolates were indistinguishable from the sequence of an electrophoretic type (ET) 1 isolate, D95-36425, from a turkey described previously by Amonsin et al. (Citation1997). However, the second Case B293-2 isolate clustered among 16S rRNA gene sequences of ET 3, ET 4, ET 5 and ET 6 isolates of ORT () previously associated predominantly with wild bird populations.
Figure 2. Phylogenetic analysis of 16S rRNA sequences of ORT isolates examined in this study in comparison with strains described previously (Amonsin et al., Citation1997). Bar = 0.0005 substitutions per nucleotide position.
Denaturing gel gradient electrophoresis and PCR testing identified M. gallisepticum in pooled respiratory tract swabs from Case B238-11, and M. synoviae, Mycoplasma gallopavonis and Mycoplasma glycophilum from Case B267-11, but no mycoplasmas were detected by this method in Case B293-2 or Case B210-12. However, M. glycophilum was detected by 16S rRNA gene testing of organisms isolated on routine culture from lung tissue from Case B210-12 Bird 3.
Virology
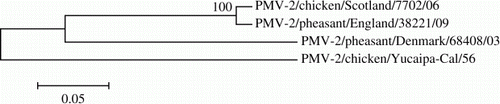
A coronavirus was isolated from pooled caecal tonsil tissue from Case B267-10, but could not be identified against a panel of known strains. No viruses were isolated from pooled tracheal tissue. A haemagglutinating virus was also isolated from pooled trachea and caecal tonsil tissue samples from Case B238-11 after inoculation into embryonated fowls' eggs. The isolated virus was then further characterized using haemagglutination inhibition testing and was identified as belonging to the APMV-2 subtype. Phylogenetic analysis revealed the virus isolated from Case B238-11 to have 97.9% identity to a PMV-2 isolate from chickens in Scotland in 2006. However, the differences between the 2009 English pheasant isolate and the 2003 Danish pheasant isolate were more noticeable, with only 76.2% identity (). The identity of the 2009 isolate with the prototype Yucaipa strain was even more remote at 70.2%, probably due to the age of the prototype virus. No viruses were detected in pooled trachea or caecal tonsil tissues from Cases B210-12 or B293-2. No aMPV was detected by real-time RT-PCR testing of oropharyngeal swabs from birds in Case B210-12 or Case B267-11.
Figure 3. Phylogenetic tree based on 1721 nucleotides of the haemagglutinin-neuraminidase gene from four APMV-2 viruses. The evolutionary history was inferred using the neighbour-joining method (Saitou & Nei, Citation1987). The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) is shown next to the branches. The evolutionary distances were computed using the Tamura three-parameter method and evolutionary analyses were conducted in MEGA5. Bar = 0.05 substitutions per nucleotide position.
Serology
Serological testing for M. gallisepticum yielded positive results in all five birds in Case B238-11 (rapid serum agglutination testing titres of 1/5 to 1/20) and all six birds in Case B293-2 (rapid serum agglutination testing titres of 1/10 to 1/80). Immunoblot testing was positive for M. gallisepticum and negative for M. synoviae in all five birds tested in Case B210-12, and positive for both M. gallisepticum and M. synoviae in the single live bird from Case B267-11. Haemagglutination inhibition testing for APMV-2 antibodies on the birds from Case B238-11 gave titres against the homologous virus of 1/128 (one bird), 1/32 (three birds) and <1/2 (one bird). All six birds from Case B293-2 and four out of five birds from Case B238-11 were serologically negative for aMPV by ELISA. However, Bird 1 from Case B238-11 gave a positive ELISA result of 47%.
Discussion
ORT is well recognized as a component of respiratory disease in domestic fowl and turkeys, but there have been few previous references to its occurrence in pheasants (Charlton et al., Citation1993; Welchman et al., Citation2002). Respiratory disease in pheasants remains predominantly associated with M. gallisepticum, aMPV, coronaviruses and syngamosis (Bradbury et al., Citation2001b; Cavanagh et al., Citation2002; Welchman et al., Citation2002; Forrester et al., Citation2011). In the birds described in the present case series, there was evidence of infection with all of these agents with the possible exception of aMPV, which was only demonstrated serologically in one bird. However, outbreaks of respiratory disease in pheasants most commonly affect the upper respiratory tract (particularly the infra-orbital sinuses), and there are few descriptions of disease affecting the lower respiratory tract (lungs and air sacs), as described in this paper. This lower respiratory tract involvement accounts for the colloquial description of a “200 yard cough” from the 1990s (Dalton et al., Citation2002). Vaccination had been initiated on some estates since that time in an attempt to control respiratory disease. Although not licensed in pheasants and of unproven efficacy (Dalton et al., Citation2002), TRT vaccination had been administered several weeks previously in at least three of the cases, and an M. gallisepticum vaccine in at least one case. However, the cases of airsacculitis and pneumonia described in this report occurred despite the use of vaccination, and were characterized by the development of lower respiratory tract disease in addition to, or as a sequel to, sinusitis.
In the present case series ORT was demonstrated in the respiratory tract of pheasants by culture, 16S rRNA gene sequencing and FISH. ORT was cultured in mixed growth with other bacteria from the affected lung tissue, and the findings from these and other cases of pneumonia investigated in pheasants (data not shown) indicate that ORT is one of a number of bacteria that can be isolated, including E. coli, P. multocida, A. gallinarum, Gallibacterium anatis as well as other Pasteurellaceae, all of which can be involved in the avian respiratory disease complex (van Empel & Hafez, Citation1999). In some instances ORT has been implicated as a primary cause of disease, including respiratory disease in broiler chickens (van Veen et al., Citation2005), otitis and cranial osteomyelitis in red legged partridges (Moreno et al., Citation2009) and respiratory disease in falcons (Hafez & Lierz, Citation2010). However, in poultry the severity of clinical signs, duration of disease and mortality associated with ORT are variable and are influenced by concurrent diseases and a range of environmental and management-related factors (van Empel & Hafez, Citation1999). Uncomplicated infections with single agents are the exception in outbreaks of respiratory disease under commercial conditions (Kleven & Glisson, Citation1997).
The pathological changes in the affected pheasants were characterized by a predominantly granulomatous pneumonia and caseous airsacculitis. The lung lesions appeared to be of a more chronic nature than those described in field cases of ORT in domestic poultry. A sequence of outbreaks in 27-week-old to 42-week-old turkey breeders was characterized histopathologically by severe fibrinoheterophilic exudation in the lungs and air sacs accompanied by gross and histopathogical changes in other viscera including the liver (De Rosa et al., Citation1996). Similar pathological findings have been described in 14-week-old to 22-week-old commercial turkeys (Roepke et al., Citation1998) and in the respiratory tract of broiler chickens (van Veen et al., Citation2000). Experimental infection of turkey poults with ORT gave rise to a caseous airsacculitis and acute fibrinopurulent pneumonia followed by development of microabscesses with necrotic cellular material surrounded by multinucleated giant cells, macrophages and a few heterophils (Sprenger et al., Citation1998). In chickens, following viral priming with Newcastle disease virus, van Empel et al. (Citation1999) described an acute granulomatous airsacculitis followed by bacterial infiltration and necrosis of the bronchus-associated lymphoid tissue in some areas of the lungs. In the pheasants, the respiratory tract lesions resembled the more chronic lesions described by Sprenger et al. (Citation1998) in younger turkeys, and lacked the acute, severe fibrinoheterophilic changes described both in experimental infections and field outbreaks in this species (De Rosa et al., Citation1996; Sprenger et al., Citation1998). The granulomatous inflammatory nature of the respiratory tract lesions thus appeared to reflect a chronic stage of progression of disease, or possibly a less fibrinous type of response to infection in this species. No gross pathological changes were seen in other viscera, unlike in field outbreaks of ORT in older turkeys (De Rosa et al., Citation1996).
ORT was detected by FISH in the bronchial exudate in three of the four cases investigated, and to the authors' knowledge this was the first time that this technique has been used to demonstrate this agent. Previous pathogenesis studies of ORT in turkeys and chickens using immunohistochemical techniques have demonstrated the organism within lung lesions, but the distribution of ORT within the respiratory tract was shown to vary with time after exposure by aerosol infection, and was detected in the air sacs before the lungs. With the passage of time, the detection of ORT became sporadic (van Empel et al., Citation1999). The timescale of ORT infection in these field outbreaks in pheasants was unknown, and therefore the distribution of the organism within diseased respiratory tract tissue was unpredictable. However, the cellular reaction within the lung tissue and the presence of well-developed granulomas with prominent borders of multinucleate giant cells suggest a relatively chronic stage of disease, which may explain the detection of ORT in cellular exudate by FISH rather than within the lung lesions themselves. The chronicity of the lesions would also contribute to the mixed nature of the bacterial flora, and ORT is best detected by bacteriology at an early stage of the disease (van Empel et al., Citation1999).
The results support previous findings that ORT often does not act as a primary aetiological agent in respiratory tract disease, but may be secondary to infection with other agents that in these cases included APMV-2, coronavirus, M. gallisepticum, M. synoviae and other bacteria. This is evidence of multicausal respiratory disease (Kleven & Glisson, Citation1997) and the synergism previously reported experimentally between ORT and other agents; for example, aMPV (Marien et al., Citation2005), Newcastle disease virus and infectious bronchitis virus (van Empel et al., Citation1996). Case reports of field outbreaks of ORT have also confirmed mixed infections affecting the severity of disease, including with E. coli in turkey breeders (De Rosa et al., Citation1996), M. synoviae in multi-age turkey flocks (Zorman-Rojs et al., Citation2000) and E. coli in broiler chickens (Sakai et al., Citation2000). Neither APMV-2 or M. synoviae appear to have been previously reported in cases of respiratory disease in pheasants, but these agents are likely to go clinically undetected in the absence of disease attributable to secondary infectious agents. An association between the culture of Pasteurella spp. in lung tissue, and the detection of M. gallisepticum by PCR in the trachea, and infection with both aMPV and coronaviruses has been reported in pheasants (Welchman et al., Citation2002). Both coronavirus and APMV-2 were demonstrated in the present cases, but there was very little evidence of exposure to aMPV and the positive ELISA reading in one bird may have been a response to vaccination. Conversely, no adverse reactions to TRT vaccination were reported in a trial in pheasants (Dalton et al., Citation2002).
APMV-2 has previously been detected in chickens in Scotland (Wood et al., Citation2008). The epidemiology of the APMV-2 infection is not clear but, because of the location, distances and time differences between the last Scottish poultry outbreaks in 2006 and this current outbreak in pheasants in 2009, it could be concluded that the infection may have been derived from a circulating infection in wild birds. The close phylogenetic relationship between these two UK APMV-2 isolates is suggestive of such a possibility. APMV-2 has been isolated from finches and other wild passerine birds (Ozdemir et al., Citation1990; Mahmood et al., Citation2010), although infections in wild birds have not yet been reported in the UK. A surveillance study of wild bird samples would be beneficial to establish whether the virus is in fact circulating within the UK wild bird population. The clinical outcome of APMV-2 infection is likely to depend on exacerbation by other infectious agents or environmental conditions (Wood et al., Citation2008).
Several factors may account for the apparent seasonality of the cases described in this paper. ORT has been shown to survive longer in the environment at lower temperatures (Lopes et al., Citation2002), which could be associated with a higher incidence of infections during the winter months (Chin et al., Citation2008). Cool and foggy weather conditions were implicated in outbreaks of ORT in turkey breeders (De Rosa et al., Citation1996) and poor weather conditions were considered to trigger an outbreak of airsacculitis caused by ORT in nestling falcons (Hafez & Lierz, Citation2010). There is also greater opportunity in the winter for contact between released pheasants and other wild birds including rooks (Corvus frugilegus) that are widespread in the area of the estates. Two different strains of ORT were detected in the pheasants, potentially from two different sources, with ET 1 being more associated with poultry and the ET 3 to ET 6 cluster more associated with wild birds, particularly rooks (Amonsin et al., Citation1997). However, it is a matter of speculation as to the role of wild birds, carrier pheasants or poultry in the origin of the ORT in the incidents investigated. ORT is suspected to be transmitted both vertically (van Empel & Hafez, Citation1999) and horizontally between birds, and possible evidence of spread over a distance of 11 km has been reported (De Rosa et al., Citation1996). Cool weather conditions may have facilitated horizontal transmission of infection between neighbouring estates, which may account for the apparent geographical clustering of three of the cases within a distance of 8 km.
A multifactorial aetiology was demonstrated in these outbreaks of respiratory disease in pheasants. ORT was part of a complex of infectious agents that included other Pasteurellaceae, E. coli, M. gallisepticum, M. synoviae and other Mycoplasma species, APMV-2, coronaviruses and S. trachea. Exposure to other potentially intercurrent factors such as cold and wet weather conditions and alimentary tract parasitism is inherent in releasing birds into the wild and may have exacerbated the severity of the disease. Although the source of ORT was not established, it is possible that infection may have become endemic in pheasants or wildlife within the geographical area of the affected estates. At the present time it appears to be an uncommon infectious component of respiratory disease in the wider pheasant population but, as an apparently new and emerging disease threat, continued investigation of field outbreaks through scanning surveillance activities (Irvine et al., Citation2010) will be required to monitor the impact of ORT and other emerging infectious agents.
Acknowledgements
The authors would like to thank Helen Davidson, Bill Cox, Amanda Hanna, Marek Slomka, Rosário Gonçalves, Miroslav Hlusek, Jakub Muchowski and Jane Errington for their technical support and Jeremy Morgan, Alan Beynan and Rita Alves for submitting birds for investigation. The authors are indebted to Alisdair Wood for his advice and encouragement. The work was supported in part by the Defra Emerging Diseases and Welfare programme under project ED1300.
References
- Amann , R.I. , Binder , B.J. , Olson , R.J. , Chrisholn , S.W. , Devereux , R. and Stahl , D.A. 1990 . Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations . Applied Environmental Microbiology , 56 : 1919 – 1925 .
- Amonsin , A. , Wellehan , J.F.X. , Li , L-L. , Vandamme , P. , Lindeman , C. , Edman , M. , Robinson , R.A. and Kapur , V. 1997 . Molecular epidemiology of Ornithobacterium rhinotracheale . Journal of Clinical Microbiology , 35 : 2894 – 2898 .
- Benčina , D. , Mrzel , I. , Zorman Rojs , O. , Bidivec , A. and Dovč , A. 2003 . Characterisation of Mycoplasma gallisepticum strains involved in respiratory disease in pheasants and peafowl . The Veterinary Record , 152 : 230 – 234 . doi: 10.1136/vr.152.8.230
- Bradbury , J.M. , Yavari , C.A. and Dare , C.M. 2001a . Detection of Mycoplasma synoviae in clinically normal pheasants . The Veterinary Record , 148 : 72 – 74 . doi: 10.1136/vr.148.3.72
- Bradbury , J.M. , Yavari , C.A. and Dare , C.M. 2001b . Mycoplasmas and respiratory disease in pheasants and partridges . Avian Pathology , 30 : 391 – 396 . doi: 10.1080/03079450120066395
- Cavanagh , D. , Mawditt , K. , Welchman , D. de B. , Britton , P. and Gough , R.E. 2002 . Coronavirus from pheasants (Phasianus colchicus) are closely related to coronaviruses of domestic fowl (infectious bronchitis) and turkeys . Avian Pathology , 31 : 81 – 93 . doi: 10.1080/03079450120106651
- Charlton , B.R. , Channing-Santiago , S.E. , Bickford , A.A. , Cardona , C.J. , Chin , R.P. , Cooper , G.L. , Droual , R. , Jeffrey , J.S. , Meteyer , C.U. , Shivaprasad , H.L. and Walker , R.L. 1993 . Preliminary characterization of a pleomorphic gram-negative rod associated with avian respiratory disease . Journal of Veterinary Diagnostic Investigation , 5 : 47 – 51 . doi: 10.1177/104063879300500111
- Chin , R.P. , van Empel , P.C.M. and Hafez , H.M. 2008 . “ Ornithobacterium rhinotracheale infection ” . In Diseases of Poultry , 12th edn , Edited by: Saif , Y.M. , Fadly , A.M. , Glisson , J.R. , McDougald , L.R. , Nolan , L.K. and Swayne , D.E . 765 – 774 . Ames : Iowa State University Press .
- Dalton , J.R.F. , Niblett , J. and Thrusfield , M.V. 2002 . Response of pheasants to live attenuated turkey rhinotracheitis vaccine . The Veterinary Record , 151 : 341 – 344 . doi: 10.1136/vr.151.12.341
- De Rosa , M. , Droual , R. , Chin , R.P. , Shivaprasad , H.L. and Walker , R.L. 1996 . Ornithobacterium rhinotracheale infection in turkey breeders . Avian Diseases , 40 : 865 – 874 . doi: 10.2307/1592311
- Forrester , C.A. , Bradbury , J.M. , Dare , C.M. , Domangue , R.J. , Windsor , H. , Tasker , J.B. and Mockett , A.P.A. 2011 . Mycoplasma gallisepticum in pheasants and the efficacy of tylvalosin to treat the disease . Avian Pathology , 40 : 581 – 586 . doi: 10.1080/03079457.2011.618822
- Gough , R.E. , Alexander , D.J. , Collins , M.S. , Lister , S.A. and Cox , W.J. 1988 . Routine virus isolation or detection in the diagnosis of diseases in birds . Avian Pathology , 17 : 893 – 907 . doi: 10.1080/03079458808436511
- Hafez , H.M. and Lierz , M. 2010 . Ornithobacterium rhinotracheale in nestling falcons . Avian Diseases , 54 : 161 – 163 . doi: 10.1637/9008-080309-Case.1
- Irvine , R.M. , Cox , W.J. , Ceeraz , V. , Reid , S.M. , Ellis , R.J. , Jones , R.M. , Errington , J. , Wood , A.M. , McVicar , C. and Clark , M.I. 2010 . Detection of IBV QX in commercial broiler flocks in the UK . The Veterinary Record , 167 : 877 – 879 . doi: 10.1136/vr.c6692
- Keymer , I.M. 1961 . Infectious sinusitis of pheasants and partridges . The Veterinary Record , 73 : 1034 – 1038 .
- Kleven , S.H. and Glisson , J.R. 1997 . “ Multicausal respiratory disease ” . In Diseases of Poultry , 10th edn , Edited by: Calnek , B.W. , Barnes , H.J. , Beard , C.W. , McDougald , L.R. and Saif , Y.M. 1008 – 1012 . Ames : Iowa State University Press .
- Lopes , V.C , Velayadhan , B. , Halvorson , D.A. and Nagaraja , K.V. 2002 . Survival of Ornithobacterium rhinotracheale in sterilized poultry litter . Avian Diseases , 46 : 1011 – 1014 . doi: 10.1637/0005-2086(2002)046[1011:SOORIS]2.0.CO;2
- Mahmood , S. , Alexander , D.J. , Slomka , M.J. , Manvell , R.J. , Hanna , A. , Fuller , C.M. and Brown , I.H. 2010 . Phylogenetic analysis of the nucleotide sequences of the HN gene of 22 avian paramyxovirus type 2 (APMV-2) viruses reveals marked heterogeneity . Avian Pathology , 39 : 453 – 458 . doi: 10.1080/03079457.2010.517514
- Marien , M. , Decostere , A. , Martel , A. , Chiers , K. , Froyman , R. and Nauwynck , H. 2005 . Synergy between avian pneumovirus and Ornithobacterium rhinotracheale in turkeys . Avian Pathology , 34 : 204 – 211 . doi: 10.1080/03079450500096414
- McAuliffe , L. , Ellis , R.J. , Ayling , R.D. and Nicholas , R.A.J. 2003 . Differentiation of Mycoplasma species by 16S ribosomal DNA PCR and denaturing gradient gel electrophoresis fingerprinting . Journal of Clinical Microbiology , 41 : 4844 – 4847 . doi: 10.1128/JCM.41.10.4844-4847.2003
- McAuliffe , L. , Ellis , R.J. , Lawes , J.R. , Ayling , R.D. and Nicholas , R.A.J. 2005 . 16S rDNA PCR and denaturing gradient gel electrophoresis; a single generic test for detecting and differentiating Mycoplasma species . Journal of Medical Microbiology , 54 : 731 – 739 . doi: 10.1099/jmm.0.46058-0
- Moreno , B. , Chacón , G. , Villa , A. , Fernández , A.I. , Vela , A.I. , Fernández-Garayzábal , J.F. , Ferré , A. and Gracia , E. 2009 . Nervous signs associated with otitis and cranial osteomyelitis and with Ornithobacterium rhinotracheale infection in red-legged partridges (Alectoris rufa) . Avian Pathology , 38 : 341 – 347 . doi: 10.1080/03079450903183686
- Nicholas , R. & Baker , S. 1998 . Recovery of mycoplasmas from animals . In R.J. Miles & R.A.J. Nicholas Mycoplasma Protocols 37 – 43 (Methods in Molecular Medicine, 104) . Totawa , NJ : Humana Press .
- OIE 2008a . Avian mycoplasmosis (Mycoplasma gallisepticum, M.synoviae) . In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals , 1 , Chapter 2.3.5., 6th edn 482 – 496 . Paris : Office Internationale des Epizooties .
- OIE 2008b . Newcastle disease . In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals , 1 , Chapter 2.3.14., 6th edn 576 – 579 . Paris : Office Internationale des Epizooties .
- Ozdemir , I. , Russell , P.H. , Collier , J. , Alexander , D.J. and Manvell , R.J. 1990 . Monoclonal antibodies to avian paramyxovirus type 2 . Avian Pathology , 19 : 395 – 400 . doi: 10.1080/03079459008418689
- Roepke , D.C. , Back , A. , Shaw , D.P. , Nagaraja , K.V. , Sprenger , S.J. and Halverson , D.A. 1998 . Isolation and identification of Ornithobacterium rhinotracheale from commercial turkey flocks in the upper Midwest . Avian Diseases , 42 : 219 – 221 . doi: 10.2307/1592601
- Saitou , N. and Nei , M. 1987 . The neighbor-joining method: a new method for reconstructing phylogenetic trees . Molecular Biology & Evolution , 4 : 406 – 425 .
- Sakai , E. , Tokuyama , Y. , Nonaka , F. , Ohishi , S. , Ishikawa , Y. , Yanaka , M. and Taneno , A. 2000 . Ornithobacterium rhinotracheale infection in Japan: preliminary investigations . The Veterinary Record , 146 : 502 – 503 . doi: 10.1136/vr.146.17.502
- Sprenger , S.J. , Back , A. , Shaw , D.P. , Nagaraja , K.V. , Roepke , D.C. and Halvorson , D.A. 1998 . Ornithobacterium rhinotracheale infection in turkeys: experimental reproduction of the disease . Avian Diseases , 42 : 154 – 161 . doi: 10.2307/1592588
- Tamura , K. , Dudley , J. , Nei , M. and Kumar , S. 2007 . MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0 . Molecular Biology and Evolution , 24 : 1596 – 1599 . doi: 10.1093/molbev/msm092
- Vandamme , P. , Segers , P. , Vancanneyt , M. , Van Hove , K. , Mutters , R. , Hommez , J. , Dewhirst , F. , Paster , B. , Kersters , K. , Falsen , E. , Devriese , L.A. , Bisgaard , M. , Hinz , K-H. and Mannheim , W. 1994 . Ornithobacterium rhinotracheale gen. nov. sp. nov., isolated from the avian respiratory tract . International Journal of Systematic Bacteriology , 44 : 24 – 37 . doi: 10.1099/00207713-44-1-24
- van Empel , P.C.M. and Hafez , H.M. 1999 . Ornithobacterium rhinotracheale: a review . Avian Pathology , 28 : 217 – 227 . doi: 10.1080/03079459994704
- van Empel , P. , van den Bosch , H. , Goovaerts , D. and Storm , P. 1996 . Experimental infection in turkeys and chickens with Ornithobacterium rhinotracheale . Avian Diseases , 40 : 858 – 864 . doi: 10.2307/1592310
- van Empel , P. , Vrijenhoek , M. , Goovaerts , D. and van den Bosch , H. 1999 . Immunohistochemical and serological investigation of experimental Ornithobacterium rhinotracheale infection in chickens . Avian Pathology , 28 : 187 – 193 . doi: 10.1080/03079459994911
- van Veen , L. , Gruys , E. , Frik , K. and Van Empel , P. 2000 . Increased condemnation of broilers associated with Ornithobacterium rhinotracheale . The Veterinary Record , 147 : 422 – 423 . doi: 10.1136/vr.147.15.422
- van Veen , L. , Nieuwenhuizen , D. , Mekkes , M. , Vrijenhoek , P. and van empel , P. 2005 . Diagnosis and incidence of Ornithobacterium rhinotracheale infections in commercial broiler chickens at slaughter . The Veterinary Record , 156 : 315 – 317 .
- Welchman , D. de B. , Bradbury , J.M , Cavanagh , D. and Aebischer , N.J. 2002 . Infectious agents associated with respiratory disease in pheasants . The Veterinary Record , 150 : 658 – 664 . doi: 10.1136/vr.150.21.658
- Wood , A.M. , Dagless , M.D. , Pirie , J.O. , Garcia-Rueda , M.C. , Manvell , R.J. , Cox , W.J. , Ceeraz , V. , Pearson , D.B. , Law , W.A. , Alexander , D.J. and Brown , I.B. 2008 . Isolations of avian paramyxovirus type 2 (PMV-2) viruses from domestic fowl in Scotland in 2002 and 2006 . The Veterinary Record , 162 : 788 – 789 . doi: 10.1136/vr.162.24.788
- Zorman-Rojs , O. , Zdovc , I. , Benčina , D. and Mrzel , I. 2000 . Infection of turkeys with Ornithobacterium rhinotracheale and Mycoplasma synoviae . Avian Diseases , 44 : 1017 – 1022 . doi: 10.2307/1593082