Abstract
Necrotic enteritis, caused by netB toxin-producing Clostridium perfringens type A, is an important disease in broiler chickens worldwide. Earlier attempts to prevent necrotic enteritis by vaccination have not sufficiently taken into account the practical limitations of broiler vaccination. In most published studies on vaccination against necrotic enteritis, multiple doses at different ages are administered, which is not practical for broilers. The aim of this study was to compare the efficacy of subcutaneous single vaccination at day 1 or day 3 and double vaccination at day 3 and day 12, using crude supernatant containing active toxin or formaldehyde-inactivated supernatant (toxoid) of a netB-positive C. perfringens strain in a subclinical necrotic enteritis model. Double vaccination with crude supernatant resulted in a significant decrease in the number of chickens with necrotic enteritis lesions. The efficacy of vaccination using toxoid was lower compared with crude supernatant. Single vaccination with crude supernatant at day 3 resulted in significant protection, while vaccination of 1-day-old chickens with crude supernatant or toxoid, as envisaged for practical field application, did not induce protection.
Introduction
Necrotic enteritis, caused by Clostridium perfringens type A, occurs in broiler chickens and emerged after the ban on the use of antimicrobial growth promoters in the European Union in 2006. C. perfringens infections in poultry may present as an acute clinical disease, with high mortality at 2 to 5 weeks of age, or in a subclinical form leading to reduced weight gain (Kaldhusdal et al., Citation2001). The disease develops when several predisposing factors are present, such as coccidial co-infection and high protein-containing, high non-starch polysaccharide-containing diet (Thompson et al., Citation2006; Gholamiandehkordi et al., Citation2007; Van Immerseel et al., Citation2009). Nowadays, necrotic enteritis is typically controlled using antibiotics and anticoccidials (Lanckriet et al., Citation2010a). Owing to concerns about the spread of antibiotic-resistant bacteria and antibiotic residues in the food chain, there is a need for alternative control strategies. The use of feed additives, including organic acids, essential oils and prebiotics, can only marginally decrease the incidence of necrotic enteritis in broilers. No feed additives are as efficient as antibiotics in controlling the disease (Lensing et al., Citation2010; Thanissery et al., Citation2010; Timbermont et al., Citation2010; Jerzsele et al., Citation2012).
Vaccination of broilers may be an interesting option for the prevention of necrotic enteritis. In the literature, vaccination trials are described using crude supernatant, inactivated supernatant and recombinant proteins; the latter being either injected or administered using a live bacterial vector. Different vaccination approaches have been used in several necrotic enteritis models in the past few years (Lee et al., Citation2011). Vaccination in broiler chickens can be done using oral, subcutaneous or intramuscular administration. All previous reports on subcutaneous or intramuscular vaccination experiments are based on double or triple vaccination schedules. Both crude supernatant and formaldehyde-inactivated supernatant (toxoid) of C. perfringens have been studied as potential vaccines for the prevention of clinical and subclinical necrotic enteritis with variable degrees of success (Kulkarni et al., Citation2007; Cooper et al., Citation2009; Jiang et al., 2009; Lanckriet et al., Citation2010b; Saleh et al., Citation2011; Jang et al., Citation2012).
All studies carried out in the past used vaccination regimens that are not applicable in the field for logistical reasons. Indeed, subcutaneous or intramuscular injection of broilers is only possible at the hatchery on day of hatch. The use of crude supernatant is not possible for safety reasons and it is thus essential to either use pure immunogenic non-toxic proteins or inactivated proteins. In Clostridium vaccines, safety is often guaranteed using formaldehyde inactivation to produce a so-called toxoid (Jones et al., Citation2008).
The objective of this study was therefore to compare the efficacy of subcutaneous vaccination with crude supernatant and toxoid, using different vaccination regimens. Broilers were either vaccinated once on day of hatch or at 3 days of age or twice at days 3 and 12.
Materials and Methods
Clostridium strains and culture conditions
C. perfringens strain 23 was used for preparing crude supernatant and toxoid vaccines. This strain is a netB-positive toxin type A strain isolated from a broiler chicken (Gholamiandekhordi et al., Citation2006; Lanckriet et al., Citation2010b). The challenge strain used in the in vivo trials, C. perfringens strain 56, was isolated from a broiler chicken with necrotic lesions and has been shown to be highly virulent in in vivo trials (Gholamiandehkordi et al., Citation2007; Timbermont et al., Citation2009; Lanckriet et al., Citation2010b). Bacteria were grown at 37°C in Brain Heart Infusion broth (Oxoid, Basingstoke, UK) supplemented with 0.375% glucose in an anaerobic (84% N2, 8% CO2 and 8% H2) workstation (Ruskinn Technology, Bridgend, UK).
Vaccines
For all in vivo trials, supernatant derived from an overnight culture of C. perfringens strain 23 was concentrated using Vivaspin containing a 5000 molecular weight cut-off polyethersulphone membrane (Sartorius Stedim Biotech GmbH, Goettingen, Germany). The protein concentration from the supernatant was determined using a commercially available BCA Protein Assay Reagent (Thermo Scientific Pierce, Rockford, Illinois, USA). The concentrated supernatant was diluted in phosphate-buffered saline (PBS) to final protein concentrations as shown in . The supernatant used for toxoid preparation was inactivated for 16 h at 37°C with 1% (Trial 1) or for 24 h at 37°C with 0.5% (Trials 2 and 3) formaldehyde solution (Sigma-Aldrich, Bornem, Belgium) to produce a toxoid. Inactivation of the supernatant was tested by analysing the inactivation of the alpha toxin and theta toxin by loss of a double haemolytic zone when plating droplets on Columbia agar containing 5% sheep blood (Columbia Blood agar base®; Oxoid, Wesel, Germany). Quil A (Brenntag Biosector, Frederikssund, Denmark) was used as adjuvant (10 mg/ml PBS solution; 50 µg/bird/vaccination) in all test groups. The total volume administered to each bird was 0.2 ml. The freshly prepared vaccines were filter-sterilized (0.2 µm).
Table 1. Description of experimental groups used in this study.
In vivo necrotic enteritis model
The in vivo necrotic enteritis model was based on the subclinical in vivo model as described previously (Gholamiandehkordi et al., Citation2007). Groups of a variable number (indicated in ) of 1-day-old Ross 308 broiler chickens were fed a wheat/rye-based (43%/7.5%) diet, with soybean meal as the protein source. The feed composition was as described elsewhere (Gholamiandehkordi et al., Citation2007). Briefly, the diet contained high levels of (animal) proteins and non-starch polysaccharides that predispose to the development of necrotic enteritis. In all trials, Nobilis Gumboro D 78 vaccine (Schering-Plough Animal Health, Brussels, Belgium) was given in the drinking water on day 16 in all groups. From day 17 onwards, soy bean meal was replaced by fishmeal (30%) as the protein source. All groups were challenged orally on days 17, 18, 19 and 20 with approximately 4×108 colony-forming units C. perfringens strain 56 bacteria per challenge dose. On day 18, all groups were inoculated orally with a 10-fold dose of Paracox-5 (Schering-Plough Animal Health).
In Trial 1, three control groups were included. Two groups were left unvaccinated (Groups 1 and 2). In Group 1 all birds were inoculated with C. perfringens once a day during four consecutive days, while in Group 2 three inoculations per day were given from day 17 to day 20. The third control group (Group 3) was vaccinated with PBS and Quil A at days 3 and 12 post hatch, and C. perfringens inoculations were done three times a day between days 17 and 20. Five test groups were included. They were vaccinated subcutaneously in the neck with a 200 µl dose. Group 4 was vaccinated with crude supernatant containing 7 and 70 µg total protein at day 3 and day 12, respectively. Group 5 was vaccinated with 7 µg crude supernatant at day 3. Group 6 was vaccinated at day 3 and day 12 with 7 and 70 µg toxoid, respectively. Groups 7 and 8 were vaccinated with 7 µg toxoid at day 3 or day 1, respectively (). On each of days 22, 23 and 24, one-third of the birds were euthanized.
Trial 2 was carried out to clarify whether vaccination at days 3 and 12 with crude supernatant yielded better protection than vaccination at days 3 and 12 using toxoid. One control group was left unvaccinated (Group 1) and another control group was vaccinated with PBS and Quil A at day 3 and day 12 post hatch (Group 2). Group 3 was vaccinated at day 3 and day 12 with 7 and 70 µg crude supernatant, respectively, while Group 4 was vaccinated at day 3 and day 12 with 7 and 70 µg toxoid, respectively (). All birds were euthanized on day 21.
Trial 3 was carried out to compare the efficacy of vaccination at day 1 with crude supernatant or toxoid at different dosages with vaccination at day 3 and 12. One control group was left unvaccinated (Group 1) and another control group was vaccinated with PBS and Quil A at day 3 and day 12 post hatch (Group 2). Groups 3 and 4 were vaccinated at day 3 and day 12 with 7 and 70 µg crude supernatant or toxoid, respectively. The other test groups (Groups 5 to 12) were vaccinated at day 1 with different doses (35 µg, 70 µg, 140 µg, 210 µg) of crude supernatant or toxoid (). On each of days 21, 22 and 23, one-third of the birds were euthanized. The bird experiments were carried out according to the recommendations and following approval from the Ethical Committee of the Faculty of Veterinary Medicine, Ghent University.
Macroscopic lesion scoring
Lesion scoring in the small intestine (duodenum, jejunum and ileum) was performed as described by Keyburn et al. (Citation2006). Chickens with a lesion score of 2 or more were classified as necrotic enteritis-positive.
Statistical analysis
A two-tailed non-parametric test (Mann–Whitney U test; Version 5.00; GraphPad Prism Software, Inc., La Jolla, USA) was used to determine whether there was a significant difference between the percentage of positive chickens and the average lesion score in the vaccinated groups and control groups. Statistical significance was determined at P<0.05.
Results
Trial 1
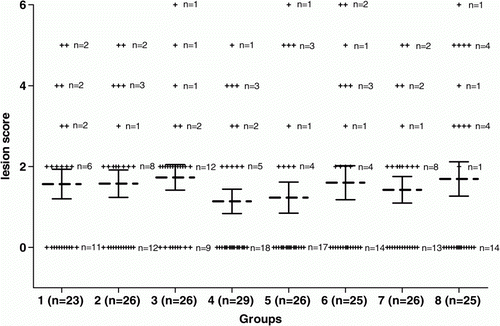
No chickens died during the challenge period. Vaccination with crude supernatant of C. perfringens strain 23 at days 3 and 12 resulted in a significant decrease (P <0.05) in the number of chickens with necrotic lesions compared with the control group vaccinated with Quil A and PBS. Single vaccination with crude supernatant at day 3 also resulted in significant protection (P<0.05) compared with the same control group. Vaccination with inactivated supernatant (toxoid) did not yield significant decreases in necrotic enteritis-positive birds (). When the average lesion score of each group of vaccinated broilers was compared with those of the control groups, no significant decreases were observed between control groups and groups vaccinated with active supernatant or toxoid (). To confirm the protective effects of administration of crude supernatant at days 3 and 12, and the loss of protection when using a toxoid, a trial with an increased number of birds per group was performed (Trial 2).
Figure 1. Lesion scores of individual broiler chickens challenged with C. perfringens in Trial 1. Dotted bars, average lesion score in each group. Solid bars, standard error of the mean (GraphPad Prism Software, Inc, USA). A description of the vaccination schedule of Groups 1 to 8 is shown in . No significant difference was seen between the groups.
Trial 2
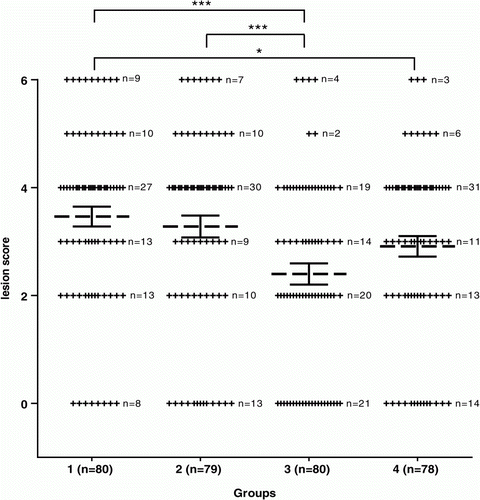
Because challenging the chickens once a day for four consecutive days with C. perfringens resulted in approximately the same average lesion score as challenging three times a day for four consecutive days (, Trial 1), in the second and third trials the challenge was only done once a day for four consecutive days. Double vaccination with crude supernatant of C. perfringens strain 23 again resulted in a significant decrease (P<0.01) of the number of chickens with necrotic lesions. Twelve chickens, originating from different groups, died during the challenge period and six moribund chickens were euthanized. All of these birds were necropsied and had the highest possible necrotic enteritis lesion score. The number of chickens with necrotic enteritis lesions was significantly lower in the group vaccinated with active supernatant at day 3 and day 12 compared with the non-Quil A treated control group. When the average lesion score from vaccinated groups was compared with those from control groups, significant differences were observed between both control groups and the group vaccinated with active supernatant at day 3 and day 12 (P<0.001). A significant difference was also observed between the non-Quil A treated positive control group and the group vaccinated with toxoid at day 3 and day 12 (P<0.05) ( and ).
Figure 2. Lesion scores of individual broiler chickens challenged with C. perfringens in Trial 2. Dotted bars, average lesion score in each group. Solid bars, standard error of the mean (GraphPad Prism Software, Inc, La Jolla, USA). A significant decrease was seen between both control groups (Groups 1 and 2) and the group vaccinated with active supernatant (Group 3) (***P<0.001). A significant difference was oserved between the unvaccinated control group (Group 1) and the group vaccinated with toxoid (Group 4) (*P<0.05). A description of the vaccination schedule of Groups 1 to 4 is shown in .
Trial 3
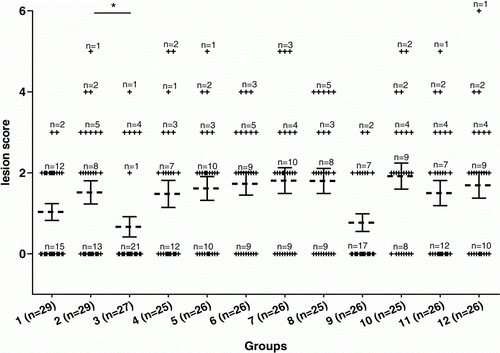
Trial 3 was performed to analyse whether a single-dose vaccination on the day of hatch could induce protection. In addition, multiple toxoid and crude supernatant vaccines containing different protein concentrations were compared. In this trial, repeated dose vaccination with active supernatant of C. perfringens strain 23, but not toxoid, protected against necrotic enteritis after challenge (P<0.05). No significant difference was seen between both positive control groups (non-Quil A treated positive control and Quil A treated positive control) and the groups that received a one-dose vaccination at day 1 with either crude supernatant or toxoid, independent of the protein concentration in the vaccines (). In the group vaccinated with the highest concentration crude supernatant (210 µg), six chickens died after vaccination. When the average lesion scores from vaccinated groups were compared with those from the control groups, a significant difference was observed between the unvaccinated control group and the group vaccinated with active supernatant at day 3 and day 12 (P<0.05). No significant differences were observed between both control groups and the groups vaccinated at day 1 ().
Figure 3. Lesion scores of individual broiler chickens challenged with C. perfringens in Trial 3. Dotted bars, average lesion score in each group. Solid bars, standard error of the mean (GraphPad Prism Software, Inc, La Jolla, USA). A significant decrease was detected between the unvaccinated control group to which Quil A was administered (Group 2), and the group vaccinated with active supernatant at day 3 and day 12 (Group 3) (*P<0.05). No significant difference was detected between the control groups and the groups vaccinated at day 1 with either crude supernatant or toxoid at different dosages (Groups 5 to 12). A description of the vaccination schedule of Groups 1 to 12 is shown in .
Discussion
Vaccination of broiler chickens at day 1 using a vaccine that is safe and does not affect their performance would be of high value as a preventive tool for necrotic enteritis. Formaldehyde inactivation has been used to produce Clostridium vaccines ensuring safety for vaccinated humans or animals (Thaysen-Andersen et al., Citation2007; Jones et al., Citation2008). Although crude supernatant of C. perfringens can induce protection against necrotic enteritis, it contains potent toxins and thus cannot be regarded as a vaccine that is safe for both the birds and the user. Indeed, we showed in our third trial that higher dosages induced mortality in the broilers. While subcutaneous administration of crude supernatant at day 3 and day 12 resulted in a significant decrease in the number of chickens with necrotic lesions, formaldehyde inactivation affected the efficacy and a toxoid was clearly less protective than the active supernatant. Formaldehyde is widely used in the production of inactivated vaccines. Although bacterial proteins treated with formaldehyde can be highly immunogenic, they often produce only low levels of neutralizing antibodies. For that reason the protection after vaccination with formaldehyde-inactivated proteins can be low (Nencioni et al., Citation1991; Petre et al., Citation1996; Jones et al., Citation2008). This is believed to be due to the cross-linking capacity of formaldehyde, with major conformational modifications of the cross-linked proteins, resulting in loss of immunogenicity of epitopes (Metz et al., Citation2004; Thaysen-Andersen et al., Citation2007; Jones et al., Citation2008). Our current findings are in agreement with this hypothesis. The importance of conformational epitopes in the protection against necrotic enteritis was already suggested by Kulkarni et al. (Citation2007), who showed that alpha-toxoid fails to offer protection.
Single vaccination on the day of hatch, even with crude supernatant, is not able to protect against necrotic enteritis in the model used, in contrast to repeated vaccination at days 3 and 12 and single vaccination at day 3, yielding partial protection. Other reports show that multiple vaccination regimens can significantly reduce necrotic lesions in challenged animals (Kulkarni et al., Citation2007; Cooper et al., Citation2009; Jiang et al., Citation2009; Lanckriet et al., Citation2010b; Saleh et al., Citation2011; Jang et al., Citation2012). The observation in Trial 1 that single vaccination at day 3 protected partially against the development of lesions is an interesting finding, but in practice administration later than day of hatch is logistically not feasible. It has been suggested already that immunization 1 day after hatching does not activate antibody production, most probably due to incomplete structural organization of the secondary lymphoid tissues in neonatal broilers (Mast & Goddeeris, Citation1999), which could explain the failure of vaccination on day of hatch. Whether the lack of protection of a toxoid vaccine and day-of-hatch vaccine regimes is also valid when using vaccine preparations derived from other C. perfringens strains is not clear and should be analysed in future studies. Indeed, the strain used for toxoid and crude supernatant preparation was isolated from a healthy chicken, and strains isolated from necrotic lesions could have been more appropriate. However, the supernatant of the strain used in the current study was shown to be superior in a comparative study using subcutaneous vaccination with supernatant from eight different strains at days 3 and 12 (Lanckriet et al., Citation2010b). For this reason the use of the vaccine preparations derived from this particular strain seems to be relevant and the data most probably can be extrapolated to the use of vaccine preparations derived from other strains.
Since vaccination on the day of hatch is not protective, there are two other options that are practical in the field. The first is immunization of parent flocks. Kulkarni et al. (Citation2007) suggested the importance of mucosal IgY, the major transferred maternal antibody, in the immunity to necrotic enteritis (Ulmer-Franco et al., Citation2012). Although vaccination can yield maternal antibodies, there is a high chance that these antibodies have disappeared by the time birds usually develop necrotic enteritis (3 to 4 weeks of age) (Lovland et al., Citation2004). Previous reports (Lovland et al., Citation2004; Crouch et al., Citation2010) described an increase in antibody response in breeder hens and partial passive protection in young chickens. Currently, there is one commercial toxoid vaccine for broiler breeder hens (Netvax®; Intervet/Schering-Plough Animal Health, Summit, New Jersey, USA) containing C. perfringens type A toxoid.
The other option that can be envisaged is the use of bacterial or viral vectors expressing recombinant proteins, provided that the strains can be given on the day of hatch (or in ovo) and persist for sufficient time to generate a protective immune response. Orally administered live vaccine strains expressing C. perfringens antigens and colonizing the intestinal tract of the broilers have been described (Kulkarni et al., Citation2006; Kulkarni et al., Citation2008; Zekarias et al., Citation2008). The protection obtained depends on the colonization level and persistence of the vaccine strains.
In conclusion, the current study shows that subcutaneous administration of crude supernatant or toxoid derived from a specific C. perfringens strain, on day of hatch, is not effective in controlling necrotic enteritis. Administration of crude supernatant at day 3, or double vaccination with crude supernatant at day 3 and day 12, was able to reduce the number of birds having necrotic enteritis lesions. In addition, subcutaneous toxoid administration was less efficient in double vaccination regimens as compared with crude supernatant.
References
- Cooper , K.K. , Trinh , H.T. and Songer , J.G. 2009 . Immunization with recombinant alpha toxin partially protects broiler chicks against experimental challenge with Clostridium perfringens . Veterinary Microbiology , 133 : 92 – 97 . doi: 10.1016/j.vetmic.2008.06.001
- Crouch , C.F. , Withanage , G.S. , de Haas , V. , Etore , F. and Francis , M.J. 2010 . Safety and efficacy of a maternal vaccine for the passive protection of broiler chicks against necrotic enteritis . Avian Pathology , 39 : 489 – 497 . doi: 10.1080/03079457.2010.517513
- Gholamiandehkordi , A.R. , Timbermont , L. , Lanckriet , A. , Van Den Broeck , W. , Pedersen , K. , Dewulf , J. , Pasmans , F. , Haesebrouck , F. , Ducatelle , R. and Van Immerseel , F. 2007 . Quantification of gut lesions in a subclinical necrotic enteritis model . Avian Pathology , 36 : 375 – 382 . doi: 10.1080/03079450701589118
- Gholamiandekhordi , A.R. , Ducatelle , R. , Heyndrickx , M. , Haesebrouck , F. and Van Immerseel , F. 2006 . Molecular and phenotypical characterization of Clostridium perfringens isolates from poultry flocks with different disease status . Veterinary Microbiology , 113 : 143 – 152 . doi: 10.1016/j.vetmic.2005.10.023
- Jang , S.I. , Lillehoj , H.S. , Lee , S.H. , Lee , K.W. , Lillehoj , E.P. , Hong , Y.H. , An , D.J. , Jeong , W. , Chun , J.E. , Bertrand , F. , Dupuis , L. , Deville , S. and Arous , J.B. 2012 . Vaccination with Clostridium perfringens recombinant proteins in combination with Montanide ISA 71 VG adjuvant increases protection against experimental necrotic enteritis in commercial broiler chickens . Vaccine , 30 : 5401 – 5406 . doi: 10.1016/j.vaccine.2012.06.007
- Jerzsele , A. , Szeker , K. , Csizinszky , R. , Gere , E. , Jakab , C. , Mallo , J.J. and Galfi , P. 2012 . Efficacy of protected sodium butyrate, a protected blend of essential oils, their combination, and Bacillus amyloliquefaciens spore suspension against artificially induced necrotic enteritis in broilers . Poultry Science , 91 : 837 – 843 . doi: 10.3382/ps.2011-01853
- Jiang , Y. , Kulkarni , R.R. , Parreira , V.R. and Prescott , J.F. 2009 . Immunization of broiler chickens against Clostridium perfringens-induced necrotic enteritis using purified recombinant immunogenic proteins . Avian Diseases , 53 : 409 – 415 . doi: 10.1637/8656-021109-Reg.1
- Jones , R.G. , Liu , Y. , Rigsby , P. and Sesardic , D. 2008 . An improved method for development of toxoid vaccines and antitoxins . Journal of Immunological Methods , 337 : 42 – 48 . doi: 10.1016/j.jim.2008.05.009
- Kaldhusdal , M. , Scneitz , C. , Hofshagen , M. and Skjerve , E. 2001 . Reduced incidence of Clostridium perfringens-associated lesions and improved performance in broiler chickens treated with normal intestinal bacterial from adult fowl . Avian Diseases , 45 : 149 – 156 . doi: 10.2307/1593022
- Keyburn , A.L. , Sheedy , S.A. , Ford , M.E. , Williamson , M.M. , Awad , M.M. , Rood , J.I. and Moore , R.J. 2006 . Alpha-toxin of Clostridium perfringens is not an essential virulence factor in necrotic enteritis in chickens . Infection and Immunity , 74 : 6496 – 6500 . doi: 10.1128/IAI.00806-06
- Kulkarni , R.R. , Parreira , V.R. , Sharif , S. and Prescott , J.F. 2006 . Clostridium perfringens antigens recognized by broiler chickens immune to necrotic enteritis . Clinical and Vaccine Immunology , 13 : 1358 – 1362 . doi: 10.1128/CVI.00292-06
- Kulkarni , R.R. , Parreira , V.R. , Sharif , S. and Prescott , J.F. 2007 . Immunization of broiler chickens against Clostridium perfringens-induced necrotic enteritis . Clinical and Vaccine Immunology , 14 : 1070 – 1077 . doi: 10.1128/CVI.00162-07
- Kulkarni , R.R. , Parreira , V.R. , Sharif , S. and Prescott , J.F. 2008 . Oral immunization of broiler chickens against necrotic enteritis with an attenuated Salmonella vaccine vector expressing Clostridium perfringens antigens . Vaccine , 26 : 4194 – 4203 . doi: 10.1016/j.vaccine.2008.05.079
- Lanckriet , A. , Timbermont , L. , De Gussem , M. , Marien , M. , Vancraeynest , D. , Haesebrouck , F. , Ducatelle , R. and Van Immerseel , F. 2010a . The effect of commonly used anticoccidials and antibiotics in a subclinical necrotic enteritis model . Avian Pathology , 39 : 63 – 68 . doi: 10.1080/03079450903505771
- Lanckriet , A. , Timbermont , L. , Eeckhaut , V. , Haesebrouck , F. , Ducatelle , R. and Van Immerseel , F. 2010b . Variable protection after vaccination of broiler chickens against necrotic enteritis using supernatants of different Clostridium perfringens strains . Vaccine , 28 : 5920 – 5923 . doi: 10.1016/j.vaccine.2010.06.035
- Lee , K.W. , Lillehoj , H.S. , Jeong , W. , Jeoung , H.Y. and An , D.J. 2011 . Avian necrotic enteritis: experimental models, host immunity, pathogenesis, risk factors, and vaccine development . Poultry Science , 90 : 1381 – 1390 . doi: 10.3382/ps.2010-01319
- Lensing , M. , van der Klis , J.D. , Fabri , T. , Cazemier , A. and Else , A.J. 2010 . Efficacy of a lactylate on production performance and intestinal health of broilers during a subclinical Clostridium perfringens infection . Poultry Science , 89 : 2401 – 2409 . doi: 10.3382/ps.2010-00942
- Lovland , A. , Kaldhusdal , M. , Redhead , K. , Skjerve , E. and Lillehaug , A. 2004 . Maternal vaccination against subclinical necrotic enteritis in broilers . Avian Pathology , 33 : 81 – 90 . doi: 10.1080/0379450310001636255
- Mast , J. and Goddeeris , B.M. 1999 . Development of immunocompetence of broiler chickens . Veterinary Immunology and Immunopathology , 70 : 245 – 256 . doi: 10.1016/S0165-2427(99)00079-3
- Metz , B. , Kersten , G.F. , Hoogerhout , P. , Brugghe , H.F. , Timmermans , H.A.M. , de Jong , A. , Meiring , H. , ten Hove , J. , Hennink , W.E. , Crommelin , D.J.A. and Jiskoot , W. 2004 . Identification of formaldehyde-induced modifications in proteins . The Journal of Biological Chemistry , 8 : 6235 – 6243 .
- Nencioni , L. , Volpini , G. , Peppoloni , S. , Bugnoli , M. , De Magistris , T. , Marsili , I. and Rappuoli , R. 1991 . Properties of pertussis toxin mutant PT-9K/129G after formaldehyde treatment . Infection and Immunity , 59 : 625 – 630 .
- Petre , J. , Pizza , M. , Nencioni , L. , Podda , A. , De Magistris , M.T. and Rappuoli , R. 1996 . The reaction of bacterial toxins with formaldehyde and its use for antigen stabilization . Developments in Biological Standardization , 87 : 125 – 134 .
- Saleh , N. , Fathalla , S.I. , Nabil , R. and Mosaad , A.A. 2011 . Clinicopathological and immunological studies on toxoids vaccine as a successful alternative in controlling clostridial infection in broilers . Anaerobe , 17 : 426 – 430 . doi: 10.1016/j.anaerobe.2011.04.019
- Thanissery , R. , McReynolds , J.L. , Conner , D.E. , Macklin , K.S. , Curtis , P.A. and Fasina , Y.O. 2010 . Evaluation of the efficacy of yeast extract in reducing intestinal Clostridium perfringens levels in broiler chickens . Poultry Science , 89 : 2380 – 2388 . doi: 10.3382/ps.2010-00879
- Thaysen-Andersen , M. , Jorgensen , S.B. , Wilhelmsen , E.S. , Petersen , J.W. and Hojrup , P. 2007 . Investigation of the detoxification mechanism of formaldehyde-treated tetanus toxin . Vaccine , 25 : 2213 – 2227 . doi: 10.1016/j.vaccine.2006.12.033
- Thompson , D.R. , Parreira , V.R. , Kulkarni , R.R. and Prescott , J.F. 2006 . Live attenuated vaccine-based control of necrotic enteritis of broiler chickens . Veterinary Microbiology , 113 : 25 – 34 . doi: 10.1016/j.vetmic.2005.10.015
- Timbermont , L. , Lanckriet , A. , Dewulf , J. , Nollet , N. , Schwarzer , K. , Haesebrouck , F. , Ducatelle , R. and Van Immerseel , F. 2010 . Control of Clostridium perfringens-induced necrotic enteritis in broilers by target-released butyric acid, fatty acids and essential oils . Avian Pathology , 39 : 117 – 121 . doi: 10.1080/03079451003610586
- Timbermont , L. , Lanckriet , A. , Gholamiandehkordi , A.R. , Pasmans , F , Martel , A. , Haesebrouck , F. , Ducatelle , R. and Van Immerseel , F. 2009 . Origin of Clostridium perfringens isolates determines the ability to induce necrotic enteritis in broilers . Comparative Immunology, Microbiology and Infectious Diseases , 32 : 503 – 512 . doi: 10.1016/j.cimid.2008.07.001
- Ulmer-Franco , A.M. , Cherian , G. , Quezada , N. , Fasenko , G.M. and McMullen , L.M. 2012 . Hatching egg and newly hatched chick yolk sac total IgY content at 3 broiler breeder flock ages . Poultry Science , 91 : 758 – 764 . doi: 10.3382/ps.2011-01757
- Van Immerseel , F. , Rood , J.I. , Moore , R.J. and Titball , R.W. 2009 . Rethinking our understanding of the pathogenesis of necrotic enteritis in chickens . Trends in Microbiology , 17 : 32 – 36 . doi: 10.1016/j.tim.2008.09.005
- Zekarias , B. , Mo , H. and Curtiss , R. 3rd . 2008 . Recombinant attenuated Salmonella enterica serovar Typhimurium expressing the carboxy-terminal domain of alpha toxin from Clostridium perfringens induces protective responses against necrotic enteritis in chickens . Clinical and Vaccine Immunology , 15 : 805 – 816 . doi: 10.1128/CVI.00457-07