Abstract
We developed a transgenic potato (TrP/R7) expressing the recombinant R7 (rR7) antigen for use as an oral vaccine to protect against a chicken protozoan disease, chicken leucocytozoonosis. The TrP/R7 potato was produced by Agrobacterium tumefaciens-mediated transformation and regeneration, and the R7 gene insertion into potato chromosomes was confirmed by genomic polymerase chain reaction and Southern hybridization. rR7 antigen expression in TrP/R7 potato was also confirmed by sandwich enzyme-linked immunosorbent assay and western blotting using an antibody against the second-generation schizont of Leucocytozoon caulleryi. A transgenic potato clone with the highest rR7 antigen expression (3 µg rR7 antigen per gram of fresh-weight potato leaves) was selected, cultivated, and used in oral administration experiments to examine its ability to boost immunity. Chickens were immunized with chicken leucocytozoonosis vaccine “Hokken” by injection, and chickens that developed moderate levels of antibody titres were fed with TrP/R7 leaves. Chickens fed with TrP/R7 leaves showed increased antibody responses. In contrast, chickens fed with non-transgenic potato leaves showed a continuous decrease in antibody titres. Furthermore, chickens fed with TrP/R7 potato leaves showed strong resistance against experimental challenge with L. caulleryi infection. This study demonstrates the use of a plant-based oral vaccine to boost immunity against a protozoan disease.
Introduction
Chicken leucocytozoonosis, caused by Leucocytozoon caulleryi protozoan infection, is observed in southeastern Asia including Japan (Mathis & Leger, Citation1909; Morii et al., Citation1981). Infected chickens develop anaemia or reduced egg production, and severe infection can cause mortality. Anti-parasitic treatments are effective against this disease (Akiba et al., Citation1964), but, because of drug residue issues, treatment with anti-parasitics in egg-producing chickens is prohibited by law in Japan. This disease is also observed in Southeast Asia, China and South Korea. Since 2004 in Thailand, regulations on the usage of anti-protozoan agents have become stricter, causing serious concern among the poultry industry regarding outbreaks of this disease. In addition, recent reports revealed that wild birds are highly infected with Leucocytozoon protozoa, and many studies have shown Leucocytozoon vectors between poultry and wild birds (Imura et al., Citation2012). Therefore, chicken leucocytozoonosis is a significant problem in the poultry industry, and immunological prevention such as vaccination is desirable. We developed a subunit vaccine (leucocytozoonosis vaccine “Hokken” [LV]) against this disease using Escherichia coli-expressed immunogenic recombinant R7 (rR7) antigen derived from the outer membrane of the second-generation schizont (2GS) of L. caulleryi (Itoh & Gotanda, Citation2002). This vaccine is already in use in Japan (Ito & Gotanda, Citation2004). The molecular structure of rR7 antigen is similar to that of candidate vaccine recombinant antigen of malaria parasites, which are closely related to L. caulleryi taxonomically, especially the circum sporozoite protein of Plasmodium vivax (Zavala et al., Citation1983). The structure, which has repeated regions at the C terminal that strongly induce humoral immunity, is specific to protozoan genomic sequences (Itoh & Gotanda, Citation2002).
We previously confirmed a strong correlation between anti-2GS antibodies detected in LV-injected chickens and disease prevention efficacy (Itoh & Gotanda, Citation2002). Chickens can therefore be protected from protozoan infection if they maintain high antibody levels. However, single administration of LV can only induce immunity for short periods. Thus, other methods of booster immunization with less stress than injectable vaccines, such as orally administered vaccines, are required. We administered lyophilized rR7 antigen expressed in E. coli with normal food to LV-vaccinated and unvaccinated chickens, and observed that only LV-injected chickens had an immune booster response (Ito et al., Citation2005). This suggested that oral immunization of rR7 antigen against leucocytozoonosis could be effective as a booster vaccine. However, considering the high quantities of antigen required for booster immunization, it is unknown whether oral administration of rR7 antigen can induce side effects such as diarrhoea caused by E. coli components. For these reasons it was desirable to develop a booster vaccine with lower antigen titres and a lower manufacturing cost.
In this study we created transgenic potatoes that expressed rR7 antigen to develop a more effective and safe oral vaccine against L. caulleryi, which does not require purifying processes. Furthermore, we evaluated the efficacy of the transgenic potatoes with chickens as the target animal, examining booster responses of humoral immunity and challenge with the parasite after oral administration of the transgenic products.
Materials and Methods
Transgenic potato expressing recombinant R7 antigen
The pTH-R7 plasmid vector (Invitrogen, Carlsbad, California, USA) that includes 2379 base pairs of R7 gene (GenBank, GI: 22026754) cloned from the 2GS of L. caulleryi was cut with restriction enzymes SmaI and SacI. Binary vector pBI2113 (Mitsuhara et al., Citation1996) containing the cauliflower mosaic virus 35S promoter, beta-glucuronidase gene (GUS) and nopaline synthetase terminator, was cut with restriction enzymes SmaI and SacI, and the DNA fragment of the R7 gene was inserted into the GUS region to obtain pBI2113/R7. This plasmid vector was inserted into Agrobacterium tumefaciens LBA4404 (Clontech, Mountain View, California, USA) using the freeze–thaw method. Transformed A. tumefaciens LBA4404 was then cultured in a flask with LB liquid medium by shaking at 28°C. After culturing, the transformed A. tumefaciens was collected by centrifuging at 3000×g at 4°C and suspended in MS medium (Sigma-Aldrich, St Louis, Missouri, USA). This bacterial suspension was used for infection of potato tubers to create the transformed potato (TrP/R7). Transformed potato tubers were passaged on MS solid medium including antibiotics, kanamycin 100 µg/ml and carbenicillin 500 µg/ml (Sigma-Aldrich) and 3% sucrose at 25°C every 2 weeks with a photoperiod of 16 h light and 8 h dark. Calli formed after 4 to 8 weeks of culture on medium discs, followed by the formation of shoots. These shoots were cut from their roots, replaced and cultured on no-hormone-containing MS solid medium including 3% sucrose, kanamycin 100 µg/ml and carbenicillin 500 µg/ml, pH 5.9. After 2 to 4 weeks, when the shoots were rooted, they were removed to 10 cm pots with compost and cultivated in an incubator with temperature, illumination, gas, and humidity control.
Confirmation of R7 gene insertion into potatoes
Leaves from TrP/R7 potatoes were frozen by liquid nitrogen and then ground and powdered in a mortar. The powdered sample was used to extract whole DNA according to the protocol in the REDExtract-N-Amp™ plant PCR Kit (Sigma-Aldrich), and genomic DNA polymerase chain reaction (PCR) was performed using primers R7f (5′-GGAAATGTGTCCTTAACTTC-3′) and R7r (5′-CTTCTTCTTCATTACTTTTTC-3′) that specifically amplify the R7 gene. The PCR conditions were as follows: hot start at 94°C for 3 min, followed by 40 cycles of 94°C for 1 min, 55°C for 1 min, and 72°C for 2 min. The obtained sample was used in electrophoresis with 1.2% agarose gel to confirm the amplified gene size. Southern hybridization was also performed as follows: extracted whole DNA (5 µg) from TrP/R7 leaves was digested with restriction enzymes XbaI and SacI. Digested DNA was then separated by electrophoresis with 1.0% agarose gel and the gel was soaked and denatured in 0.5 M NaOH and 1.5 M NaCl for 30 min. The fragmented DNA was shaken and neutralized in 0.5 M Tris hydrochloride and 1.5 M sodium chloride solution (pH 7.5) for 30 min and transferred to Hybond-N+(Amersham Bioscience, Piscataway, New Jersey, USA) by capillary transfer in 20× SSC solution (3 M NaCl and 0.3 M citric acid) overnight. After capillary transfer, the fragmented DNA was fixed by ultraviolet. Preparation of the probe, hybridization and detection of signals were performed as per the protocol for the DIG High Prime DNA Labeling and Detection Starter Kit II (Roche Diagnostics, Basel, Switzerland). PCR was then performed with plasmid pBER7 as a template and primer that amplified 276 base pairs at the 3′ side of the R7 gene (5′-CTCCCGGGAAGAGCATGAAATGGCTGTAACA-3′, 5′-GGGAGCTCCTTCTTCTTCATTCATTTTTC-3′), and the amplified product was labelled with digoxigenin. The signal was detected using anti-digoxigenin antibody (Roche Diagnostics).
Confirmation of recombinant R7 antigen expression in TrP/R7
One gram of TrP/R7 leaves was ground with 3 ml phosphate-buffered saline Tween buffer (135 mM NaCl, 1.5 mM NaH2PO4, 2.7 mM NaHPO4, 0.05% (v/v) Tween-20, pH 7.2). The TrP/R7 mixture was centrifuged at 3000×g for 15 min, and TrP/R7 extract was obtained as the mixture's supernatant. Using this extract, rR7 antigen was detected by sandwich enzyme-linked immunosorbent assay (ELISA) with monoclonal antibody R1 (Gotanda et al., Citation2002) against R7 antigen as previously described (Itoh & Gotanda, Citation2002). Moreover, 5 g TrP/R7 that shows a high titre in the sandwich ELISA was used for extraction using 50 ml TE buffer (50 mM Tris hydrochloride, 1 mM ethylenediamine tetraacetic acid, pH 8.0). The extract was purified by ion exchange chromatography using DEAE-Sephacel (Amersham Bioscience). Sodium dodecyl sulphate-polyacrylamide gel electrophoresis was performed using this purified extract and proteins were transferred to nitrocellulose membrane, followed by western blotting using the ECL Advance Western Blotting Detection Kit (Amersham Bioscience). For the detection of antigen, anti-2GS polyclonal antibody obtained from a rabbit immunized with 2GS antigen purified with sonication, and anti-rabbit horseradish peroxidase labelled IgG were used.
Evaluation of booster immunization by oral administration of TrP/R7 leaves to chickens
A half-dose of LV was injected intramuscularly to eight 42-day-old specific-pathogen-free white leghorn chickens (Nippon Institute for Biological Science, Tokyo, Japan). After immunization, blood was collected from chickens periodically and the presence of anti-2GS antibody was determined by ELISA using 2GS antigen as previously described (Isobe & Suzuki, Citation1986). Chickens were divided into two groups (Group A and Group B) 8 weeks post immunization when the mean of anti-2GS antibody titres from tested chickens was 8000 to 9000. Group A chickens were fed normal food prepared with powdered, lyophilized TrP/R7 leaves. The amount of powdered TrP/R7 leaves per chicken per day was 5 g (containing about 150 µg rR7 antigen, equivalent to about 20 doses of LV) added to 65 g normal feed. The mixed feed was given to group A chickens for 7 days continuously, and normal feed was given for the next 7 days, which was followed by 7 more days in which chickens were fed TrP/R7 leaves again. The total amount of freeze-dried TrP/R7 leaves per chicken was 70 g over 14 days. Group B chickens had the same administration schedule and administration amounts, except they had powdered, lyophilized non-transgenic potato leaves in their feed. Blood was collected on days 0, 4, 7, 11, 14, 18, 21, and 25 after the beginning of oral administration of leaves, and anti-2GS antibody titres in blood were determined for each chicken. The booster response of humoral immunity was thus evaluated.
Efficacy of prevention of protozoan infection in chickens fed with TrP/R7 leaves
L. caulleryi was prepared for the challenge experiment as follows: we collected 200 Culicoides arakawae, which transmit L. caulleryi, and colonized them in culture as described by Morii & Kitaoka (Citation1968), in a small case that had a mesh cover on one side. The small case with C. arakawae was then placed on the chest of an infected chicken in which gametocytes of L. caulleryi Shizuoka strain were present (Morii, Citation1972) for 3 min to allow them to draw blood. The L. caulleryi-infected C. arakawae were cultured for 3 days at 25°C until L. caulleryi became sporozoites in the midges. C. arakawae was homogenized with 199 medium (GIBCO® Cell Culture, Carlsbad, California, USA), and then sporozoites were counted and used for laboratory challenge experiments.
All chickens tested (in both Groups A and B) were challenged at 25 days after oral administration started, with intravenous injection of 3000 sporozoites of L. caulleryi per chicken. After challenge with parasites, all chickens were observed for specific clinical signs of leucocytozoonosis such as anaemia, discharge of green faeces, and depression until the experiment ended. Blood was collected from all chickens at day 13 post challenge for the detection of serum-soluble antigen by agar gel precipitation that reflected propagation of 2GS (Morii, Citation1972). Furthermore, on days 14 and 19 post challenge, a blood smear was prepared to detect second-generation merozoites and gametocytes, respectively (Akiba, Citation1960). All parasitemia observed in tested chickens was compared between Group A and Group B.
Statistical analysis
The means of anti-2GS antibody titres in chickens after TrP/R7 or non-transgenic potato leaf administration were calculated using a one-sided unpaired Student's t test to determine the significant difference between two groups. Levels of parasitaemia observed after protozoan challenge in chickens were measured using Fisher's exact test (detection of serum-soluble antigen) and one-sided unpaired Student's t test (detection of second-generation merozoites and gametocytes) for significant differences.
Results
Genomic PCR and southern hybridization using TrP/R7 leaves
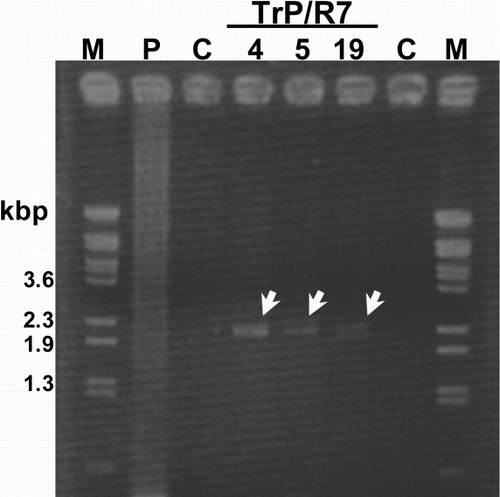
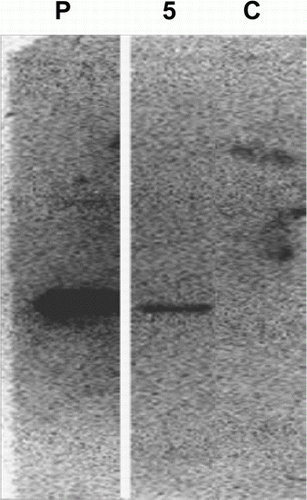
shows the results of genomic PCR using TrP/R7 leaves. In three of 76 regenerated transgenic potatoes, an amplified gene of approximately 2.3 kilobase pairs was observed and confirmed as the R7 gene. In Southern hybridization using the R7 gene cut from pBE2113 plasmid vector by restriction enzymes (XbaI and SacI), a signal was detected at the desired size in one out of 76 regenerated plants (No. 5) (). We thus confirmed that one TrP/R7 potato received the complete R7 gene in its chromosomes by gene insertion.
Sandwich ELISA and western blotting using TrP/R7 leaves
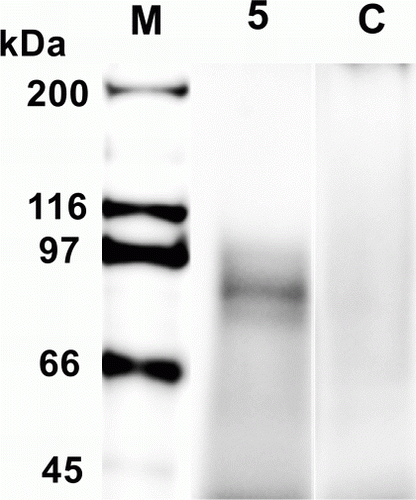
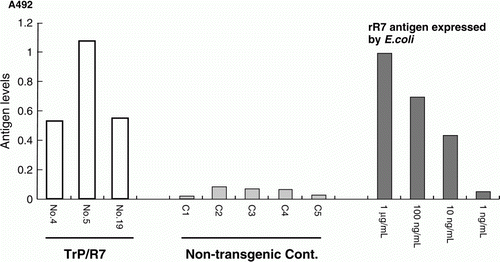
rR7 antigen expression was demonstrated in one transgenic potato with high optical density values by sandwich ELISA using TrP/R7 extracts from a regenerated potato (No. 5) (). We found that TrP/R7 No. 5 expressed 3 µg of rR7 antigen in 1 g fresh leaves. Western blotting was performed using purified extracts of this ELISA-positive TrP/R7 potato, and a strong signal was observed around 90 kDa that was equal to the approximate R7 molecular weight as estimated from its amino acid sequence. No signal was observed from non-transgenic potatoes by western blotting ().
Figure 3. Detection of rR7 antigen in TrP/R7 leaves by sandwich ELISA using monoclonal antibody R1. The rR7 antigen detected is shown as: white bar, regenerated TrP/R7 potato; grey bar, non-transgenic potato as control; and dark grey bar, E. coli expressed. The number displayed on graph represents the individual number of TrP/R7 or non-transgenic potato leaves.
Selection of TrP/R7 with high recombinant R7 antigen expression
We selected TrP/R7 No. 5 based on the confirmation of R7 gene insertion by genomic PCR, southern hybridization, sandwich ELISA and western blotting. Large-scale cultivation of TrP/R7 No. 5 was performed in an incubator with temperature, illumination, gas, and humidity controls, and was used for oral administration experiments.
Booster responses of antibodies in TrP/R7-fed chickens
shows the duration of anti-2GS antibody titres in each group after oral administration of lyophilized TrP/R7 leaves or non-transgenic potato leaves mixed feeds. The mean antibody titres of both Group A (TrP/R7 leaves-fed group) and Group B (non-transgenic potato leaves-fed group) were about 7800 on day 0. Antibody titres of Group B chickens decreased with time and the mean antibody titres on day 25 since oral administration started was 1903. In contrast, antibody titres of Group A chickens that had TrP/R7 feed were maintained at the same level or were elevated. During the second oral administration period, from day 14 to day 21, the antibody titres of Group A chickens decreased slightly (mean antibody titre=5382), but were significantly higher compared with Group B chickens (P <0.05).
Table 1. Titres of anti-2GS antibodies in chickens orally administered transgenic/non-transgenic leaves as booster vaccination.
Evaluation of protective efficacy in TrP/R7-treated chickens
shows the clinical signs observed and parasitemia level in all chickens of each group after challenge infection of L. caulleryi on day 25 post oral administration. In Group B, receiving lyophilized non-transgenic potato leaves, all chickens developed specific signs of disease such as anaemia, green faeces, and depression. In contrast, Group A chickens, receiving TrP/R7 feed, did not develop any clinical signs (). In addition, Group A chickens had significantly lower parasitemia levels than Group B chickens (P<0.01), especially when detecting second-generation merozoites and gametocytes, which are present during the erythrocyte-infecting stages and cause anaemia.
Figure 5. Chickens were fed TrP/R7 leaves (left) or non-transgenic potato leaves (right) and observed day 19 post experimental challenge with L. caulleryi. The non-transgenic potato leaf-administered chickens developed specific signs of disease such as anaemia (whitened comb) caused by protozoan infection.

Table 2. Protective effects of orally administered TrP/R7 leaves against experimental protozoan infection in chickens.
Discussion
LV, a recombinant subunit vaccine against protozoan disease, was the first animal biological approved and is now used in Japan. LV uses an oil adjuvant, and provides chickens with protective immunity that lasts for 5 months from a single injection (Itoh & Gotanda, Citation2002). In Japan, the peak of chicken leucocytozoonosis is observed during the summer when C. arakawae (Akiba, Citation1970), the transmitting vector of this disease, spreads. Initially, it was thought that 5 months was long enough to maintain protective immunity against the disease. However, large poultry farms control their numbers of chickens so that they always hold chickens with a high egg-producing rate (200 to 300 days old) and keep egg productivity stable. Normally, almost all vaccination programmes for chickens are set before the laying period, and therefore some lots of chickens cannot maintain immunity against leucocytozoonosis from LV injection at high enough levels during leucocytozoonosis-spreading periods. Therefore, some chickens will have little or no immunity and are at high risk for infection, causing a loss in egg productivity, one of the most important problems in the poultry industry. Our study provides a solution, whereby chickens receive primary immunization with LV during the rearing period, and maintain immunity by oral administration of TrP/R7 during their laying period when chickens cannot be vaccinated by injection because of the stress from vaccine shots.
In this study, primary vaccination of chickens used one-half of the usual dose of LV, and we evaluated the efficacy of booster immunization using TrP/R7 leaves. This was due to financial considerations as we felt the total cost of the combined plant-based vaccine and LV should not be higher than that of LV only. The use of half-doses of LV injection resulted in a reduced period of stable antibody titres at moderate levels compared with the normal single dose (data not shown). However, we found that oral administration of TrP/R7 prevented further decrease in antibody titres. Moreover, we observed no clinical signs in TrP/R7-administrated chickens after experimental challenge with L. caulleryi. Based on these results, it may be possible to reduce costs of LV vaccination by one-half when plant-based oral vaccines are used as booster vaccinations. Thus, the total cost for leucocytozoonosis prevention may not be higher than the current cost, even if we use the plant-based oral vaccine.
Recent studies have shown the efficacy of recombinant plant-based vaccines against several pathogens including protozoa. However, vaccines were administered by injection and also required purification steps (Farrance et al., Citation2011). It has been suggested that the use of plant-based oral vaccines for booster immunization, with injected vaccination for primary immunization, may be more effective than current strategies (Wu et al., Citation2004; Thanavala et al., Citation2005). Here we present data on the evaluation of efficacy, especially infection-preventing effectiveness, of a plant-based oral vaccine against protozoan infectious disease. Our results demonstrate the advantages in combining current injected vaccines and plant-based oral vaccines.
Here, we scheduled two 1-week oral administration periods with a 1-week interval (non-oral administration period). This was due to the possibility of tolerance caused by long-term administration of the plant-based vaccine (Ogra et al., Citation2001; Streatfield & Howard, Citation2003). We previously confirmed that intermittent oral administration of E. coli-expressed rR7 antigen over a short period could boost sufficient protective immunity (Ito et al., Citation2005). We plan further studies for the development of a more effective and economical administration programme. In this study we prepared 70 g of TrP/R7 mixed feeds (5 g lyophilized TrP/R7 potato leaves and 65 g normal feeds) per chicken per day, which contained 150 µg of rR7 antigen, the maximum amount of feed a chicken can eat per day. However, it may be possible to obtain sufficient efficacy using smaller quantities of TrP/R7 to boost immunity to prevent infection.
Our report presents strong data for plant-based oral vaccines that may be used to develop other vaccines against protozoan infectious diseases as a proof-of-concept study. However, our experimental data merely confirmed protective effects immediately after the oral administration of transgenic plants as a booster vaccine that was administered about 2 months after primary vaccination by injection. Further experiments to examine booster efficacy of transgenic plant vaccines over a long period should thus be performed with more chickens per group. In addition to this, we will consider improving our transgenic vaccine using mucosal adjuvants such as synthetic oligodeoxynucleotides containing CpG motifs (CpG-ODNs) so that the vaccine can provide stronger booster responses (Ameiss et al., Citation2006).
Acknowledgements
The authors are deeply grateful to Dr Tsutomu Morii for the distribution of L. caulleryi used in this study. They are also grateful to Dr Masatoshi Tanida and Dr Katsumi Kume for their many suggestions during this study.
References
- Akiba , K. 1960 . Studies on the Leucocytozoon found in the chicken, in Japan. II. On the transmission of L. caulleryi by Culicoides arakawae . Japanese Journal of Veterinary Science , 22 , 309 – 317 , plate 1 . doi: 10.1292/jvms1939.22.309
- Akiba , K. 1970 . Leucocytozoonosis of chickens . National Institute of Animal Health Quarterly , 10 : 131 – 147 .
- Akiba , K. , Ebisawa , S. , Nozawa , S. , Komiyama , T. and Minai , T. 1964 . Preventive effects of pyrimethamine and some sulfonamides on Leucocytozoon caulleryi in chickens . National Institute of Animal Health Quarterly , 122 : 222 – 228 .
- Ameiss , K.A. , El Attrache , J. , Barri , A. , McElroy , A.P. and Caldwell , D.J. 2006 . Influence of orally administered CpG-ODNs on the humoral response to bovine serum albumin (BSA) in chickens . Veterinary Immunology and Immunopathology , 110 : 257 – 267 . doi: 10.1016/j.vetimm.2005.10.011
- Farrance , C.E. , Rhee , A. , Jones , R.M. , Musiychuk , K. , Shamloul , M. , Sharma , S. , Mett , V. , Chichester , J.A. , Streatfield , S.J. , Roeffen , W. , van de Vegte-Bolmer , M. , Sauerwein , R.W. , Tsuboi , T. , Muratova , O.V. , Wu , Y. & Yusibov , V. 2011 . A plant-produced Pfs230 vaccine candidate blocks transmission of Plasmodium falciparum . Clinical and Vaccine Immunology , 1351 – 1357 . doi: 10.1128/CVI.05105-11
- Gotanda , T. , Doi , M. , Eiguchi , Y. , Tanaka , Y. , Kobayashi , S. and Fujisaki , Y. 2002 . Characterization of monoclonal antibodies against the second-generation schizonts of Leucocytozoon caulleryi . Journal of Veterinary Medical Science , 64 : 281 – 283 . doi: 10.1292/jvms.64.281
- Imura , T. , Suzuki , Y. , Ejiri , H. , Sato , Y. , Ishida , K. , Sumiyama , D. , Murata , K. and Yukawa , M. 2012 . Prevalence of avian haematozoa in wild birds in a high-altitude forest in Japan . Veterinary Parasitology , 183 : 244 – 248 .
- Isobe , T. and Suzuki , K. 1986 . Enzyme-linked immunosorbent assay for detection of antibody to Leucocytozoon caulleryi . Avian Pathology , 15 : 199 – 211 . doi: 10.1080/03079458608436281
- Ito , A. and Gotanda , T. 2004 . Field efficacy of recombinant R7 vaccine against chicken leucocytozoonosis . Journal of Veterinary Medical Science , 66 : 483 – 487 . doi: 10.1292/jvms.66.483
- Ito , A. , Gotanda , T. , Kobayashi , S. , Kume , K. , Sugimoto , C. and Matsumura , T. 2005 . Increase of antibody titer against Leucocytozoon caulleryi by oral administration of recombinant R7 antigen . Journal of Veterinary Medical Science , 67 : 211 – 213 . doi: 10.1292/jvms.67.211
- Itoh , A. and Gotanda , T. 2002 . The correlation of protective effects and antibody production in immunized chickens with recombinant R7 vaccine against Leucocytozoon caulleryi . Journal of Veterinary Medical Science , 64 : 405 – 411 . doi: 10.1292/jvms.64.405
- Mathis , C. and Leger , M. 1909 . Leucocytozoon de la poule. CR . Society of Biology (Paris) , 67 : 470 – 472 .
- Mitsuhara , I. , Ugaki , M. , Hirochika , H. , Ohshima , M. , Murakami , T. , Gotoh , Y. , Katayose , Y. , Nakamura , S. , Honkura , R. , Nishimiya , S. , Ueno , K. , Mochizuki , A. , Tanimoto , H. , Tsugawa , H. , Otsuki , Y. and Ohashi , Y. 1996 . Efficient promoter cassettes for enhanced expression of foreign genes in dicotyledonous and monocotyledonous plants . Plant & Cell Physiology , 37 : 49 – 59 . doi: 10.1093/oxfordjournals.pcp.a028913
- Morii , T. 1972 . Presence of antigens and antibodies in the sera of chickens infected with Akiba caulleryi . National Institute of Animal Health Quarterly , 12 : 161 – 167 .
- Morii , T. and Kitaoka , S. 1968 . The laboratory colonization of Culicoides arakwae (Diptera. Ceratopogonidae) . National Institute of Animal Health Quarterly , 8 : 26 – 30 .
- Morii , T. , Shiihara , T. , Lee , Y.C. , Manuel , M.F. , Nakamura , K. , Iijima , T. and Hoji , K. 1981 . Seroimmunological and parasitological surveys of Leucocytozoon caulleryi . The International Journal for Parasitology , 11 : 187 – 190 . doi: 10.1016/0020-7519(81)90047-3
- Ogra , P.L. , Faden , H. and Welliver , R.C. 2001 . Vaccination strategies for mucosal immune responses . Clinical Microbiology Reviews , 14 : 430 – 445 . doi: 10.1128/CMR.14.2.430-445.2001
- Streatfield , S.J. and Howard , J.A. 2003 . Plant-based vaccines . The International Journal for Parasitology , 33 : 479 – 493 . doi: 10.1016/S0020-7519(03)00052-3
- Thanavala , Y. , Mahoney , M. , Pal , S. , Scott , A. , Richter , L. , Natarajan , N. , Goodwin , P. , Arntzen , C.J. and Mason , H.S. 2005 . Immunogenicity in humans of an oral vaccine for hepatitis B . Proceedings of the National Academy of Sciences of the United States of America , 102 : 3378 – 3382 . doi: 10.1073/pnas.0409899102
- Wu , H. , Singh , N.K. , Locy , R.D. , Scissum-Gunn , K. and Giambrone , J.J. 2004 . Immunization of chickens with VP2 protein of infectious bursal disease virus expressed in Arabidopsis thaliana . Avian Diseases , 48 : 663 – 668 . doi: 10.1637/7074
- Zavala , F. , Cochrane , A.H. , Nardin , E.H. , Nussenzweig , R.S. and Nussenzweig , V. 1983 . Circumsporozoite proteins of malaria parasites contain a single immunodominant region with two or more identical epitopes . The Journal of Experimental Medicine , 157 : 1947 – 1957 . doi: 10.1084/jem.157.6.1947