Abstract
An ostrich farm of 929 birds that tested polymerase chain reaction-positive for highly pathogenic avian influenza H5N2 in a single sample was designated for culling, despite no evidence of sero-conversion as assessed by haemagglutination inhibition (HI) tests. A month later and immediately prior to culling, all birds were bled and tested with an IDEXX avian influenza virus (AIV) nucleoprotein (NP)-specific enzyme-linked immunosorbent assay (ELISA) and a high sero-prevalence was detected. To address the question of whether the NP-specific antibodies detected indicated exposure to H5 or non-H5 subtypes (H6N2 and H1N2 strains were also circulating regionally at the time), we developed two H5-specific ELISAs, both based on a recombinant H5 HA1 antigen. The H5 indirect ELISA used a horseradish peroxidase ostrich IgY conjugate that we produced in chicken eggs. The single-chain variable fragment (scFv) competitive ELISA (H5 scFv cELISA) used a scFv derived from an H5-immune chicken scFv library. By comparing IDEXX AIV ELISA results with those of the two H5-specific ELISAs and HI tests, we determined that up to 89% of the flock had been exposed to H5N2 AIV. We also detected evidence of suspected vaccination, since 17% of sera contained antibodies against the H5 glycoprotein but not the NP protein. Comparative analytical sensitivity indicated that HI tests are likely to miss up to 35% of H5-positive samples, and thus we consider that H5/H7-specific ELISAs should replace HI tests for ostrich testing in future.
Introduction
Highly pathogenic avian influenza (HPAI) viruses of the H5 and H7 subtypes can cause serious disease in most types of poultry and occasionally mammals, including humans. Both HPAI and the low-pathogenic avian influenza forms of H5 and H7 viruses, termed “notifiable” avian influenza (NAI), are subject to official control (OIE, Citation2012). Farmed ostriches (Struthio camelus) are classified as poultry by the World Animal Health Organization (OIE) and the European Union, and are thus subject to the reporting and control procedures that apply to NAI (Toffan et al., Citation2010). Unlike other poultry, however, adult ostriches show few clinical signs following infection with HPAI and mortality is usually low even when the infecting strains are highly pathogenic in/virulent to chickens (Manvell et al., Citation1998, Citation2003; Capua et al., Citation2000; Olivier, Citation2006). Ostriches younger than 6 months may show clinical signs that include respiratory difficulty, green urine, depression, acute hepatitis, peritonitis and mortality. Factors such as infection with other pathogens, high bird densities and cold weather spells can increase the severity of the disease (Oliver, Citation2006).
The ostrich farming industries of South Africa's Western and Eastern Cape provinces account for at least 65% of global ostrich production, with 62% of income derived from meat, much of which is exported to Europe (SA Ostrich Business Chamber, Oudtshoorn, Western Cape Province, South Africa). The seasonal appearance of avian influenza is associated with the clinical signs described above. Numerous low-pathogenic avian influenza strains have been isolated from ostriches since 1991 (Olivier, Citation2006; Abolnik et al., Citation2010). In recent years, outbreaks of HPAI H5N2 in the Western and Eastern Cape provinces have had devastating effects on the ostrich industry (Abolnik, Citation2007; Abolnik et al., Citation2009). The most recent outbreak affected the Western Cape Province during 2011 and caused a loss of export revenue due to a trade embargo that was estimated at more than €10 million per month (South African Ostrich Business Chamber). As with previous outbreaks, the disease was controlled by stamping out, since vaccination of ostriches against avian influenza is not currently permitted in South Africa.
Serology indicated that the progenitor to the 2011 HPAI H5N2 outbreak virus was probably present in 2010. Compulsory bi-annual surveillance, pre-movement, pre-slaughter and outbreak response testing of sera was conducted by haemagglutination inhibition (HI) assay (H5/H7) using an H5N2 antigen isolated from ostriches in 2006 that was demonstrated to be sufficiently reactive with antibodies from circulating outbreak strains in 2011, as well as an H7N1 strain from 1991. When the first HPAI H5N2 reverse-transcription polymerase chain reaction (RT-PCR)-positive case in the outbreak was detected in early March 2011, a control area was established and all farms within this zone were tested. A total of 43 farms, 41 of which were within the control area, tested H5N2-positive using RT-PCR or serology from February 2011 to February 2012 (Western Cape Department of Agriculture outbreak situation reports) and co-circulation of H1N2 and H6N2 was detected on some farms (Abolnik et al., Citation2012).
On 26 April 2011, 60 serum samples and 12 cloacal swab pools (five swabs per pool) were collected for testing on farm AI18 within the control area. Serum samples were negative for H5 and H7 specific antibodies on the HI assay but one swab pool tested positive for the presence of the HPAI H5N2 virus (unpublished data). A further 60 sera (56 from the same birds) collected a day later also tested H5/H7-negative on HI assay. In accordance with regulations, the farm was designated for slaughter and birds sent to the abattoir on 26 May 2011. Blood samples were collected from the entire flock prior to slaughter and the sera were tested by enzyme-linked immunosorbent assay (ELISA) by a private laboratory for the presence of anti-avian influenza virus (anti-AIV) antibodies (IDEXX AIV Ab ELISA). This nucleoprotein (NP)-based ELISA indicated a high proportion of sero-positives (unpublished) but since none of the birds on the farm had shown any clinical signs and H5/H7 HI tests were regarded as negative, it was presumed that the high AIV sero-positivity was due to circulation of non-NAI viruses (e.g. H1N2 and H6N2). Since all official surveillance testing at the time was carried out using only HI, we wished to establish whether ELISA could be used as an alternative assay. Accordingly we developed two H5-specific ELISA assays and compared ELISA with traditional HI tests. Both H5 immunoassays used a recombinant haemagglutinin (HA) produced in eukaryotic cells, but we compared two different formats to determine which was more suitable for ostrich sera. One was an indirect ELISA in combination with a chicken anti-ostrich IgY to detect serum antibodies, while the other aimed to exploit a recombinant antibody specific for H5 in a competitive ELISA. Our objectives were to re-test the sera and to confirm whether H5 was the circulating serotype on farm AI18 at the time of slaughter.
Materials and Methods
Samples
Farm AI18 is a typical concentrating unit that receives chicks from a number of chick-rearing farms outside the Klein Karoo region, where 60% of all slaughter birds are farmed. Keeping chick producers outside this area mitigates the disease threats associated with high ostrich population densities. Farm AI18 received chicks of 4 to 5 months in age from four chick-rearing farms over a period of 3 months. Chicks were weighed and marked on the chick farms prior to transport to Farm AI18. At the time of testing, 929 ostriches of 5 to 9 months of age were housed in seven fenced camps. Sera collected from the right jugular veins were collected in 4 ml ethylenediamine tetraacetic acid tubes stored at −20°C until testing.
AIV group-specific nucleo protein competitive ELISA
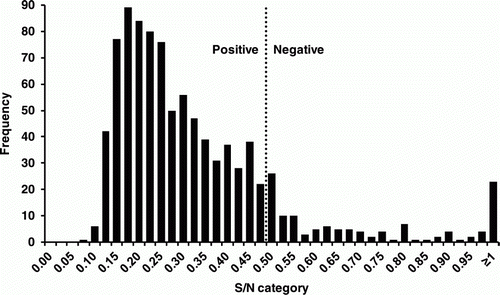
Serum samples (n = 929) were tested in duplicate using the IDEXX Influenza A antibody test kit (product no. 53101-02; IDEXX Laboratories, Johannesburg, Gauteng Province, South Africa) according to the recommended method. The optical density (OD) was measured at A650 nm on an ELx808 plate reader (BioTek, Winooski, VT, USA) and results were expressed as the sample/negative (S/N) ratio. S/N values of 0.5 and below are classified as positive. Three hundred and sixty-six samples were randomly selected for further testing.
Development of H5-specific indirect ELISA (H5 iELISA)
Chicken anti-ostrich IgY conjugate
The intact yolk of a fresh ostrich egg was washed with distilled water to remove egg white. Lipids were precipitated by diluting 200 ml yolk 10× with distilled water to a total volume of 2 l. Following incubation at 4°C overnight, the suspension was centrifuged at 8000×g for 25 min, after which the supernatant was removed and stored at −20°C. Ostrich IgY was purified by thiophillic adsorption with a HiTrap IgY Purification HP 5ml column (GE Healthcare, Pittsburg, PA, USA), coupled to a peristaltic pump and fraction collector. Briefly, the column was equilibrated with 25 ml binding buffer (20 mM phosphate buffer, 0.5 M (NH4)2SO4, pH 7.5) before 50 ml of the de-lipidized ostrich egg yolk was passed through. The column was washed with 10 column volumes (50 ml) of binding buffer and 2 ml fractions were collected and spectrophotometrically analysed until A280 OD protein values had dropped to below 0.1. Fifty millilitres of elution buffer (phosphate buffer, pH 7.5) were added to the columns and 2 ml fractions were collected and analysed by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (Criterion 10% Bis-Tris precast gels; BioRad, Hercules, CA, USA). IgY was detected in fractions 13 to 15 as confirmed by the photometric peak and visualization of heavy and light chains at the predicted sizes of 67.5 kDa and 27 kDa, respectively, on reducing 10% polyacrylamide gel electrophoresis gel (data not shown). These fractions were pooled and the IgY concentration determined using the Bradford method (Quick Start Bradford Protein Assay; BioRad).
A 10-week-old specific pathogen free White Leghorn hen was inoculated via the intramuscular route with 3 µg purified ostrich IgY (600 µl) mixed with an equal volume of the adjuvant Montanide ISA 70 (Seppic, Paris France), and boosted with the same amounts at days 21 and 35 post initial inoculation. The hen started to lay eggs at 22 weeks of age and eggs were collected and stored at 4°C until use. All bird experimentation was carried out with the approval of the ARC-OVI Animal Ethics Committee and birds were allowed access to feed and water ad libitum.
Four chicken egg yolks were washed in distilled water and the volume was diluted 10× with distilled water. The yolks were then filtered through gauze to remove membranes and HCl was added to a final concentration of 3 mM. The pH of the solution was adjusted to 5.0 with 10% HAc, allowed to stand for 1 h, and centrifuged at 8000×g for 10 min. An EGGstract® IgY Purification System (Promega, Madison, WI, USA) was used according to the recommended protocol for the isolation of egg yolk IgY.
To produce conjugated chicken anti-ostrich IgY, 10 mg horseradish peroxidase (HRP; Faizyme, Cape Town, Western Cape Province, South Africa) was dissolved in 1.9 ml distilled water to which 600 µl of 0.1 M NaIO4, 10 mM NaP, pH 7 was added. While this was incubated in the dark with shaking for 30 min, a PD-10 desalting column (GE Healthcare) was equilibrated with 20 ml of 1 mM NaOAc, pH 4.2. The buffer of the activated HRP was exchanged to 1 mM NaOAc, pH 4.2 by loading a volume of 2.5 ml of the HRP solution and eluting with 3.5 ml of 1 mM NaOAc, pH 4.2. Another PD-10 column was equilibrated with 20 ml of 20 mM carbonate buffer, pH 9.6, and the buffer of the IgY preparation was exchanged by loading 2.5 ml onto the column and eluting with 3.5 ml of 20 mM carbonate buffer, pH 9.6. The IgY concentration was adjusted to 10 mg/ml with the same buffer. For the coupling step, 1 ml of 10 mg/ml antigen was added to 10 mg activated HRP and incubated with shaking in the dark at room temperature. Two hundred microlitres of a 100 mg/ml glycine solution were added and incubated with shaking in the dark for 30 min. The buffer of the IgY–HRP conjugate was changed as described before to storage buffer (10 mM HEPES, pH 7.5, 150 mM NaCl, 1% bovine serum albumin), and an equal volume of 100% glycerol was added. Stocks were stored at −20°C.
The conjugate was tested by ELISA for specificity against ostrich and chicken sera, and no cross-reaction with chicken sera was detected (data not presented).
rH5 HA1 antigen
A pUC-based plasmid containing a cloned full-length HA gene of strain A/ostrich/South Africa/AI1091/2006 (H5N2) was provided to Creative Diagnostics (New York, USA). A poly-histidine-tagged recombinant HA1 fragment of the gene (388 amino acids with a molecular mass of 38.2 kDa) was expressed in human cells (data not shown). The rH5 HA1 antigen was reconstituted in phosphate-buffered saline (PBS; Sigma-Aldrich, Johannesburg, Gauteng Province, South Africa) for use as a coating antigen in the ELISAs.
H5 indirect ELISA
Nunc PolySorp immuno plates (Sigma-Aldrich) were coated overnight with 100 µl of 5 ng/µl rH5 HA1 antigen in 0.05 M carbonate–bicarbonate buffer, pH 9.6 (Sigma). Wells were washed three times using TST buffer (50 mM Tris–HCl, 150 mM NaCl, 0.05% Tween 20, pH 8.0) and an ELx50 auto strip washer (Bio-Tek Instruments, Inc). Three hundred microlitres of blocking solution (20% Elite Fat Free milk powder in TST buffer) was added to each well and incubated with shaking for 1 h at 37°C (Thermostar plate incubator; BMG Labtech, Ortenberg, Germany). The plate was washed as before. Ostrich sera were diluted 1:50 in blocking buffer into wells, with appropriate controls, in duplicate. Following incubation for 1 h and washing as before, 100 µl each of a 1:1000 dilution of the chicken anti-ostrich IgY–HRP conjugate in blocking buffer was added to each well. Following incubation for 1 h and washing as before, 100 µl TMB substrate (ready-to-use Single Solution; Zymed, San Francisco, CA, USA) was added to each well and left in the dark at room temperature for 10 min, before the reaction was stopped with 2 N H2SO4. OD values were measured at A450 with a Wallac Victor2 1420 multi label counter (Perkin Elmer, Midrand, Gauteng Province, South Africa). Results are expressed as percent positive (PP) values, calculated as the sample OD mean divided by the difference of the mean plate positive minus mean negative control ODs. A panel of 300 known H5-positive ostrich sera, confirmed by HI, as well as 60 negative sera from a Zimbabwean flock, confirmed by IDEXX AIV Ab ELISA, were used to validate for both H5-specific ELISAs. The positive cut-off value for the H5 indirect ELISA (H5 iELISA) was chosen at <9.0 as determined by receiver operating characteristic (ROC) analysis with GraphPad Prism software, edition 5.0 (GraphPad Software, Inc., La Jolla, California USA). At this cut-off value, the sensitivity was 98.33% (95% confidence interval = 96.15 to 99.46%) and the specificity was 98.44% (95% confidence interval = 91.60 to 99.96%), with a likelihood ratio of 62.93. The area under the ROC curve was 0.9951.
Development of H5-specific competitive ELISA (H5 scFv cELISA)
Isolation of a recombinant single-chain antibody fragment specific for H5 HA1
A hen was immunized with betapropiolactone-inactivated H5N2 virus (strain A/ostrich/South Africa/N227/2004) and boosted at 21 days with a second dose, with approval from the Onderstepoort Veterinary Institute's Animal Ethics Committee. After the hen was euthanized the spleen was collected, sliced into small fragments and stored in RNAlater (Qiagen, Hilden, Germany) at −70°C. A phage-displayed single-chain fragment variable (scFv) library was constructed using the repertoire of the immunized chicken as described before (van Wyngaardt et al., Citation2004; Chiliza et al., Citation2008). Phages were rescued from the library of 3.82×107 primary clones and 5×1012 phages were subjected to selection on rH5 HA1. Selection and clone characterization was as described previously (van Wyngaardt et al., Citation2004) with minor adjustments. For the first round of selection, an immunotube (Nunc Maxisorp) was coated with 20 µg/ml rH5 HA1. The subsequent rounds were performed in immunoplates (Nunc Maxisorp). Three wells were coated with 20 µg/ml for round two and 10 µg/ml HA1 for round three.
H5-specific competitive ELISA
One of the scFvs (1F11) selected from the H5N2 immune antibody phage display library that reacted specifically to rH5 HA1, functioned well as a soluble protein and was stable. It was possible to inhibit the binding of scFv 1F11 to rH5 HA1 with ostrich sera known to be positive for H5 by HI but not by H6 and H7-positive sera. Thus a competitive ELISA was developed to detect H5-specific antibodies in ostrich sera. Large-scale preparation of soluble scFv 1F11 was performed as described previously (van Wyngaardt et al., Citation2004). The scFvs in the culture supernatant were used for the ELISA. Maxisorp immunoplate wells were coated with 50 µl of 2.5 µg/ml rH5 HA1 overnight at 4°C. The rest of the incubation steps were for 1 h at 37°C and, except for the blocking step, 50 µl reaction volumes were used. After coating, the wells were washed once with PBS containing 0.05% Tween (PBST) and filled with 2% milk powder in PBS (MP-PBS) to block. After one wash, serum samples, diluted 1:25 in MP-PBS, were added to the wells. The wells were washed three times with PBST. ScFv 1F11 was diluted 1:1 with 4% MP-PBS and added to the wells. The wells were washed three times. Anti-c-myc-peroxidase (Roche, Johannesburg, South Africa), which recognizes the c-myc epitope tag on the scFv, was used to detect 1F11. After the final wash, substrate was added as described (van Wyngaardt et al., Citation2004) and absorbance measured at 492 nm with a Multiskan Ex (Thermo Electron Corporation, Elandsfontein, Gauteng Province, South Africa). Results are expressed as the S/N ratio. The positive S/N cut-off value for the H5-specific competitive ELISA (H5 scFv ELISA) was chosen at ≤1.01 as determined by ROC analysis. At this cut-off value, the sensitivity was 96.96% (95% confidence interval = 94.31 to 98.60%) and the specificity was 95.59% (95% confidence interval = 87.64 to 99.08%) with a likelihood ratio of 21.98. The area under the ROC curve was 0.9890.
Haemagglutinin inhibition assay
HI tests were conducted according to the OIE (Citation2012) recommended procedure for HA and HI testing of ostrich sera, briefly described as follows. A heat inactivation and red blood cell (RBC) adsorption step was included to remove non-specific haemagglutinating and inhibiting factors. Five hundred microlitre aliquots of ostrich test sera were heat-inactivated at 56°C for 30 min, allowed to cool for 10 min, and 25 µl packed RBCs were added. Following 30 min incubation at room temperature with occasional mixing, sera were centrifuged at 800×g for 5 min and the supernatant collected for testing. Two-fold serial dilutions of 25 µl test sera in PBS were prepared in V-bottomed plates with appropriate controls.
Two test antigens, A/ostrich/South Africa/AI1091/2006 (H5N2) and A/ostrich/South Africa/AI2114/2011 (H5N2), were diluted to 4 haemagglutination units (4 HA units), and 25 µl were aliquotted into each test well. Following incubation for 30 min at room temperature, 25 µl of 1% (v/v) chicken RBCs was added to each well, and after mixing was left at room temperature. After 40 min, agglutination was assessed by streaming of RBCs in tilted plates. Only wells in which the RBCs stream at the same rate as the cell control wells were considered positive, and a sample was only considered positive if it caused HA activity of 4 HA units at a titre of at least 1:16 (24).
Analytical sensitivity
A two-fold serial dilution of a strong positive H5 ostrich serum in negative ostrich serum (samples A to J, ) was tested in parallel using the three ELISA assays and HI. For HI, the testing laboratory was requested to use two H5N2 antigens, instead of a heterologous N-type pair. The first antigen, A/ostrich/South Africa/AI1091/2006 (H5N2), was used routinely in the national laboratory for HI screening of ostriches at the time of the outbreak. The second antigen, A/ostrich/South Africa/AI2114/2011 (H5N2), was an outbreak strain and an antigenic match to the sera being tested. The HA proteins of these two viruses share 94.92% identity at the nucleotide sequence level, and 94.86% identity at the amino acid level (pair-wise alignments not shown), and the field sera were expected to react more strongly with the corresponding 2011 field antigen compared with the 2006 antigen. Cut-off values were established according to kit instructions for the AIV group-specific nucleocapsid protein competitive ELISA (AIV NP cELISA), OIE recommendations for the HI tests and those determined in this study by ROC analysis (H5 scFv and iELISAs).
Table 1. Relative sensitivity of AIV antibody detection assays.
Ostrich antisera raised against non-H5/H7 AIV subtypes experimentally are unavailable and the high volumes of sera required for HI typing preclude routine identification of non-H5/H7-positive sera using a full panel of H1 to H16 antigens. The H5 scFv and iELISAs were therefore validated with known field H5-specific, H6-specific and H7-specific antisera.
Statistical analyses
Quantitative test result data were descriptively presented as histograms and scatter plots. Agreement between test results was assessed on the positive and negative classifications using pairwise kappa statistics and on the quantitative scale using Spearman's correlation statistic (rho). Data were analysed in commercially available software—IBM SPSS Statistics Version 20 (International Business Machines Corp., Armonk, New York, USA) and Epi Info, version 6.04 (CDC, Atlanta, Georgia, USA)—and results interpreted at the 5% level of significance.
Table 2. Results of serological testing where disagreement between the three ELISA assays was obtained.
Results
Analytical sensitivity
represents the comparative analytical sensitivity of the ELISA assays and HI as assessed by a serial dilution of H5-positive serum. The H5 iELISA detected the lowest antibody titres at a 1:64 dilution, which was four-fold better than the AIV NP cELISA that detected antibodies up to dilution 1:16. The H5 scFv cELISA only detected antibodies up to the 1:8 dilution. Since this ELISA uses a serum dilution of 1:25 compared with the AIV NP cELISA serum dilution of 1:10, the detection limits of these two assays under the current cut-off values are similar. The H5 iELISA not only detected the highest dilution, but the test serum was diluted double that of the H5 scFv cELISA and five-fold more than the IDEXX AIV NP cELISA.
Neither of the H5-specific ELISAs detected H6-specific or H7-specific antibodies in ostrich H6-positive and H7-positive reference antisera. H6-positive reference sera were obtained from a field infection in 2010 and identified by HI, and H7-positive reference sera were generated experimentally at ARC-OVI.
Test comparison on a flock level
A total of 929 ostrich sera were tested by IDEXX AIV NP cELISA. Of these, 830 (89.3%) tested positive and 99 (10.7%) tested negative (). A subset of 366 samples containing mostly IDEXX ELISA-positive (n = 305) but also some negative (n = 61) samples were selected and were tested by the two H5-specific ELISA assays and HI in order to establish whether the serological response was due to H5 or non-H5 infection.
Figure 1. Distribution of IDEXX AIV NP cELISA results for 929 ostriches on an AIV-infected farm in South Africa.
Of these 366 samples, 292 (79.78%) achieved consensus across all three ELISAs; that is, samples that tested positive on the IDEXX AIV NP cELISA also tested positive on the two H5-specific ELISAs (data not shown). Many of these samples also tested H5-positive on the HI assay (13 insufficient sera, 89 testing positive on either one or both of the H5N2 antigens with the highest titre of 1:256).
Four samples (Samples 89, 136, 146 and 154; ) were positive on the IDEXX AIV NP cELISA (S/N values of 0.496, 0.142, 0.202 and 0.376, respectively) as well as positive on the H5 scFv cELISA (low positive S/N values of 0.984, 0.929, 0.909 and 0.943, respectively) but negative on the H5 iELISA having PP values of 7.87, 8.21, 2.75 and 2.1, respectively. These samples were probably from birds that were exposed to non-H5 influenza, which could account for the reasonable NP cELISA titres or that they have very low H5 AIV titres (perhaps due to an early immune response) and the serum dilution used in the H5 iELISA precluded detection of the H5-specific antibodies. The positive HI reactions with H5N2 viruses for Sample 146 might be explained by a possible previous exposure to H1N2 or H6N2, producing cross-reactions with the neuraminidase antibodies.
Nine samples (Samples 100, 104, 107, 128, 130, 132, 171, 173 and 259) were clearly positive on both the AIV NP cELISA (S/N values in the range of 0.449 to 0.128) and the H5 iELISA (PP values in the range of 41 to 116) but negative on the H5 scFv cELISA with S/N values ranging from 1.01 to 1.17. This may be explained by the lower analytical sensitivity of the H5 scFv cELISA compared with the H5 iELISA.
Fifteen samples (Samples 307, 311, 316, 317, 318, 327, 330, 331, 332, 335, 336, 339, 340, 359 and 364) were negative on the AIV NP cELISA and the H5 scFv cELISA but positive on the H5 iELISA. Most of these were at the lower end of the positive range, with PP values of 9.19 to 114.59 (with the majority PP <40). Given the lower analytical sensitivity of the H5 scFv cELISA compared with the H5 iELISA as well as the differences in dilution factors, this set of 15 samples may be included in the final group discussed below.
Thirty-seven samples tested negative on the IDEXX AIV NP cELISA but positive on both H5 ELISAs. These samples were re-tested on AIV NP cELISA with the same results and HI results confirmed the presence of anti-H5 antibodies. For example, Sample 281 had a negative AIV NP cELISA S/N value of 1.339, a strong positive S/N value of 0.323 for the H5 scFv cELISA, strong positive PP value of 141.257 for the H5 iELISA and HI titres of 1:64 and 1:128 for the two HI H5N2 antigens, respectively. Positive H5 HI titres were also obtained for Samples 278, 291, 310, 324, 337, 343, 349, 353, 360 and 361. Weak HI titres (below the positive cut-off threshold of 1:16) were observed in numerous of the aforementioned 15 samples as well as the current group of 37. The presence of H5-specific antibodies but lack of NP-specific antibodies in these 15 + 37 samples (52/306; 17%) was an interesting finding that is discussed in the next section.
None of the 306 AIV NP cELISA-positive samples were negative in the H5-specific ELISA. However, in certain cases high AIV NP cELISA S/N values were obtained that were low positive on the two H5-specific ELISAs; for example, Sample 350 had a strong positive AIV NP cELISA S/N result of 0.17, whereas the H5 scFv cELISA S/N value was 0.99 and the H5 iELISA PP value was 25.88. Other examples included Samples 137, 187, 208, 346, 150, 280 et al. (data not shown). This suggests that a percentage of the ostriches in the flock had previous exposure to non-H5 AIV serotypes, but that all had become infected with H5 AIV prior to the time of sampling.
HI results were sometimes atypical, with stronger reactions observed to the 2006 antigen than the homologous 2011 outbreak antigen. This was probably due to errors in diluting the test antigens to 4 HA units. Re-testing was not possible due to limited serum volumes. This also illustrates the susceptibility to technical error to which the HI assay is prone.
Statistical results
Kappa statistics suggested moderate to good agreement between the HI tests and poor to fair agreement for all other test pairs (). Evaluation on the continuous scale suggested good correlation between the two H5-specific ELISAs but substantially weaker correlation between other test pairs (, and ).
Figure 2. Scatter plot of IDEXX AIV NP cELISA and H5 scFv cELISA results for 366 ostriches from an AIV-infected farm in South Africa. Spearman's rho = 0.330; P <0.001.
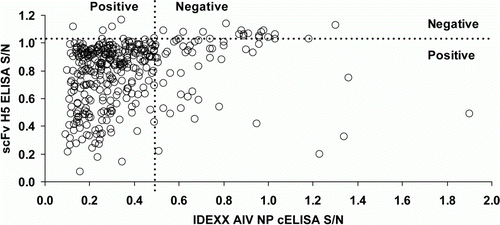
Figure 3. Scatter plot of IDEXX AIV NP cELISA and H5 iELISA results for 366 ostriches from an AIV-infected farm in South Africa. Spearman's rho = − 0.391; P <0.001.
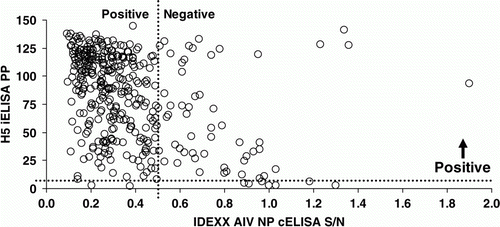
Figure 4. Scatter plot of H5 scFv cELISA and H5 iELISA results for 366 ostriches from an AIV-infected farm in South Africa. Spearman's rho = − 0.769; P <0.001.
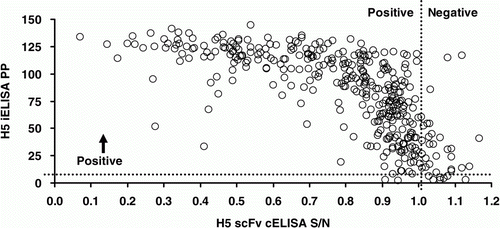
Table 3. Overall agreement between AIV test results from a subset of 366 ostriches from an infected farm in South Africa.
Discussion
A single swab pool collected on Farm AI18 tested RT-PCR-positive for the HPAI H5N2 virus during the 2011 outbreak, but serological testing by HI at the time yielded negative results. A month later when the entire flock of 929 ostriches was sent to slaughter, the farmer, sceptical of the RT-PCR test results and infection status of the flock, arranged for the entire flock to be bled and tested by a private laboratory. Laboratory test results conducted with the IDEXX AIV NP cELISA indicated a high incidence of AIV exposure, but since the assay detects group-specific nucleoprotein antibodies, and H6N2 plus H1N2 was known to be co-circulating with the HPAI H5N2 outbreak strain in the region, the farmer was still convinced that his birds had been culled unfairly. To address the question of whether the NP-specific antibodies detected were raised following exposure to H5 or non-H5 subtypes, we developed two H5-specific ELISAs (H5 iELISA and H5 scFv cELISA), both based on rH5 HA1 as the coating antigen.
The H5 iELISA relies on an anti-ostrich immunoglobulin HRP conjugate, produced in a chicken and purified from its eggs. No cross-reactivity was observed between a commercial anti-chicken IgY conjugate and ostrich IgY and similarly no reaction was seen between the anti-ostrich IgY conjugate described here and chicken IgY. This illustrates that antibodies to an antibody of one avian species could be raised in another avian species, and this reaffirms the phylogenetic distance between ostriches and chickens (Pereira & Baker, Citation2006). Producing an IgY conjugate using IgY isolated from chicken eggs has several benefits. This approach allows the non-invasive, low-cost collection of a large quantity of antibodies from chicken egg yolks. The amount of IgY in egg yolks collected from a single chicken in one month corresponds to the amount of immunoglobulin present in approximately half a litre of immune serum (Jensenius et al., Citation1981). It is not necessary to bleed the birds for evaluation of their immune status and IgY is easily purified by a variety of methods. IgY does not-cross react with mammalian antibodies and is therefore highly suitable for use in assays based on antibodies from two species (Devergne et al., Citation1981). The absence of interactions between IgY and protein A (Kronvall et al., Citation1970) allows immunodiagnostic applications that are not possible with immunoglobulins that do bind to protein A. The chicken-anti ostrich IgY conjugated to HRP appears to be highly stable since it was produced in 2007 without a detectable drop in reactivity in 2012 (results not presented), and it could be used for the detection ostrich antibodies to any pathogen for which a coating antigen is available.
The H5 scFv cELISA uses a scFv derived from an immune chicken scFv library. A major benefit is that ELISAs based on well-defined recombinant proteins and antibodies can be standardized worldwide. The reagents are renewable and can even be “reconstructed” from the DNA sequence if needed. This cELISA is slightly more sensitive than the gold standard HI but much less time consuming. Another consideration is that in a cELISA no species-specific conjugate is required. Thus sera from any animal could be tested, but this needs to be confirmed experimentally. The reaction of the scFv with an epitope is inhibited and the presumption would be that the animal recognizes that same epitope; however, this is not always the case (Bentley et al., Citation2000).
By comparing IDEXX AIV NP cELISA results with those of the two H5-specific ELISAs and HIs, we determined that up to 89% of the flock had been exposed to H5N2 AIV by the time the birds were slaughtered, where a month prior 60 serum samples had tested H5 HI-negative. From the comparative analytical sensitivity data we presented here, HI is likely to miss up to 35% of H5-positive samples. The PCR-positive result was probably captured very early in the infection of the flock, but a month later the infection had spread and almost the entire flock had sero-converted. This implies that AIV spreads much more rapidly between ostriches than previously reported (Oliver, Citation2006) and we propose that the most likely primary route of transmission is through the watering troughs where the ostriches congregate, as opposed to the faecal–oral route. Shedding of H5 was detected from both tracheal and cloacal swabs during the outbreak (Abolnik et al., Citation2012) but other studies have shown that ostriches shed AIV from the trachea for longer periods, and that, of the two sources of samples, successful isolations are made more frequently from the trachea (Manvell et al., Citation1998; Clavijo et al., Citation2003). Ensuring that drinking water is adequately disinfected may thus be the single most important method of preventing AIV from spreading within an ostrich flock, but this remains to be demonstrated experimentally.
Other groups (e.g., Toffan et al., Citation2010) have maintained that the HI test remains a sensitive and specific test to monitor the circulation of AIV of known subtype in ostriches, especially when performed with the homologous antigen. Our results, which were generated from the largest study group of field sera to date (929 ostrich sera), demonstrated that only 64.7% of the H5-specific ELISA-positive samples would have been identified as H5-positive by HI (187 HI-positives out of 289, 17 samples of the total 306 positive were of insuffiecient quantity for HI testing). This apparent low sensitivity of HI for ostrich sera is troubling because HI has been the standard test of NAI screening for over a decade in South Africa. The possibility exists that numerous positive cases have been missed during routine surveillance, pre-movement and outbreak response testing in the past, resulting in the undetected incidence of the disease. It must be noted, however, that the 2004, 2006 and 2011 HPAI H5N2 ostrich outbreaks have been unrelated, since genomic segments have displayed reassortment, and different lineages of H5 and N2 have been involved (Abolnik, Citation2007; Abolnik et al., Citation2009; Abolnik et al., Citation2012).
The limitations of HI testing for high-throughput screening of ostrich sera were recognized, thus, following extensive validation, the national authorities approved the IDEXX AIV NP cELISA for use with ostrich sera in September 2011. The proviso is that this ELISA is only considered a screening assay and that all positive reactors must be screened by H5 and H7-specific HI assays before a diagnosis can be made. Most national laboratories use this strategy now, and it significantly reduces the workload and improves test turnaround times, but the caveat of missing positive NAI due to insensitivity of HI remains.
The detection of 17% of sera on Farm AI18 that contained H5-specific antibodies but no NP-specific antibodies was an intriguing discovery. It is possible that some of the sera did contain NP-specific antibodies, albeit at undetectable levels given the difference in relative sensitivity of the NP ELISA. In many of these cases, however, disproportionately higher levels of H5-specific antibodies were detected. Anti-NP antibodies are produced in the host following exposure to the virus, either with a live replicating virus or an inactivated virus (chemically inactivated or otherwise), since NP is a structural component of the virus (Suarez, Citation2005). One explanation is that these birds were exposed to recombinant vaccine expressing the H5 antigen. Numerous recombinant H5 vaccines (e.g. Fowlpox-H5) are commercially available internationally but not registered for use in South Africa, since vaccination for AIV is not permitted. Rumours of vaccination of ostriches persisted during the time of the outbreak, but these are unsubstantiated at present. Testing for antibodies against the suspected “backbones” (e.g. fowlpoxvirus, Marek's disease virus or Newcastle disease virus) might not be significant since the normal exposure of ostriches to these viruses is unknown, and ostriches are routinely vaccinated for Newcastle disease virus anyway.
AIV can spread rapidly in an ostrich flock at points of contact, and sensitive, high-throughput methods such as ELISA are vital to detect and control incursion of the disease following point introductions by wild birds. The next step is implementing H5/H7 ELISA assays to replace HI, towards earlier detection and improved control of AI in the valuable ostrich farming industry.
Acknowledgements
Marco Romito is thanked for assisting with immunization of the hens. Thandeka Phiri is thanked for assisting with IDEXX ELISA assays and Denise Petlele and Mpho Molefe for HI assays. Alex Caron is thanked for providing AI-negative Zimbabwean ostrich sera. Dion du Plessis is thanked for critical revision of the manuscript. This work was funded by the National Department of Agriculture, Forestry and Fisheries.
References
- Abolnik , C. 2007 . Molecular characterization of H5N2 avian influenza viruses isolated from South African ostriches in 2006 . Avian Diseases , 51 : 873 – 879 . doi: 10.1637/7953-022107-REGR.1
- Abolnik , C. , Gerdes , G.H. , Sinclair , M. , Ganzevoort , B.W. , Kitching , J.P. , Burger , C.E. , Romito , M. , Dreyer , M. , Swanepoel , S. , Cumming , G.S. and Olivier , A.J. 2010 . Phylogenetic analysis of influenza A viruses (H6N8, H1N8, H4N2, H9N2, H10N7) isolated from wild birds, ducks, and ostriches in South Africa from 2007 to 2009 . Avian Diseases , 54 : 313 – 322 . doi: 10.1637/8781-040109-Reg.1
- Abolnik , C. , Londt , B.Z. , Manvell , R.J. , Shell , W. , Banks , J. , Gerdes , G.H. , Akol , G. and Brown , I.H. 2009 . Characterisation of a highly pathogenic influenza A virus of subtype H5N2 isolated from ostriches in South Africa in 2009 . Influenza and Other Respiratory Viruses , 13 : 63 – 68 . doi: 10.1111/j.1750-2659.2009.00074.x
- Abolnik , C. , Olivier , A.J. , Grewar , J. , Gers , S. and Romito , M. 2012 . Molecular analysis of the 2011 HPAI H5N2 outbreak in ostriches, South Africa . Avian Diseases , 56 : 865 – 879 . doi: 10.1637/10171-041012-Reg.1
- Bentley , L. , Fehrsen , J. , Jordaan , F. , Huismans , H. and du Plessis , D.H. 2000 . Identification of antigenic regions on VP2 of African horsesickness virus serotype 3 using phage displayed epitope libraries . Journal of General Virology , 81 : 993 – 1000 .
- Capua , I. , Mutinelli , F. , Bozza , M.A. , Terregino , C. and Cattoli , G. 2000 . Highly pathogenic avian influenza (H7N1) in ostriches (Struthio Camelus) . Avian Pathology , 29 : 643 – 646 . doi: 10.1080/03079450020016913
- Chiliza , T.E. , Van Wyngaardt , W. and du Plessis , D.H. 2008 . Single-chain antibody fragments from a display library derived from chickens immunized with a mixture of parasite and viral antigens . Hybridoma , 27 : 413 – 421 . doi: 10.1089/hyb.2008.0051
- Clavijo , A. , Riva , J. and Pasick , J. 2003 . Pathogenicity of a ratite-origin influenza A H5 virus in ostriches (Struthio camelus) . Avian Diseases , 47 : 1203 – 1207 . doi: 10.1637/0005-2086-47.s3.1203
- Devergne , J.C. , Cardin , L. , Burckard , J. and van Regenmortel , M.H.V. 1981 . Comparison of direct and indirect ELISA for detecting antigenically related cucumoviruses . Journal of Virological Methods , 3 : 193 – 199 . doi: 10.1016/0166-0934(81)90070-7
- Jensenius , J. Chr. , Andersen , I. , Hau , J. , Crone , M. and Koch , C. 1981 . Eggs: conveniently packaged antibodies. Methods for purification of yolk IgG . Journal of Immunological Methods , 46 : 63 – 68 . doi: 10.1016/0022-1759(81)90333-1
- Kronvall , G. , Seal , U.S. , Finstad , J. and Williams , R.C. 1970 . Phylogenetic insight into evolution of mammalian Fc fragment of gamma G globulin using staphylococcal protein. A . Journal of Immunology , 104 : 140 – 147 .
- Manvell , R.J. , English , C. , Jorgensen , P.H. and Brown , I.H. 2003 . Pathogenesis of H7 influenza A viruses isolated from ostriches in the homologous host infected experimentally . Avian Diseases , 47 : 1150 – 1153 . doi: 10.1637/0005-2086-47.s3.1150
- Manvell , R.J. , Jørgensen , P.H. , Nielsen , O.L. and Alexander , D.J. 1998 . Experimental assessment of the pathogenicity of two avian influenza A H5 viruses in ostrich chicks (Struthio camelus) and chickens . Avian Pathology , 27 : 400 – 404 . doi: 10.1080/03079459808419358
- OIE . 2012 . Manual of Diagnostic Tests and Vaccines for Terrestrial Animals: Avian Influenza . Retrieved from http://www.oie.int
- Olivier , A.J. 2006 . Ecology and epidemiology of avian influenza in ostriches . Developments in Biologicals , 124 : 51 – 57 .
- Pereira , S.L. and Baker , A.J. 2006 . A mitogenomic timescale for birds detects variable phylogenetic rates of molecular evolution and refutes the standard molecular clock . Molecular Biology and Evolution , 23 : 1731 – 1740 . doi: 10.1093/molbev/msl038
- Suarez , D.L. 2005 . Overview of avian influenza DIVA test strategies . Biologicals , 33 : 221 – 226 . doi: 10.1016/j.biologicals.2005.08.003
- Toffan , A. , Olivier , A. , Mancin , M. , Tuttoilmondo , V. , Facco , D. , Capua , I. and Terregino , C. 2010 . Evaluation of different serological tests for the detection of antibodies against highly pathogenic avian influenza in experimentally infected ostriches (Struthio camelus) . Avian Pathology , 39 : 11 – 15 . doi: 10.1080/03079450903431390
- van Wyngaardt , W. , Malatji , T. , Mashau , C. , Fehrsen , J. , Jordaan , F. , Miltiadou , D. and Du Plessis , D.H. 2004 . A large semi-synthetic single-chain Fv phage display library based on chicken immunoglobulin genes . BMC Biotechnology , 4 : 6 doi: 10.1186/1472-6750-4-6