Abstract
Five phytochemicals/extracts (an extract from Echinacea purpurea, a β-glucan-rich extract from Shiitake, betaine [Betain™], curcumin from Curcuma longa [turmeric] powder, carvacrol and also a recombinant fungal immunomodulatory protein [FIP] from Ganoderma lucidum) cloned and expressed in Escherichia coli were investigated for their anticolibacillosis potential in three chicken experiments, which were conducted in floor pens. Birds that were inoculated with E. coli intratracheally were treated with the phytochemicals/extracts or the FIP and compared with doxycycline-medicated and non-medicated infected broilers. Non-medicated and non-infected birds were used as negative controls. Mortality, colibacillosis lesions and body weight gains were used as parameters. Considering the sum of dead birds and chickens with generalized colibacillosis per group, there was no significant difference between the positive control groups and birds treated with phytochemicals/extracts or the FIP. In contrast, doxycycline-treated birds showed significantly lower mortality and generalized colibacillosis. Moreover, none of the phytochemicals/extracts and the FIP improved recovery from colibacillosis lesions, while all doxycycline-treated broilers recovered completely. The negative control birds and doxycycline-treated groups consistently showed the highest weight gains. Pulsed-field gel electrophoresis of reisolates showed that they were genetically indistinguishable from the inoculation strain. In conclusion, none of the tested phytochemicals/extracts and the FIP significantly reduced the E. coli-induced mortality and generalized colibacillosis, and nor did they improve recovery from colibacillosis lesions.
Introduction
The drawbacks of antibiotic use in farm animals are well known by scientists and consumers alike. Amongst them is the increased concern for residues in the food chain and the transfer of resistant bacteria from animals to humans (World Health Organization, Citation2011). Aiming at reducing the usage of antibiotics in farm animals, alternative treatment and prevention strategies are becoming increasingly popular. Amongst these, natural plant-derived and fungus-derived compounds and extracts are gaining popularity due to a renewed interest in natural medicine.
Colibacillosis is a disease of poultry with great economic impact, which is responsible for the use of very significant volumes of antibiotics in commercial poultry farming. It is the disease that is most frequently found at post-mortem and at slaughter, 43% of condemned carcasses having Escherichia coli lesions. Colibacillosis has been found in 18% of submissions of broiler chickens to GD – Animal Health Service (Deventer, the Netherlands) during 2008 (unpublished results). Antibiotics such as trimethoprim-sulfa, doxycycline and ampicillin are often applied for the treatment of colibacillosis in our country (KNMvD, Citation2012).
The occurrence of resistance to antibiotics is common (Gyles, Citation2008; Persoons et al., Citation2010) and will most probably further increase in the future paralleling their use (Maran reports, Citation2002–2009).
Although numerous studies have shown in vitro antimicrobial efficacy for phytochemicals, in vivo evidence is scarce (Cowan, Citation1999; Windisch et al., Citation2008; Cravotto et al., Citation2009). Therefore, based on recent literature, in vivo screening of five phytochemicals/extracts and a fungal immunodulatory protein (FIP) (Guo et al., Citation2004, Citation2005; Dalloul et al., Citation2006) was performed. Root extracts from Echinacea purpurea (Allen, Citation2003), β-glucan from Lentinula edodes (Smith & Ovington, Citation1996; Yun et al., Citation2003; Huff et al., Citation2006; Stuyven et al., Citation2009), betaine from Beta vulgaris (Augustine et al., Citation1997; Matthews et al., Citation1997; Waldenstedt et al., Citation1999; Matthews & Southern, Citation2000; Klasing et al., Citation2002; Fetterer et al., Citation2003; Eklund et al., Citation2005), curcumin from Curcuma longa (Allen et al., Citation1998) and carvacrol from Origanum vulgare (Giannenas et al., Citation2003; Sokovic et al., Citation2010) were studied. The in vivo antimicrobial activity of all products was assessed using a bird model for colibacillosis in broilers (Van Eck & Goren, Citation1991) and by comparing their effect on E. coli to traditional prevention and therapy; that is, an antibiotic (doxycycline). In total, three chicken trials were conducted.
Materials and Methods
Phytochemicals/extracts, fungal immunomodulatory protein and antibiotic
Commercial phytochemicals/extracts
The following phytochemicals were purchased: extract of E. purpurea (E. purpurea root, 4:1, standardized to 6% total polyphenols; NatuurApotheek, Pijnacker, the Netherlands), betaine (Betain™ 96, batch number 812040000; Trouw Nutrition, Putten, the Netherlands), curcumin (C. longa [turmeric] powder (batch number C1386; Sigma-Aldrich Chemie B.V., Zwijndrecht, the Netherlands), carvacrol (Carvacrol ≥98%, FCC, Kosher, batch number W224502; Sigma-Aldrich Chemie B.V., Zwijndrecht, the Netherlands) and β-glucan from L. edodes.
The concentrations in the feed were assessed by chemical analysis. The standardized extract of E. purpurea root was characterized by determination of the presence of alkylamides and quantification of total phenolics, cichoric acid, chlorogenic acid, and total sugar/carbohydrate content. Alkylamides in the extract were identified by the method described in the European Pharmacopoeia (Citation2010a). The total phenolic content was determined using a modified version of the Folin–Ciocalteu assay as described by Singleton et al. (Citation1999). Cichoric acid and chlorogenic acid content were determined by high-performance liquid chromatography (HPLC) analysis using a slightly modified version of the method described by Chen (Citation2006). Total sugar/carbohydrate content was determined using a modified version of the anthrone reaction as described by Dreywood (Citation1946).
The content of standardized E. purpurea root extract in chicken feed samples was determined by HPLC analysis of cichoric acid and by semiquantitative thin-layer chromatographic analysis of alkylamides. For the HPLC analysis, 10 g unsupplemented control feed samples, Echinacea supplemented feed samples, and control feed samples spiked with 1250 mg/kg Echinacea were extracted in 20 ml methanol 30% (v/v) for 20 min at room temperature by ultrasonic extraction. After centrifugation (13,500×g, 10 min), supernatants were analysed for cichoric acid content using a slightly modified version of the method described by Chen (Citation2006). For the semiquantitative thin-layer chromatographic analysis, 10 g feed samples were extracted in 75 ml dichloromethane for 60 min at room temperature under continuous shaking. After filtration, organic solvent was evaporated under reduced pressure and the residues were dissolved in 10 ml dichloromethane. Extracts were applied to silica 60 F254 plates (20 µl) and chromatographed using n-hexane:ethyl acetate (2:1) as the mobile phase. Alkylamide spots were visualized under ultraviolet 254 nm and after spraying with anisaldehyde reagent (Bauer & Remiger, Citation1989). Intensity of the alkylamide spots was digitally recorded and analysed using CAMAG (Muttenz, Switzerland) video scan TLC/HPTLC software. Echinacea content was quantified using a calibration curve of the standardized E. purpurea root extract.
The content of betaine in chicken feed samples was determined by a colorimetric assay based on the periodide reaction of quaternary nitrogen compounds (Wall et al., Citation1960). Unsupplemented control feed samples, betaine supplemented feed samples (1500 mg/kg), and unsupplemented control feed samples spiked with 1500 mg/kg betaine (10 g each) were extracted in 30 ml distilled water for 30 min at room temperature under continuous shaking. After filtration, potassium triiodide solution was added to appropriate dilutions of extracts. The mixture was shaken and left for 90 min in ice. Subsequently, reaction mixtures were extracted with 1,2-dichloroethane and absorption of the organic phase was measured at 365 nm as previously described by Storey & Wyn Jones (Citation1977). Betaine was quantified using a betaine calibration curve.
The content of curcumin in chicken feed samples was determined by spectrophotometric analysis. Unsupplemented control feed samples, curcumin supplemented feed samples (500 mg/kg), and control feed samples spiked with 500 mg/kg curcumin (10 g each) were extracted in dichloromethane for 30 min under reflux boiling. After filtration, organic solvent was evaporated under reduced pressure and the residues were dissolved in 10 ml dichloromethane. Extracts were appropriately diluted, absorption was measured at 345 nm (absorption maximum of curcumin), and curcumin content was quantified using a curcumin calibration curve (European Pharmacopoeia, Citation2010c).
The content of carvacrol in chicken feed samples was determined by semiquantitative thin-layer chromatographic analysis. Unsupplemented control feed samples, carvacrol supplemented feed samples (200/1000 mg/kg), and control feed samples spiked with 200 or 1000 mg/kg carvacrol (10 g each) were extracted in 75 ml dichloromethane for 60 min at room temperature under continuous shaking. After filtration, organic solvent was evaporated under reduced pressure and the residues were dissolved in 10 ml dichloromethane. Extracts were applied to silica 60 F254 plates (5 µl) and chromatographed using dichloromethane as the mobile phase. Carvacrol spots were visualized after spraying with anisaldehyde reagent (European Pharmacopoeia, Citation2010b). Colour intensity of carvacrol spots was digitally recorded and analysed using CAMAG video scan TLC/HPTLC software. Carvacrol content was quantified using a carvacrol calibration curve.
Isolated phytochemical/extract
The rich sample of Shiitake (β-glucan) was isolated basically according to Yap & Ng (Citation2001) with some small adaptations as described before (Tomassen et al., Citation2011). Because of the presence of maize, wheat, soy and peas polysaccharides present in the feed of which cross-reactivity will occur with β-glucan analysis of the mixed Shiitake polysaccharides, no attempts have been performed to analyse the final concentration of β-glucan in feed. Based on the starting concentration the amount will be at a maximum of 200 mg/kg.
Fungal immunomodulatory protein
Based on degenerated primers designed towards the LZ8 gene, encoding a FIP of Ganoderma lucidum (Kino et al., Citation1989), a fragment was amplified, cloned and sequenced. The cloned FIP sequence had 88% homology to the LZ8 gene and was named LZ9. The gene was cloned in pET101 TOP expression vector and fused in frame to the V5/HIS tag. E. coli BL21Star cells were used for expression. The expressed FIP was isolated via Ni-chromatography (Ni-NTA Purification System; Life Technologies Ltd, Paisley, UK) and was found to possess in vitro haemagglutinating properties (data not shown). For the feeding experiments, the transgenic clone was cultured to the exponential growth, induced with isopropyl β-D-1-thiogalactopyranoside, and cells were subsequently harvested 4 h after induction. Cells were then lysed using extraction buffer (50 mM NaH2PO4, 0.5 M NaCl, 10 mg/ml lysozyme [pH 8]), sonicated and freeze dried. The amount of FIP present in the sample was determined using immunodot blot (see below). As the crude FIP will contain E. coli-derived compounds that might have an influence on the response of the broilers, a control feed sample was prepared using a transgenic E. coli BL21 strain expressing a FIP with a point mutation in the start of the gene introducing a frame shift and a premature stop of the protein production. This sample could therefore be used as a control for the FIP responses and was named FIPcontr.
To analyse the amount of FIP in the crude sample that was mixed with the feed, and to analyse the stability of the FIP in the feed delivered to the birds, immunodot blot analysis was used. The recombinant FIP is fused to a V5 epitope, making it possible to detect the protein with a commercial anti-V5 antibody (V5 mouse mAb, AP Conjugate; Invitrogen, Burlington, Canada). A secondary antibody (anti-mouse IgG; Sigma-Aldrich, St Louis, MO, USA), which is capable of binding to the anti-V5 antibody, covers a peroxidase conjugate and this conjugate was used for the colorimetric detection of the FIP.
Extraction and detection were performed by dissolving the crude sample or feed with 50 mM Tris-buffered saline (pH 7.4) followed by a 30-min incubation on a rotating shaker at room temperature. Then the extract was centrifuged during 15 min at 20,800×g and the clear supernatant used for quantification. For detection, the supernatants were spotted in dilution series on protran BA/optritan BA-S nitrocellulose membrane (Whatman, Schleicher & Schuell, Keene, NH, USA). After drying and blocking of the membranes, the membranes were incubated with anti-V5 antibody and the secondary antibody, each performed for 1 h at room temperature, and every incubation step was followed by standard washing procedure with phosphate-buffered saline (PBS) with 0.05% Tween. Subsequently, spots were detected with Sigma Fast BCIP/NBT (Sigma-Aldrich) and the blots were scanned to quantify the intensity of the spots.
Positope, a protein with a V5 epitope, was used as positive control and was used as the calibration protein. It was spiked in feed to which no FIP was added and subsequently treated the same as the other samples.
Antibiotic
Doxycycline (Doxycycline 50% WSP, batch number O9C30-09/1; Dopharma B.V., Raamsdonksveer, the Netherlands) in a dose of 125 or 250 mg/l drinking water was given to the birds of the antibiotic treatment group. The administration of doxycycline started on the day of the E. coli inoculation and lasted 4 days. In the first chicken experiment a dose of 125 mg/l was used, while in the other experiments a dose of 250 mg/l was used.
Doxycycline in water was quantified using liquid chromatography–mass spectrometry/mass (Waters Acquity Ultra performance liquid chromatography [UPLC] with Micromass Quattro Ultima Pt, Etten-Leur, the Netherlands). The UPLC column was the Acquity UPLC 1.8 µm 2.1×100 mm, temperature set at 15°C; the mobile phase consisted of 0.1% formic acid in ultrapure water and 0.1% formic acid in acetonitrile. A gradient was applied from 90% water to 95% acetonitrile. The quantification was done against doxycycline hyclate. The standard was diluted to a final concentration range of 1 to 10 mg/l. Water samples were diluted 100 times to reach an expected concentration of about 2.5 mg/l.
Preparation of the medicated feed
A complete formulated broiler mash free of antibiotic products () was mixed with the phytochemicals/extracts and the FIPs to produce the desired concentrations of the medicated rations. The required amount of phytochemicals/extracts and the FIPs was pre-mixed with approximately 2.5 kg mash in order to safeguard homogeneous distribution in the feed. Thereafter, it was further mixed with the remainder of the feed in a blender (Research Diet Services B.V., Wijk bij Duurstede, the Netherlands).
Table 1. Ingredients and composition of the feed used in three broiler experiments in which birds were inoculated intratracheally with E. coli strain 506.
presents an overview of the intended and obtained concentrations of the phytochemicals/extracts and the FIP in feed, and of the antibiotic drug in drinking water used in the trials.
Table 2. Intended and obtained concentrations of phytochemicals/extracts and FIP in the feed (mg/kg) and doxycycline in the drinking water (mg/l) in three broiler experiments in which birds were inoculated intratracheally with E. coli strain 506.
To assess the mixing procedure of the phytochemicals/extracts and the FIP in the feed, and the antibiotic drug in the drinking water, samples of feed charges and drinking water were subjected to chemical analysis as reported before.
Escherichia coli culture, bacteriological analysis and pulsed-field gel electrophoresis
Inocula
The E. coli strain 506 (serotype O78K80) used is a flumequine-resistant and doxycycline intermediary sensitive strain isolated from an inflamed pericardium from a commercial broiler suffering from natural colibacillosis (Van Eck & Goren, Citation1991). Its colibacillosis inducing potency was confirmed experimentally earlier (Matthijs et al., Citation2003; Velkers et al., Citation2005; Ask et al., Citation2006; Dwars et al., Citation2009). The inocula were prepared from a deep-frozen stock culture (–70°C) of this strain. Frozen beads with E. coli strain 506 were submerged in glucose peptone broth and incubated during 20 h at 37°C.
In Experiment 1, a dilution of 1:100 of the culture was made in glucose peptone broth. In all other experiments the same culture dilution was made, but using physiological saline solution instead. Birds were individually inoculated intratracheally with 0.3 ml E. coli suspension. The birds in the negative control group were placebo inoculated with 0.3 ml glucose peptone broth (Experiment 1) or a sterile physiological saline solution (other experiments). The intratracheal inoculations were performed using a knobbed curved stainless steel cannula size 1.5×45 mm (item number 14186, AUV Group, Cuijk, the Netherlands). Assessment of the bacterial concentration of inocula was performed at the start and the end of the inoculation procedure according to international standards (ISO 7402, Citation1985).
Bacteriological analysis
Sagitally cut femur physis of one or two birds or organs (pericardium, liver or air sac) of one or two surviving birds per group were sterilized with a hot scalpel blade, after which a sample was collected with a wire. A sheep-blood agar plate (K004P090; Biotrading, Mijdrecht, the Netherlands) was then inoculated, subsequently incubated overnight at 37°C and thereafter the purity of colony growth was visually assessed. Biochemical identification of colonies was performed using the indole and β-glucuronidase test.
Pulsed-field gel electrophoresis
The pulsed-field gel electrophoresis (PFGE) technique of contour-clamped homogeneous electric field (CHEF) was used for the genomic typing of the reisolates of all experiments. Genomic DNAs were digested in agarose plugs with XbaI (10 U; Roche Diagnostics, Mannheim, Germany). The resulting fragments were resolved by CHEF-PFGE with a CHEF DR-III apparatus (Bio-RAD Laboratories, Richmond, CA, USA) at a constant voltage of 200 V for 20 h at 13°C and a linearly ramped pulse time of 2.2 to 54.2 sec. The fingerprints generated were processed using Bionumerics software (Applied Maths, Kortrijk, Belgium). Isolates were considered “indistinguishable” if 100% of the fragments were identical.
Chickens, experimental design and parameters
Chickens, housing and ethics
In the first and second chicken experiments, 30 specific pathogen free male broiler chickens (GD – Animal Health Service) per group were used. In the third experiment, 30 commercial male broiler chicks per experimental group were used. In all three chicken experiments, birds were housed in floor pens with wooden shavings. Each floor pen measured 1.0×1.5 m, with walls 0.5 m high. Feed and water were provided ad libitum. The housing temperature was 34°C at 1 day of age and was gradually reduced to 24°C at 15 days of age. A scheme of 22 h light per 24 h was applied.
Experiments were approved by the Institutional Animal Experimental Committee, DEC-Consult Foundation, according to Dutch law on experimental animals (Wet op de dierproeven).
Experimental design
Three chicken experiments were performed. The first and second experiments were carried out with the phytochemicals/extracts E. purpurea, betaine, curcumin, two doses of carvacrol and a FIP with its FIPcontr. In the third experiment, the influence of betaine, curcumin, FIP, one dose of carvacrol and β-glucan on colibacillosis in broilers was investigated. In all experiments the antibiotic doxycycline was used as a control treatment ().
One-day-old birds were tagged with Swiftack®, (Heartland Animal Health Inc., Fair Play, MO, USA) weighed, divided in weight classes and thereafter distributed proportionally to the experimental groups in order to avoid differences in average weight between groups at start. The chicks were given feed supplemented with the phytochemicals/extracts or the FIP, except for the antibiotic-treated birds and the positive and negative control groups. At the age of 8 days, birds were individually weighed and the corresponding groups inoculated intratracheally with 0.3 ml suspension containing E. coli strain 506 (Van Eck & Goren, Citation1991). At the age of 15 days (7 days post inoculation [p.i.]) birds were weighed again and subjected to post-mortem examination to determine the E. coli lesions scores as described by Van Eck & Goren (Citation1991).
Efficacy of treatments was evaluated based on the parameters mortality, mean body weight gain between 8 and 15 days of age, colibacillosis lesion scores and generalized colibacillosis (Velkers et al., Citation2005; Ask et al., Citation2006).
In the third study, which was performed as a split design experiment, the influence of phytochemicals/extracts and the FIP on the recovery of diseased birds was also assessed. Thus, identical treatment groups were housed in two similar separate experimental rooms. Birds in experimental room 1 (Experiment 3A) were used to determine the severity rate of colibacillosis at the post-mortem examination performed at 15 days of age (7 days p.i.) similarly to the previously performed chicken experiments. The birds in experimental room 2 (Experiment 3B) were used to determine the effect of phytochemicals/extracts and the FIP on the recovery of birds between 16 and 36 days of age (8 to 28 days p.i.).
From the results of Experiment 3A, the expected number of broilers with colibacillosis lesions at 15 days of age can be calculated for the groups of Experiment 3B. Subtraction of mortality from 16 to 36 days of age and the number of birds with colibacillosis lesions at 36 days of age from the expected number of broilers with lesions at 15 days of age indicates the number of birds that recovered between 16 and 36 days of age. The method to calculate recovery rates is outlined in .
Table 3. Effect of phytochemicals/extracts and FIP on the recovery of male commercial broilers from experimentally induced colibacillosis between 16 and 36 days of age in Experiments 3A and 3B.
Parameters
Birds were weighed at day 0, day 8 and day 15 of age. Only the chickens in part B of the third experiment were weighed at day 36. The mean body weight gain (± standard error of the mean [SEM]) per experimental group was calculated from 8 until 15 days of age (0 to 7 days p.i.) or from 8 until 36 days of age (0 to 28 days p.i.) in part B of Experiment 3.
Birds were observed daily. Dead birds were collected and stored at –20°C until post-mortem examination at day 15 and day 36 of age.
Colibacillosis lesions were scored macroscopically in the right and left thoracic airs sac, the pericardium and the liver. The scoring system described by Van Eck & Goren (Citation1991) was used. Briefly, 0 = no lesions; 0.5 = at least one pinhead-size spot; 1 = two or more one pinhead-size spots; 2 = some fibrinous patches; and 3 = widespread evidence of fibrin and exudation. A maximal score of 12 per bird could be obtained. Scoring of colibacillosis lesions was performed double blind.
Birds with lesions in the pericardium and/or liver were considered to have generalized infection. Mean colibacillosis lesion scores (MLS) were calculated per group. Examinations were performed blind. Birds that died during the experiment were also necropsied, but their lesion scores were not included in the calculation of MLS.
In all trials, birds were stunned with a mixture of carbon dioxide and oxygen and bled by an incision of the vena jugularis before post-mortem examination.
Statistical analyses
Differences in mean body weight gain (days 8 to 15 in Experiments 1, 2 and 3A, and days 8 to 36 in Experiment 3B) between experimental groups and MLS were assessed using the non-parametric Kruskal–Wallis one-way analysis of variance test. Mortality, number of colibacillosis-affected birds, number of birds with generalized colibacillosis lesions and number of dead birds plus birds with generalized colibacillosis were analysed using Fisher's exact test (User's manual Statistix 8.2 for Windows®, Citation2010). Differences were considered significant if P <0.05.
Results
Concentration of commercial phytochemicals/extracts in the feed
The standardized extract of E. purpurea root was chemically characterized by the presence of alkylamides (not quantified), total phenolic content (7.9±0.1%, n=3), cichoric acid and chlorogenic acid content (0.99±0.11%, n=4 and 0.28±0.02%, n=3, respectively), and total sugar/carbohydrate content (58.6±0.1%, n=3).
The content of standardized E. purpurea root extract in chicken feed samples could not be established by HPLC analysis of cichoric acid since the amount of cichoric acid in the extracts of the feed samples was found to be below the detection limit of the method used (approximately 10 to 25 ng cichoric acid). Unlike the HPLC method, semiquantitative thin-layer chromatographic analysis of alkylamides proved to be an adequate method to determine the content of standardized E. purpurea root extract in chicken feed samples. Thus, the content of standardized E. purpurea root extract in the Echinacea-supplemented feed samples was determined to be 1151±82 mg/kg for the Echinacea-supplemented chicken feed samples (1250 mg/kg). These concentrations were 92.1±6.6% of the desired amount of Echinacea.
Quantitative analysis of the chicken feed samples supplemented with betaine (1500 mg/kg), curcumin (500 mg/kg), and carvacrol (200 mg/kg and 1000 mg/kg) showed concentrations of 1490±327 mg/kg (n=3), 500±5 mg/kg (n=3), 208±1 mg/kg (n=2), and 1090±160 mg/kg (n=3), respectively. These concentrations were 99.3±21.8%, 99.9±1.0%, 104.0±0.5%, and 109.0±16.0% of the desired amount of phytochemicals, respectively. None of the phytochemicals/extracts used in this study, were detected in the unsupplemented control feed samples.
Fungal immunomodulatory protein concentration in the feed
Based on the FIP immune blot analysis, using the positope protein as reference and correcting for the background signal of the E. coli sample not expressing FIP (FIPcontr sample), the calculated amount of FIP in the crude sample used for supplementing feed was 9.3 µg FIP/mg powder. This will have led to a little lower than the attempted FIP concentration in the feed at the start of the experiment but still in the range of 96% (first experiment) to 87% (third experiment) of the desired quantities.
Several months after the experiment, the stored feed samples were analysed for the FIP concentration. After correction for the background signal, a concentration of 4.0 mg FIP/kg feed was detected in the feed originating from the third experiment, indicating that after 4 months the remaining FIP concentration was 40%. It was therefore assumed that FIP concentrations were probably in range of the desired concentrations during the short experimental periods.
Antibiotic concentration in the drinking water
The results of the analysis of the antibiotic (doxycycline) concentration (mg/l) given through the drinking water to the birds during the first, second and third chicken experiments are presented in . The amount of doxycycline detected was 94.8%, 108.4% and 103.4%, respectively.
Table 4. Doxycycline concentration in the drinking water of broilers during treatment in three experiments in which birds were inoculated intratracheally with E. coli strain 506.
Escherichia coli concentration of the inocula, bacterial analysis and PFGE
The inoculation doses obtained for Experiments 1, 2 and 3 were 105.9 to 107.5, 105.9 to 106.2 and 106.2 to 106.4 colony-forming units (CFU)/bird, respectively.
E. coli was reisolated from bone marrow or affected organs of one to four birds per E. coli challenged group, except the doxycycline-treated groups of Experiments 3A and 3B and the positive control group of Experiment 3A (see ), indicating that the mortality and lesions were specific.
Figure 1. The restriction endonuclease digestion patterns obtained by PFGE of reisolated E. coli bacteria from Experiments 1, 2 and 3 (n=≥1 reisolate per experimental group). Patterns were identical to the inoculation strain 506 and therefore considered clonal. The control isolate originating from a broiler flock with colibacillosis appeared genetically unrelated. Reisolates are identified by experiment number, treatment and dose (mg/kg feed, except doxycycline where dose is mg/l drinking water).
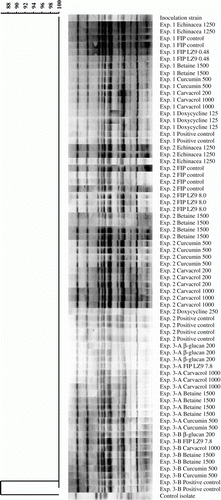
In contrast to the control isolate, all reisolates showed the same banding pattern as the parent E. coli strain 506 after PFGE analysis and were therefore considered genetically indistinguishable ().
Chicken experiments
First experiment
No mortality was recorded in the negative control group, while 28% of the non-treated E. coli inoculated birds died. Doxycycline-treated chicks showed 7% mortality, while mortality in birds treated with phytochemicals/extracts and FIP LZ9 including its control ranged from 31 to 67%. The latter percentages, including that of doxycycline, were not significantly different from the positive control birds except betaine and carvacrol (200 mg/kg), which showed significantly higher mortality.
Although mild lesions occurred in the birds treated with one-half the recommended doxycycline dose, this group was not significantly different from the negative control birds where no lesions were found. However, antibiotic-treated birds did not differ significantly from the broilers treated with Echinacea, FIP LZ9 (nor its control FIPcontr), carvacrol (low dose) and carvacrol (high dose). Differences were significant with respect to betaine and curcumin. The positive control group did not differ significantly from the groups treated with phytochemicals/extracts, but differed significantly from the negative control group.
Generalized colibacillosis did not occur in the negative control group, while in the positive control it was found in 86% of the birds. In the doxycycline-treated birds it occurred in 8% of cases, and in the birds receiving phytochemicals it ranged from 62 to 90%. There was no significant difference between the positive control group and the groups treated with phytochemicals/extracts, while the negative control birds and the birds receiving doxycycline showed significantly less generalized colibacillosis.
Considering the relevance of E. coli-induced mortality and generalized colibacillosis, the sum of both was also analysed. Birds with E. coli mortality and generalized colibacillosis were not found in the negative control group, while in the positive control group 90% belonged to this category. In doxycycline-treated birds 14% of birds were affected, while in the broilers fed phytochemicals it ranged from 79 to 97%. No significant differences were found between the positive control birds and those treated with phytochemicals, while the negative controls and doxycycline-treated birds showed significantly lower percentages.
Comparison of growth showed that positive control birds did not differ significantly from birds treated with phytochemicals/extracts and FIP LZ9, and its control, while the negative controls and doxycycline-treated chicks showed a significantly higher growth. However, there was no significant difference between the doxycycline group and the birds fed FIP LZ9, FIPcontr or betaine. The FIP LZ9 treatment had the highest body weight gain in this growth period. This effect might be partly the result of E. coli-derived triggers (from the recombinant FIP expression) as the FIPcontr treatment is somewhat higher than the control (but not significant). However, this might be caused by the bioactive effects of FIP because the FIP LZ9 is also higher than FIPcontr although this effect is not significant.
The results of the first chicken experiment are summarized in .
Table 5. Effect of treatment with phytochemicals/extracts and FIP on experimentally induced colibacillosis in specific pathogen free male broilers of Experiments 1 and 2.
Second experiment
No mortality was recorded in the negative control group or in the birds treated with doxycycline, while 33% of the non-treated E. coli inoculated birds died. Mortality percentages in birds treated with phytochemicals/extracts and FIP LZ9 including its control ranged from 13 to 20% and were not significantly different from the positive control birds. However, the positive controls differed significantly from the negative controls and doxycycline-medicated birds regarding mortality.
The MLS of the negative control group was zero and did not differ significantly from the doxycycline-treated birds. The latter did not differ significantly from the chicks treated with Echinacea, curcumin, carvacrol 200 mg/kg and carvacrol 1000 mg/kg, and from the positive control. Doxycycline-medicated chicks differed significantly from FIPcontr, FIP LZ9 and betaine, which all had a higher MLS. The positive control group did not differ significantly from groups fed phytochemicals/extracts, FIP LZ9 and FIPcontr, but differed significantly from the negative control group.
Generalized colibacillosis in surviving birds did not occur in the negative control group, while in the positive control birds it was 30%. In the doxycycline-treated birds it was 0%, and in the birds receiving phytochemicals/extracts it ranged from 27 to 48%. There was no significant difference between the positive control group and the groups treated with phytochemicals/extracts, while the negative control birds and the birds receiving doxycycline showed significantly less generalized colibacillosis.
Considering the relevance of E. coli-induced mortality and generalized colibacillosis, the addition of both has also been analysed here. Birds with E. coli mortality and generalized colibacillosis were not found in the negative control group, while in the positive controls 53% belonged to this category. In doxycycline-treated birds 0% was affected, while in the chicks fed phytochemicals/extracts and FIP LZ9 including its control it ranged from 37 to 57%. No significant differences were found between the positive control group and the latter treatment groups, while the negative controls and doxycycline-treated birds showed significantly lower percentages.
Although growth was the highest in the negative control group and doxycycline-medicated birds, there were no significant differences between any of the experimental groups.
The results of the second chicken experiment are summarized in .
Third experiment
Part A
Non-specific mortality occurred in one bird of the negative control group, while not a single bird died in the doxycycline-treated chicks. In the positive control group 10% of birds died. Mortality in birds treated with phytochemicals/extracts and FIP LZ9 ranged from 10 to 23%. No significant differences regarding mortality were found between any of the experimental groups.
The mean lesion score of the negative control group was 0 and did not differ significantly from the doxycycline-treated birds. However, the latter did not differ significantly from the chicks treated with FIP LZ9 and betaine. Doxycycline-medicated chicks differed significantly from β-glucan, carvacrol 1000 mg/kg and curcumin. The positive control group did not differ significantly from groups fed phytochemicals and FIP LZ9, but was significantly different from the negative control and doxycycline-treated birds.
Generalized colibacillosis in surviving birds did not occur in the negative control group, while in the positive control birds it was 56%. In the doxycycline-treated birds it was 0% and in the birds receiving phytochemicals/extracts it ranged from 24 to 57%. There was no significant difference between the positive control group and the groups treated with phytochemicals/extracts and FIP LZ9, while the negative control birds and the birds receiving doxycycline showed significantly less generalized colibacillosis than the former group.
Considering the relevance of E. coli-induced mortality and generalized colibacillosis, the addition of both has also been analysed here. Birds with E. coli mortality and generalized colibacillosis were not found in the negative control group except one bird with non-specific mortality, while in the positive controls 60% belonged to this category. In doxycycline-treated birds 0% was affected, while in the chicks fed phytochemicals/extracts it ranged from 43 to 67%. No significant differences between the positive controls and the latter treatment groups were found, while the negative controls and doxycycline-treated birds showed significantly lower percentages of affected birds than the former groups.
The highest growth was found in the negative control group and doxycycline-treated birds. However, there was no significant difference between the former and FIP LZ9, curcumin and doxycycline-treated birds. Positive controls only differed significantly from the negative controls.
The results of part A of the third chicken experiment are summarized in .
Table 6. Effect of treatment with phytochemicals/extracts and FIP on experimentally induced colibacillosis in commercial male broilers in Experiment 3A.
Part B
No mortality occurred in the negative control group or in the doxycycline-treated chicks. Taking the results of mortality of both periods together (day 8 to and including day 36), 20% of the non-treated E. coli inoculated birds died. Mortality in birds treated with phytochemicals/extracts and FIP LZ9 ranged from 20 to 43%. Positive control birds did not differ significantly from chicks fed phytochemicals/extracts. However, positive controls and birds given phytochemicals and FIP LZ9 differed significantly from the negative controls and doxycycline-medicated chicks.
The mean lesion score of the negative control group and doxycycline-treated birds was zero. However, the mean lesion score of these groups did not differ significantly from any of the other experimental groups except FIP LZ9, which had a higher mean lesion score.
Generalized colibacillosis in surviving birds did not occur in the negative control group, while in the positive control birds it was 21%. In the doxycycline-treated birds it was 0%, and in the birds receiving phytochemicals/extracts and FIP LZ9 it ranged from 29 to 47%. There was no significant difference between the positive control group and the groups treated with phytochemicals/extracts and FIP LZ9, while the negative control birds and the birds receiving doxycycline showed significantly less generalized colibacillosis than the former groups.
Considering the relevance of E. coli-induced mortality and generalized colibacillosis, the sum of both has also been analysed here. Birds with E. coli mortality and generalized colibacillosis were not found in the negative control group, while in the positive controls 37% belonged to this category. In doxycycline-treated birds 0% was affected, while in the chicks fed phytochemicals/extracts and FIP LZ9 it ranged from 53 to 67%. No significant differences between the positive controls and the latter treatment groups were found except for FIP LZ9 fed birds, which showed a significantly higher percentage mortality and generalized colibacillosis. The negative controls and doxycycline-treated birds showed significantly lower percentages than the positive control group and the birds treated with phytochemicals/extracts and FIP LZ9.
The highest growth was found in the negative control group and doxycycline-treated birds, which did not differ from each other significantly. The doxycycline-medicated birds only differed significantly from FIP LZ9, curcumin and the positive controls. The negative control only differed significantly with FIP LZ9, which showed lower growth. The positive controls only differed significantly from the doxycycline-treated birds.
The results of part B of the third chicken experiment are summarized in .
Table 7. Effect of treatment with phytochemicals/extracts and FIP on experimentally induced colibacillosis in commercial male broilers in Experiment 3B.
Recovery of birds
There was no significant difference between the positive control group and the various treatments regarding the percentage of recovered birds, except for the doxycycline-medicated group where the percentage recovery amounted to 100%. In the negative control groups no colibacillosis-affected birds were found ().
Discussion
The selection of phytochemicals/extracts tested was made based on the literature (Peek, Citation2010) and availability. Echinacea (Allen et al., Citation1998; Allen, Citation2003) and betaine (Augustine et al., Citation1997; Matthews et al., Citation1997; Waldenstedt et al., Citation1999; Matthews & Southern, Citation2000; Klasing et al., Citation2002; Fetterer et al., Citation2003) were previously reported to be active against coccidiosis and colibacillosis, while curcumin (Allen et al., Citation1998) and carvacrol (Giannenas et al., Citation2003) have been known to induce immunomodulatory stress proteins. Mushroom extracts (Guo et al., Citation2004, Citation2005; Dalloul et al., Citation2006) and β-glucan (Smith & Ovington, Citation1996; Huff et al., Citation2006) have been shown in several studies to be potential alternatives for antibiotics. β-glucans have been tested in in vivo enterotoxigenic E. coli infection models using piglets. Stuyven et al. (Citation2009) showed that the type and concentration of processed glucans such as MCG from Saccharomyces cerevisia and G3 from Sclerotium rolfsii resulted in a significant protective effect against enterotoxigenic E. coli. In this study β-glucan from Shiitake was used because many bioactive effects have been published, including protective effect towards tuberculosis infection in mice (Markova et al., Citation2003), prophylactic potential to malaria tested in mice (Zhou et al., Citation2009) and many more in which most effects are mediated via the inactivation of macrophages. FIPs were never tested in an E. coli infection model but have been tested in animal allergy models (Li et al., Citation2011; Wichers & Mes, Citation2011). These studies resulted in the hypothesis that these FIPs are potent immunomodulators skewing the immune balance towards Th1, which has an effect on the anaphylactic shock but might also modulate the immune response against intestinal infections.
Phytochemical or FIP treated unchallenged groups were not included in the study as they were not necessary to address the question whether these compounds could prevent or significantly reduce colibacillosis in broilers, and we were not interested in other effects of the phytochemicals and the FIP. The doses used for these products were obtained from the literature and had not been reported as toxic; moreover, none of the phytochemicals and the FIP had a significant effect on mortality and body weight at day 8 (day of E. coli challenge) (data not shown). The specificity of mortality and lesions was assessed by sampling a number of birds of each treatment group for bacteriology. E. coli was reisolated from bone marrow or organs with lesions of at least one bird, but often from more birds, per E. coli challenged group, indicating that the mortality and lesions were specific. Additionally, PFGE was performed on all reisolates to study their clonality with respect to the inoculation strain. The banding patterns of reisolates appeared to be identical to that of the inoculated E. coli strain 506.
The FIP analysis indicated that the V5 epitope protein fragment and therefore probably the FIP was present in the feed and remained present for a prolonged period although it had decreased to less than 50% within 4 months. The observed reduction in the feed could have been due to degradation of the protein or due to the fact that FIP has lectin activity, which results in strong binding to sugars at the surface of the feed particle, and may have impaired its release during the extraction. These results strengthen previous studies on the processing tolerance of FIPs, which indicated a high temperature, acid, and dehydration stability (Ou et al., Citation2009). However, this is based on the presence of the protein and not on bioactivity as no haemagglutination assays could be performed with these crude extracts, making it impossible to detect parts of its possible bioactivity.
The inability to detect standardized E. purpurea root extract in chicken feed samples by HPLC analysis of cichoric acid was not related to insufficient sensitivity of the analytical method. Since cichoric acid was not detectable in extracts of the control chicken feed spiked with 1250 mg/kg Echinacea (with a theoretical amount of 250 ng cichoric acid, which exceeds the detection limit by 10 to 25 times), it was concluded that cichoric acid (but probably also other polyphenolic constituents present in the standardized E. purpurea root extract) was bound to the feed matrix and could not be extracted. Most likely, the high content of proteins and/or polysaccharides in the feed samples play a role in this respect as it is well known that these macromolecules can interact with and precipitate polyphenols (see, for instance, McManus et al., Citation1985). The ineffectiveness of Echinacea-supplemented feeds as demonstrated in this study may, at least in part, be attributed to this limited bioavailability of polyphenolic constituents from the feed matrix since cichoric acid (and related constituents) is generally considered one of the active principles in this plant (Barnes et al., Citation2005).
The relatively high standard deviation in betaine content of the feed samples is probably due to the low specificity of the colorimetric reaction used; in addition to betaine, other quaternary ammonium compounds (amongst which choline) also react with potassium triiodide and result in a high background signal (Wall et al., Citation1960; Storey & Wyn Jones, Citation1977).
The relatively high standard deviation observed for the quantitative analysis of the chicken feeds supplemented with high carvacrol concentrations is related to the incidental and partial overlap of the carvacrol spots with spots of other constituents in the feed.
Chemical analyses of feeds and drinking water have shown that the desired concentration of phytochemicals/extracts, FIP LZ9 and doxycycline had been obtained ( and ).
The inoculum of the first experiment was prepared and diluted in glucose peptone broth, resulting in an initial inoculation dose of 105.9 CFU/bird. However, during the lengthy inoculation procedure, bacterial growth occurred, as established by means of bacterial counting, resulting in a final inoculation dose of 107.5 CFU/bird. This might explain the variation in mortality between some treatment groups. Nevertheless, in groups with lower mortality more generalized colibacillosis occurred, while the contrary happened in the groups with high mortality. Since the relevance of colibacillosis in terms of economic damage is determined by both mortality and generalized colibacillosis, the latter resulting in condemnations at slaughter, the addition of both parameters illustrates very well the global impact of the infection, which was similar for all experiment groups despite the higher infection doses of some groups in this experiment. Nevertheless, in subsequent experiments, inocula were prepared using sterile physiological salt solution and kept on ice. Bacterial concentrations remained stable during the inoculation procedure and ranged from 105.9 to 106.2 CFU/bird and from 106.2 to 106.4 CFU/bird in Experiments 2 and 3, respectively.
Birds fed with FIP LZ9 showed a relatively high body weight gain during the first 8 to 15 days of life, whereas in part B of the third experiment, as birds grew older, treatment with this FIP was associated with the lowest mean body weight gain. This indicates that long-term exposure of broilers to FIP LZ9 may be detrimental for body weight gain. Therefore other doses and duration of treatment should be considered in future experiments.
In Experiments 1 and 2 FIPcontr was included in order to assess whether E. coli-derived fractions such as lipopolysaccharide (LPS), which are likely to be present in the FIP LZ9 due to the fact that it is a recombinant product expressed in E. coli, influenced the experimental results. However, no significant differences were found between FIP LZ9 and FIPcontr fed birds.
In the third experiment, no FIPcontr was used to compensate for adverse effects of E. coli-derived fractions such as LPS (which are in the sample because of the recombinant expression in E. coli), therefore it cannot be ruled out that possible positive effects of FIP were counteracted by negative effects of LPS. In future, it will be wise to express FIPs in Pichia that do not produce challengers like LPS. Xue et al. (Citation2008) and Liang et al. (Citation2009) have shown that these FIPs can be expressed in Pichia, while retaining bioactivity. Although studied FIPs such as LZ8, FIP-five, FIP-Vvo and all other FIPs identified have a high degree of similarity at the protein level, they all have different specificity and activity in haemagglutination depending on the species origin of the blood (Li et al., Citation2011). It would be worth testing the specificity of the LZ9 and other FIPs towards chicken red blood cells and based on these results select a potential best-fitted FIP for future experiments with birds.
Colibacillosis was successfully induced in broilers of the positive control group in all three experiments and the negative control birds remained free of E. coli lesions and mortality in all cases.
In the first part of the third experiment (Experiment 3A), no significance difference between groups was found regarding mortality. This was attributed to the low mortality in general and the fact that non-specific mortality occurred in one bird of the negative control group.
The colibacillosis parameter of ultimate concern for the field is E. coli-induced mortality and generalized colibacillosis together. It ranged from 79 to 97% in Experiment 1, from 37 to 57% in Experiment 2, from 43 to 67% in Experiment 3A and from 37 to 67% in Experiment 3B. The higher percentages found in Experiment 1 were explained by the increasing bacterial concentrations of the inocula.
In any case, phytochemicals/extracts were unable to significantly reduce the incidence of E. coli mortality and generalized colibacillosis when compared with the positive control birds. Moreover, they were unable to show any positive effect on the recovery of colibacillosis-affected broilers. In contrast, doxycycline proved to be highly efficient at controlling mortality and colibacillosis lesions in infected birds, both at one-half of the recommended dose as well as at full dose. It also greatly favoured the recovery of birds (100%) with colibacillosis.
In conclusion, in contrast to doxycycline, the selected phytochemicals/extracts and the FIP failed to reduce E. coli mortality and generalized colibacillosis and cannot be used to replace antibiotic drugs. However, this does not rule out the possibility that these compounds can have other positive effects on bird health not analysed in this study.
Acknowledgements
This study was funded by the Dutch Ministry of Economic Affairs, Agriculture and Innovation, coordinated by the Immuno Valley foundation and embedded in the research programme ALTANT (ALTernatives for ANTibiotics) “Modulation with immune stimulating phytochemicals (MODIPHY) as alternative for antimicrobial treatment”. The authors also thank all participants in this project—Dr R. Adriaansen-Tennekes, Dr C.J. Beukelman, Dr S.A. Burt, Dr R.H.H. Pieters, Prof. Dr H.F.J. Savelkoul, Dr A.J.J. van den Berg, Dr R. van der Zee, Prof. Dr W. van Eden, Prof. Dr F. van Knapen, Dr L. Vervelde, and Prof. Dr H.J. Wichers—for fruitful discussions and Dr J.H.H. van Eck for his assistance with the post-mortem examinations and critically reading the manuscript.
References
- Allen , P.C. 2003 . Dietary supplementation with Echinacea and development of immunity to challenge infection with coccidia . Parasitology Research , 91 : 74 – 78 . doi: 10.1007/s00436-003-0938-y
- Allen , P.C. , Danforth , H.D. and Augustine , P.C. 1998 . Dietary modulation of avian coccidiosis . International Journal for Parasitology , 28 : 1131 – 1140 . doi: 10.1016/S0020-7519(98)00029-0
- Ask , B. , van der Waaij , E.H. , van Eck , J.H.H. , van Aarendonk , J.A.M. and Stegeman , J.A. 2006 . Defining susceptibility of broiler chicks to colibacillosis . Avian Pathology , 35 : 147 – 153 . doi: 10.1080/03079450600597998
- Augustine , P.C. , McNaughton , J.L. , Virtanen , E. and Rosi , L. 1997 . Effect of betaine on the growth performance of chicks inoculated with mixed cultures of avian Eimeria species and on invasion and development of Eimeria tenella and Eimeria acervulina in vivo and in vitro . Poultry Science , 79 : 802 – 809 .
- Barnes , J. , Anderson , L.A. , Gibbons , S. and Phillipson , J.D. 2005 . Echinacea species (Echinacea angustifolia (DC.) Hell., Echinacea pallida (Nutt.) Nutt., Echinacea purpurea (L.) Moench): a review of their chemistry, pharmacology, and clinical properties . Journal of Pharmacy and Pharmacology , 57 : 929 – 954 . doi: 10.1211/0022357056127
- Bauer , R. and Remiger , P. 1989 . TLC and HPLC analysis of alkamides in Echinacea drugs . Planta Medica , 55 : 367 – 371 . doi: 10.1055/s-2006-962030
- Chen , P. 2006 . Development and validation of a high-throughput based on liquid chromatography with ultraviolet absorption and mass spectrometry detection method for quantitation of cichoric acid in Echinacea purpurea aerial-based dietary supplements . Journal of AOAC International , 89 : 612 – 618 .
- Cowan , M.M. 1999 . Plant products as antimicrobial agents . Clinical Microbiological Reviews , 12 : 564 – 582 .
- Cravotto , G. , Boffa , L. , Genzini , L. and Garella , D. 2009 . Phytotherapeutics: an evaluation of the potential of 1000 plants . Journal of Clinical Pharmacy and Therapeutics , 35 : 11 – 48 . doi: 10.1111/j.1365-2710.2009.01096.x
- Dalloul , R.A. , Lillehoj , H.S. , Lee , J.S. , Lee , S.H. and Chung , K.S. 2006 . Immunopotentiating effect of a Formitella fraxinea-derived lectin on chicken immunity and resistance to coccidiosis . Poultry Science , 85 : 446 – 451 .
- Dreywood , R. 1946 . Qualitative test for carbohydrate material. Industrial and Engineering Chemistry . Analytical Edition , 18 : 499 – 499 . doi: 10.1021/i560156a015
- Dwars , R.M. , Matthijs , M.G.R. , Daemen , A.J.J.M. , van Eck , J.H.H. , Vervelde , L. and Landman , W.J.M. 2009 . Progression of lesions in the respiratory tract of broilers after single infection with Escherichia coli compared to superinfection with E. coli after infection with infectious bronchitis virus . Veterinary Immunology and Immunopathology , 127 : 65 – 76 . doi: 10.1016/j.vetimm.2008.09.019
- Eklund , M. , Bauer , E. , Wamatu , J. and Mosenthin , R. 2005 . Potential nutritional and physiological functions of betaine in livestock . Nutrition Research Reviews , 18 : 31 – 48 . doi: 10.1079/NRR200493
- European Pharmacopoeia . ( 2010a ). Purple coneflower root; Echinaceae purpureae radix. Monograph no. 01/2008:1824 corrected 6 . 0 6th edition 2010 (6.8) . EDQM Council of Europe , Strasbourg , , France .
- European Pharmacopoeia . ( 2010b ). Oregano; Origani herba. Monograph no. 07/2010:1880 6th edition (6.8) . EDQM Council of Europe , Strasbourg , , France .
- European Pharmacopoeia . ( 2010c ). Turmeric, Javanese; Curcumae xanthorrhizae rhizoma. Monograph no. 01/2008:1441 6th edition 2010 (6.8) . EDQM Council of Europe , Strasbourg , , France .
- Fetterer , R.H. , Augustine , P.C. , Allen , P.C. and Barfield , R.C. 2003 . The effect of dietary betaine on intestinal and plasma levels of betaine in uninfected and coccidia-infected broiler chicks . Parasitology Research , 90 : 343 – 348 . doi: 10.1007/s00436-003-0864-z
- Giannenas , I. , Florou-Paneri , P. , Papazahariadou , M. , Christaki , E. , Botsoglou , N.A. and Spais , A.B. 2003 . Effect of dietary supplementation with oregano essential oil on performance of broilers after experimental infection with Eimeria tenella . Archives of Animal Nutrition , 57 : 99 – 106 . doi: 10.1080/0003942031000107299
- Guo , F.C. , Kwakkel , R.P. , Williams , B.A. , Paramentier , H.K. , Li , W.K. , Yang , Z.Q. and Verstegen , M.W. 2004 . Effects of mushroom and herb polysaccharides on cellular and humoral immune responses of E. tenella-infected chickens . Poultry Science , 83 : 1124 – 1132 .
- Guo , F.C. , Kwakkel , R.P. , Williams , B.A. , Suo , X. , Li , W.K. and Verstegen , M.W.A. 2005 . Coccidiosis immunization: effects of mushroom and herb polysaccharides on immune responses of chickens with Eimeria tenella . Avian Diseases , 49 : 70 – 73 . doi: 10.1637/7227-062504R1
- Gyles , C.L. 2008 . Antimicrobial resistance in selected bacteria from poultry . Animal Health Research Reviews , 9 : 149 – 158 . doi: 10.1017/S1466252308001552
- Huff , G.R. , Huff , W.E. , Rath , N.C. and Tellez , G. 2006 . Limited treatment with beta 1,3/1,6-glucan improves production values of broiler chickens challenged with E. coli . Poultry Science , 85 : 613 – 618 .
- ISO 7402 1985 . Microbiology – General Guidance for the Enumeration of Enterobacteriaceae without Resuscitation – MPN Technique and Colony Count Technique , 1st edn . Geneva : International Standard Organization .
- Kino , K. , Yamashita , A. , Yamaoka , K. , Watanabe , J. , Tanaka , S. , Ko , K. , Shimizu , K. and Tsunoo , H. 1989 . Isolation and characterization of a new immunomodulatory protein, ling zhi-8 (LZ-8), from Ganoderma lucidum . Journal of Biological Chemistry , 264 : 472 – 478 .
- Klasing , K.C. , Adler , K.L. , Remus , J.C. and Calvert , C.C. 2002 . Dietary betaine increases intraepithelial lymphocytes in the duodenum of coccidial-infected chicks and increases functional properties of phagocytes . Journal of Nutrition , 132 : 2274 – 2282 .
- KNMvD . 2012 . Koninklijke Nederlandse Maatschappij voor Diergeneeskunde (Royal Dutch Veterinary Association). Formularium pluimvee (formulary for poultry), version 31 July 2012. http://www.knmvd.nl/wvab/formularia/formularia .
- Li , Q.Z. , Wang , X.F. and Zhou , X.W. 2011 . Recent status and prospects of the fungal immunomodulatory protein family . Critical Reviews in Biotechnology , 31 : 365 – 375 . doi: 10.3109/07388551.2010.543967
- Liang , C. , Zhang , S. , Liu , Z. and Sun , F. 2009 . Ganodema lucidum immunomodulatory protein (Lz-8) expressed in Pichia pastoris and the identification of immunocompetence . Sheng Wu Gong Cheng Xue Bao , 25 : 441 – 447 .
- Maran reports . ( 2002–2009 ). Monitoring of antimicrobial resistance and antibiotic usage in animals in the Netherlands. Retrieved from http://www.cvi.wur.nl/UK/publications/otherpublications/maran/
- Markova , N. , Kussovski , V. , Drandarska , I. , Nikolaeva , S. , Georgieva , N. and Radoucheva , T. 2003 . Protective activity of Lentinan in experimental tuberculosis . International Immunopharmacology , 3 : 1557 – 1562 . doi: 10.1016/S1567-5769(03)00178-4
- Matthews , J.O. and Southern , L.L. 2000 . The effect of dietary betaine in Eimeria acervulina infected chicks . Poultry Science , 79 : 60 – 65 .
- Matthews , J.O. , Ward , T.L. and Southern , L.L. 1997 . Interactive effects of betaine and monensin in uninfected and Eimeria acervulina-infected chicks . Poultry Science , 76 : 1014 – 1019 .
- Matthijs , M.G.R. , van Eck , J.H.H. , Landman , W.J.M. and Stegeman , J.A. 2003 . Ability of Massachusetts-type infectious bronchitis virus to increase colibacillosis susceptibility in commercial broilers: a comparison between vaccine and virulent field virus . Avian Pathology , 32 : 473 – 481 . doi: 10.1080/0307945031000154062
- McManus , J.P. , Davis , K.G. , Beart , J.E. , Gaffney , S.H. , Lilley , T.H. & Haslam , E. 1985 . Polyphenol interactions. Part I. Introduction: some observations on the reversible complexation with proteins and polysaccharides . Journal of the Chemical Society, Perkin Transactions II, (9) , 1429 – 1438 . doi: 10.1039/p29850001429
- OU , C.C. , Hsiao , Y.M. , Wang , W.H. , Ko , J.L. and Lin , M.Y. 2009 . Stability of fungal immunomodulatory protein, FIP-gts and FIP-fve, in IFN-gamma production . Food and Agricultural Immunology , 20 : 319 – 332 . doi: 10.1080/09540100903247688
- Peek , H.W. 2010 . Resistance to anticoccidial drugs: alternatives strategies to control coccidiosis in broilers . PhD thesis, ISBN 978-90-393-5272-4. Utrecht University, Utrecht, the Netherlands .
- Persoons , D. , Dewulf , J. , Smet , A. , Herman , L. , Heyndrickx , M. , Martel , A. , Catry , B. , Butaye , P. and Haesebrouck , F. 2010 . Prevalence and persistence of antimicrobial resistance in broiler indicator bacteria . Microbial Drug Resistance , 16 : 67 – 74 . doi: 10.1089/mdr.2009.0062
- Singleton , V.L. , Orthofer , R. and Lamuela-Raventós , R.M. 1999 . Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent . Methods in Enzymology , 299 : 152 – 178 .
- Smith , N.C. and Ovington , K.S. 1996 . The effect of BCG, zymosan and Coxiella burnetti extract on Eimeria infections . Immunology and Cell Biology , 74 : 346 – 348 . doi: 10.1038/icb.1996.61
- Sokovic , M. , Glamoclija , J. , Marin , P.D. , Brkic , D. and van Griensven , L.J.L.D. 2010 . Antibacterial effects of the essentials oils of commonly consumed medicinal herbs using an in vitro model . Molecules , 15 : 7532 – 7546 . doi: 10.3390/molecules15117532
- Storey , R. and Wyn Jones , R.G.W . 1977 . Quaternary ammonium compounds in plants in relation to salt resistance . Phytochemistry , 16 : 447 – 453 . doi: 10.1016/S0031-9422(00)94326-7
- Stuyven , E. , Cox , E. , Vancaeneghem , S. , Arnouts , S. , Deprez , P. and Goddeeris , B.M. 2009 . Effect of ß-glucans on an ETEC infection in piglets . Veterinary Immunology and Immunopathology , 128 : 60 – 66 . doi: 10.1016/j.vetimm.2008.10.311
- Tomassen , M.M.M. , Hendrix , E.A.H.J. , Sonnenberg , A.S.M. , Wichers , H.J. & Mes , J.J. 2011 . Variation of bioactive lentinan-containing preparations in Lentinula edodes strains and stored products . Proceedings of the 7th International Conference on Mushroom Biology and Mushroom Products (ICMBMP7) (pp. 259 – 267 ). Belgrade , Serbia .
- User's manual Statistix 8.2 for Windows®. [Computer software] . 2010 . Tallhassee , FL : Analytical Software .
- Van Eck , J.H.H. and Goren , E. 1991 . An Ulster 2C strain derived Newcastle disease vaccine: vaccinal reaction in comparison with other lentogenic Newcastle disease vaccines . Avian Pathology , 20 : 497 – 507 . doi: 10.1080/03079459108418787
- Velkers , F.C. , teLoo , A.J.H. , Madin , F. and van Eck , J.H.H. 2005 . Isopathic and pluralist homeopathic treatment of commercial broilers with experimentally induced colibacillosis . Research in Veterinary Science , 78 : 77 – 83 . doi: 10.1016/j.rvsc.2004.06.005
- Waldenstedt , L. , Elwinger , K. , Lunden , A. , Thebo , P. and Uggla , A. 1999 . Effect of betaine supplement on broiler performance during an experimental coccidial infection . Poultry Science , 78 : 182 – 189 .
- Wall , J.S. , Christianson , D.D. , Dimler , R.J. and Senti , F.R. 1960 . Spectrophotometric determination of betaines and other quaternary nitrogen compounds as their periodides . Analytical Chemistry , 32 : 870 – 874 . doi: 10.1021/ac60163a044
- Wichers , H.J. and Mes , J.J. 2011 . “ Immunomodulation by food: allergy mitigation by dietary components ” . In Food Allergies: Symptoms, Diagnosis and Treatments , 1 ed. , Edited by: Rodgers , P.M. 79 – 94 . New York : Nova Science Publishers Inc., ISBN: 978-1-61728-748-0 .
- Windisch , W. , Schedle , K. , Plitzner , C. and Kroismayr , A. 2008 . Use of phytogenic products as feed additives for swine and poultry . Journal Animal Science , 86 : 40 – 148 .
- World Health Organization . 2011 . Tackling Antibiotic Resistance from a Food Safety Perspective in Europe . Copenhagen : World Health Organization, Regional Office for Europe . Retrieved from http://www.euro.who.int/en/what-we-publish/abstracts/tackling-antibiotic-resistance-from-a-food-safety-perspective-in-europe
- Xue , Q. , Ding , Y. , Shang , C. , Jiang , C. and Zhao , M. 2008 . Functional expression of LZ-8, a fungal immunomodulatory protein from Ganodema lucidum in Pichia pastoris . Journal of General and Applied Microbiology , 54 : 393 – 398 . doi: 10.2323/jgam.54.393
- Yap , A.T. and Ng , M.L. 2001 . An improved method for the isolation of lentinan from the edible and medicinal shiitake mushroom, Lentinus edodes (Berk.) Sing. (Agaricomycetideae) . International Journal of medicinal mushrooms , 3 : 9 – 19 . doi: 10.1615/IntJMedMushr.v3.i1.20
- Yun , C.H. , Estrada , A. , Van Kessel , A. , Park , B.C. and Laarveld , B. 2003 . Beta-glucan, extracted from oat, enhances disease resistance against bacterial and parasitic infections . FEMS Immunology and Medical Microbiology , 35 : 67 – 75 . doi: 10.1016/S0928-8244(02)00460-1
- Zhou , L.D. , Zhang , Q.H. , Zhang , Y. , Liu , J. and Cao , Y.M. 2009 . The shiitake mushroom-derived immuno-stimulant lentinan protects against murine malaria blood-stage infection by evoking adaptive immune-responses . International Immunopharmacology , 9 : 455 – 462 . doi: 10.1016/j.intimp.2009.01.010