Abstract
Wild-type (WT) and small-colony-variant (SCV) strains of Streptococcus equi subsp. zooepidemicus have recently been isolated from a layer flock in Denmark experiencing high mortality. To investigate the disease-causing potential of SCV compared with WT, a 2-week long infection study was performed in 45-week-old brown layer chickens. Four groups of 11 chickens each were inoculated with a WT or SCV strain by the intravenous or intra-tracheal route: WT-IV, SCV-IV or WT-IT, SCV-IT, respectively. Clinical signs were observed in most chickens in the WT-IV group (9/11). Mortality was observed in the SCV-IV (4/11) and WT-IV (2/11) groups. Ten chickens in the WT-IV and WT-IT groups, respectively, developed gross lesions including oophoritis/peritonitis, hepatitis and airsacculitis cervicalis. Bronchopneumonia was common in the SCV-IT group (6/11), and valvular endocarditis in the SCV-IV group (4/11). Histological lesions in liver tissue were frequently observed in the chickens of the SCV-IV group (9/11), followed by the WT-IT (7/11), WT-IV (6/11), and SCV-IT (2/11) groups. The lesions in the SCV-IV group were dominated by deposition of eosinophilic material with infiltration of inflammatory cells (6/9). Bacteriological re-isolation of either strain type was achieved from all chickens of the WT-IV and WT-IT groups, and from nine and seven out of 11 chickens for each of the SCV-IV and SCV-IT groups, respectively. In summary, we were able to reproduce clinical signs and lesions as observed during the natural outbreak, which included an overall initial onset in WT-infected chickens as opposed to a late onset and possible recurring infection seen in the SCV-infected chickens.
Introduction
Streptococcus equi subsp. zooepidemicus has been associated with infections in adult hens with variable mortality, ranging from 11 to 80% (Genest & Nadeau, Citation1944; Buxton, Citation1952; Sato et al., Citation1960; Thayer et al., Citation2008; Bisgaard et al., Citation2012). Recently flock infections have been described that subsequently continued for several weeks and contained different clinico-pathological patterns including sub-acute septicaemia, respiratory disturbances, fever and diarrhoea, in agreement with previous reports (Edwards & Hull, Citation1937; Buxton, Citation1952; Sato et al., Citation1960; Peckham, Citation1966), and the recent natural infection was associated with wild-type (WT) and small-colony-variant (SCV) strains of S. zooepidemicus (Bisgaard et al., Citation2012).
In addition to the typical WT colony appearance, some strains of S. zooepidemicus and Staphylococcus aureus are capable of producing pinpoint colonies—known as SCVs (Proctor et al., Citation2006; Sendi & Proctor, Citation2009; Bisgaard et al., Citation2012). SCVs are generally regarded as slow-growing sub-populations of bacteria with distinctive phenotypic and pathogenic traits, which are easily overlooked unless examined closely (Proctor et al., Citation2006). The SCV phenotype has been proposed to represent a microbial cell type adapted to the intracellular environment, favouring persistent or recurring sub-acute infections (Eiff et al., Citation2006; Proctor et al., Citation2006; Allegrucci & Sauer, Citation2007). In the case of S. aureus the altered phenotype of SCV includes increased expression of clumping factor and fibronectin binding protein, whereas this cell type has been demonstrated to cause less damage to host cells due to decreased production of toxins (Sendi & Proctor, Citation2009). The pathogenic potential of S. aureus SCVs has been studied in human infections, and animal models (Proctor et al., Citation2006) and recently SCVs of Enterococcus faecalis were isolated from chickens suffering from amyloid arthropathy (Petersen et al., Citation2008) and from humans with endocarditis (Kaase et al., Citation2004). The clinical importance and pathogenic potential of SCVs are still controversial due to a lack of relevant studies aiming at reproducing the clinical signs and lesions observed during natural infections. Thus, in the present investigation, we aimed at reproducing the course of infection with S. zooepidemicus appearing in two forms, WT and SCV, respectively. The isolates were obtained from chickens in a Danish organic layer farm, experiencing an accumulated mortality of 80% (Bisgaard et al., Citation2012). The outbreak was characterized by an initial acute phase, associated with hepato-spleno-reno-megaly, peritonitis, and transudate in the pericardial sac and peritoneal and thoracic cavities, which was superseded by a chronic phase with chickens suffering from salpingitis, oophoritis, airsacculitis, endocarditis and liver infarcts. A large proportion of the chickens in the flock were emaciated and somnolent. In addition to the typical mucoid colonies, an increasing number of SCVs were isolated during the chronic phase of the outbreak, but-their contribution to the observed lesions was not clearly understood. Transmission via droplet infection was suspected to be the predominant route between the birds.
Owing to the slow bacterial growth, nonspecific lesions and signs of SCV infections are likely to be overlooked (Eiff et al., Citation2006; Proctor et al., Citation2006; Allegrucci & Sauer, Citation2007). Early detection may, however, be a very effective way to prevent increased mortality and condemnations in chickens.
Here, we characterize the pathogenic potential of S. zooepidemicus WT and SCV, respectively, in adult layer chickens infected by intravenous (i.v.) and intra-tracheal (i.t.) routes, on the basis of clinical signs, macroscopic and microscopic lesions, and bacteriological re-isolation rates.
Materials and Methods
WT and SCV strains of S. zooepidemicus
One WT strain (strain F122 Hj5) (a) and one SCV strain (strain F122 Abscess 115) (b) of S. zooepidemicus were used for this study. Both strains were initially isolated by Bisgaard et al. (Citation2012) from a natural outbreak in chickens at an organic layer farm in Denmark.
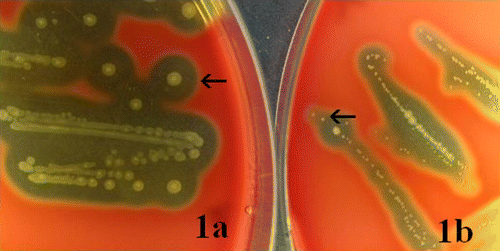
Figure 1. Colonies of the WT and SCV strains of S. zooepidemicus. Arrows indicate typical colonies of the respective strains. 1a: WT: after 48 h of incubation at 37°C, the easily visible typical colonies of the WT appeared with whitish to yellowish colour, circular and convex shape, single or short-chain form, and each of 1 to 2 mm in diameter, an entire margin, mucoid capsule and clear β-haemolytic zone (WT colonies were also observed at 24 h of incubation). 1b SCV: colonies of the SCV were observed after 48 h of incubation at 37°C as grey transparent to white dry pin-points, sometimes too tiny to see with the naked eye, circular and convex shape, mostly in chain form, and each up to 0.5 mm in diameter, an entire margin, faint β-haemolytic zone and without capsule (SCV colonies were not shown following 24 h of incubation; however, in very few cases they were observed as around 0.1 mm in diameter).
Groups of chickens
A randomized experimental study was made in 45-week-old Lohmann brown layer chickens obtained from a commercial flock with no history of disease. The chickens had been vaccinated according to the general recommendations in Denmark, which includes vaccination against Marek's disease, coccidiosis, infectious bronchitis, Newcastle disease, infectious bursal disease, chickens anaemia virus, avian encephalomyelitis, EDS78, and avian pneumovirus. The chickens were randomly divided into four experimental groups, referred to as WT-IV, WT-IT, SCV-IV and SCV-IT, respectively, depending on the bacterial type (WT, SCV) and route of infection (i.v., i.t.). Each group comprised 11 chickens whereas two uninfected control groups, Control-IV and Control-IT, contained six and five chickens, respectively. Each group of chickens were housed separately and provided ad libitum with standard feed and water.
Experimental infections and sample collections
All experimental procedures and bird management protocols were undertaken in accordance with legislation of the Ministry of Justice, Denmark (approval number: 2008/561-1481).
After 1 week of acclimatization, each bird was bled through the brachial vein to collect 1 ml blood for bacteriological examination. Subsequently, chickens belonging to the WT-IV and SCV-IV groups were injected intravenously with 0.5 ml bacterial suspension (109 colony-forming units/ml in brain–heart infusion broth) of the WT or SCV strain, respectively. Chickens belonging to the WT-IT and SCV-IT groups were inoculated with a similar dose via the i.t. route. Each bird of the control groups received 0.5 ml sterile brain–heart infusion broth. At day 1 post infection (p.i.), two birds from each group were randomly selected, euthanized and submitted to post-mortem examinations. Two additional birds were examined at day 3 p.i., whereas the remaining birds were examined at day 14 p.i. Prior to euthanasia, 1 ml blood was collected from each bird of the WT-IV and SCV-IV groups for bacteriological culture. Tracheal swabs were taken from each bird of the WT-IT and SCV-IT groups at day 14 p.i. prior to euthanasia. A swab was also taken for bacteriological culture when any lesions were encountered in an organ during post-mortem examination.
Clinical signs, gross pathology and bacteriology
All ante-mortem and post-mortem abnormalities including mortality were carefully noted during the entire experiment. Swabs for bacteriological culture were smeared on blood agar base (CM 55; Oxoid, Basingstoke, UK) with 5% citrated bovine blood added, and incubated at 37°C in sealed plastic bags for 24 to 48 h. For blood cultures, 1 ml citrated blood was added to 9 ml brain–heart infusion broth in a test tube, incubated at 37°C for the same period, and subsequently examined for bacterial growth, which, if positive, was inoculated on 5% bovine blood agar. Any growth resembling S. zooepidemicus was identified according to the previously reported characteristics (Bisgaard et al., Citation2012).
Histopathology
For histopathological examination, a piece of liver was fixed in 10% neutral buffered formalin solution for 24 h, subsequently trimmed, dehydrated and embedded in paraffin wax prior to preparation of 3 to 5 µm thick sections, which were mounted on adhesive slides (Super Frost/Plus; Menzel-Gläser, Braunschweig, Germany). All sections were stained with haematoxylin and eosin. In addition, a subset of sections was stained with Congo Red and phosphotungstic acid–haematoxylin preparation, according to Bancroft & Gamble (Citation2007).
Statistical analysis
R software (version 2.12.2; R Software, Vienna, Austria) was used for data analyses. Pair-wise comparison of categorical data was performed using Fisher's exact test. In case of multiple comparison within the same dataset (e.g. WT-IV against WT-IT and WT-IV against SCV-IT), the standard critical P value of 0.05 was adjusted to 0.0127 to avoid false positive results according to Motulsky (Citation2010).
Results
No chickens in the two control groups showed any clinical signs or lesions and remained culture-negative for S. zooepidemicus during the entire experiment. In contrast, different clinical signs and pathology were observed in chickens belonging to each of the experimental groups. The results have been summarized and compared in .
Table 1. Pathogenicity study of the WT and SCV strains of S. zooepidemicus in adult layer chickens infected through i.v. and i.t. routes.
Clinical signs and mortality
Common clinical signs recorded in the experimental chickens were depression, a soiled cloacal region and a negatively affected body condition. In the WT-IV group, nine out of 11 birds showed some kind of clinical signs, and the remaining two birds were found dead at day 1 p.i. without preceding clinical signs. Two somnolent birds in the SCV-IV group were euthanized at day 10 and 11 p.i., respectively. In addition, two birds of this group died on days 9 and 11 p.i.—the first had a soiled cloacal region and the second had no noticeable signs. The remaining seven birds did not show clear clinical signs during the experiment.
Gross pathological changes
Among the different pathological lesions observed in this study, the most frequently recorded were oophoritis associated with peritonitis (oophoritis/peritonitis), liver necrosis, bronchopneumonia, spleen necrosis, airsacculitis cervicalis, and valvular endocarditis. A significantly higher fraction of the birds in both the WT-IV (10/11) and WT-IT (10/11) groups were associated with at least one of these lesions as compared with the SCV-IV (4/11) group (P=0.012). The birds of the WT-IV group had liver and spleen necroses, oophoritis associated with chronic adhesive and fibrino-purulent peritoneum, and fibrino-purulent airsacculitis cervicalis. The liver and spleen necroses were mostly associated with organo-megaly. In the liver, coagulation necrosis appeared in different forms: irregular, pinpoint, confluent or focal. Proliferation of the white pulp was observed in some spleens. In the WT-IT group, the birds had lesions in the liver, ovary, peritoneum, air sacs and lungs. The latter was characterized by unilateral and focal fibrino-purulent bronchopneumonia associated with acute haemorrhagic lesions in the ventral part of the left lung. In the SCV-IV group, only the dead or somnolent birds had lesions in the liver, spleen, ovary and peritoneum, and in the heart appearing as valvular verrucous endocarditis in the mitral valves. In six out of 11 birds of the SCV-IT group bronchopneumonia and airsacculitis cervicalis were the predominating lesions. Bronchopneumonia was more common in the SCV-IT group compared with the SCV-IV group (P=0.012), whereas oophoritis associated with peritonitis in the WT-IT group was more frequent than in the SCV-IT group (P=0.003).
Of minor lesions, nephropathy, ovarian regression, sero-gelatinous exudates in the pericardial sac, vascular collapse with sero-haemorrhagic fluid in the abdomen, focal-fibrinous peri-hepatitis, and splenomegaly without necrosis were observed sporadically in all the groups. Neither minor nor major gross lesions were found in the birds of the SCV-IV group at day 1 or 3 p.i.
Microscopic lesions
The microscopic lesions observed in the livers were categorized into four major types: type A included micro-multifocal degeneration consisting of small yet widespread areas of hepatocytes that had acquired ghost-cell or necrotic appearance (a); type B consisted of foci of necrotic hepatocytes and/or separated hepatocytes infiltrated by heterophils (lesion type Ba) and occasionally with other inflammatory cells (mononuclear cells and/or lymphoid cells; lesions type Bb) (b); type C consisted of foci of amorphous eosinophilic material replacing hepatocytes and infiltration of a few heterophils (lesion type Ca) and/or with other inflammatory cells (mononuclear cells and/or lymphoid cells; lesion type Cb) (c); and type D consisted of foci of central necrosis with infiltration of heterophils, peripheral macrophages and giant cells (d). The acute inflammatory lesions were characterized by the infiltration of heterophils only, whereas the chronic lesions also contained mononuclear or lymphoid cells. Thus, the different lesions represented different stages of inflammatory responses, with types B and C either acute or chronic, type D a chronic form, and type A considered as degeneration with a potential of becoming inflammatory. A chronic-active type of process, characterized by presence of the all kind of inflammatory cells, was also observed in some lesions.
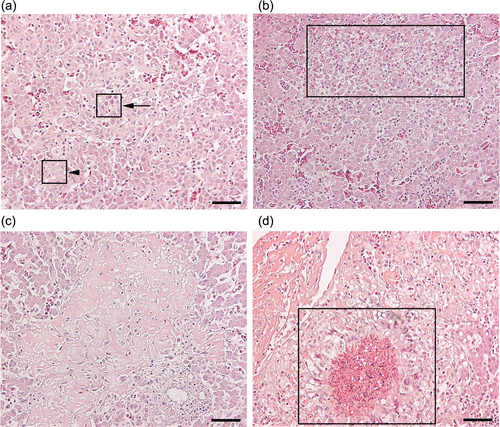
Figure 2. Microscopic lesions observed in livers of the chickens infected by the WT and SCV strains of S. zooepidemicus. 2a: Lesion type A, micro-multifocal degeneration- very tiny areas of hepatocytes with a ghost-cell or necrotic appearance, (i.e. disintegrated eosinophilic hepatocytes with fading nuclear staining or disappearance of nuclei in some cells) (arrow-head), as opposed to normal appearing hepatocytes (arrow). 2b: Lesion type B, necrotic and/or separation of hepatocytes with infiltration of heterophils, mononuclear and lymphoid cells (box). 2c: Lesion type C, amorphous eosinophilic material replacing hepatocytes with infiltration of mononuclear cells, lymphoid cells and few heterophils. 2d. Lesion type D, pyo-granulomas consist of central necrosis with infiltration of heterophils, peripheral macrophages and giant cells (box). Haematoxylin and eosin stain. Scale bar: 50 micrometers.
The number of chickens with microscopic liver lesions was higher in the SCV-IV group (9/11) compared with the SCV-IT (2/11) group (P=0.008). In the SCV-IV group, 4/11, 3/11, 2/11 and 2/11 chickens had type A, type Ca, type Cb and type D liver lesions, respectively. Both type Ba and type Bb lesions were dominating in the WT-IT group. Type C lesions were frequently found in the dead chickens of the SCV-IV (3/4) and WT-IV (1/2) groups, whereas the somnolent chickens that were euthanized had type D lesions. Type A lesions were only present at days 9 to 14 p.i. Congo Red and phosphotungstic acid–haematoxylin staining was negative in the liver section stained, suggesting absence of amyloid and fibrin.
Bacteriological re-isolation rates and the clinico-pathological findings
Most of the infected chickens were found culture-positive; however, the bacteriological re-isolation rates varied over the trial period (). All WT-IV-infected chickens remained culture-positive during the entire experimental period, but two birds became blood culture-negative at day 14 p.i. Organ samples tested from all the chickens in the WT-IT group were culture-positive. In the same group, tracheal swabs of 4/7 chickens were culture-positive, while one of them was in full lay and had a normal body condition and absence of any gross or microscopic lesions in any organs at day 14 p.i. One bird at day 14 p.i. was tested culture-positive from several organs, without showing any clinical signs or pathological lesions. In the SCV-IV group, most of the chickens (9/11) were blood culture-positive; the two chickens sacrificed at day 1 p.i. were culture-negative, and the remaining birds became culture-negative at day 14 p.i. However, only 7/11 chickens in group SCV-IT were culture-positive in bacteriology during the entire period—five chickens in the tracheal swabs at day 14 p.i., and three chickens in the organ swabs. In the same group, one bird at day 14 p.i. had a culture-positive tracheal swab and microscopic lesions in the liver, but did not show any gross lesions in any organs. All of the blood samples tested at day 0 were culture-negative.
Discussion
In the present study we reproduced the clinical findings caused by the WT and SCV strains of S. zooepidemicus as described by Bisgaard et al. (Citation2012) from a natural outbreak in organic layers in Denmark. In addition to this, we showed that the WT and SCV strains had different disease-causing potentials in adult layer chickens. Clinical signs and pathology attributable to each strain varied according to the routes of infection. Surprisingly, the SCV strain caused a higher mortality (36%) in the intravenously infected chickens than the WT strain, although the appearance of abnormalities including clinical signs, gross and histological lesions in different organs, as well as the bacteriological re-isolation rates were proportionately more severe in the WT-infected chickens. The clinical signs, gross and microscopic pathology, and bacteriology indicated that the WT strain promoted an initial onset whereas the SCV caused a late onset of infection, which is in accordance with the observed acute and chronic phases during the natural outbreak (Bisgaard et al., Citation2012).
In addition to the experimental reproduction of the clinical findings, the gross lesions recorded in the liver tissues of chickens infected with both WT and SCV strains (initial-onset and late-onset infection, respectively) in the present study mimicked the findings at corresponding times during the spontaneous course of the infection. Similarly, other lesions observed in the late phase following WT and SCV infections, respectively, correlated very well between the experimental and spontaneous infection. For example, airsacculitis cervicalis and oophoritis/peritonitis were mainly detected during the late phase and associated with both strains, which closely resemble the reported lesions observed in the chronic phase in the natural outbreak. Endocarditis was only found in the experimentally infected birds receiving the SCV strain during the later phases, again corresponding to observations reported by Bisgaard et al. (Citation2012). Although the lesions in the spleen were mainly observed at late phases with both strains, these appeared in the acute phase of the WT strain in the natural infection. Somnolent birds were seen in the SCV-injected groups at late phases, and this was mainly observed during the chronic phase in the outbreak. The per-acute death of chickens without preceding clinical signs was restricted to the WT strain during the very initial phase (day 1 p.i.) in the experimental study, corresponding to the observations in the acute phase during the outbreak (Bisgaard et al., Citation2012). Thus, generally, there was a very good correlation between the clinic–pathological finding in the experimentally infected chickens and the chickens infected spontaneously. Consequently, the present study also demonstrated the pathogenic potential of both WT and SCV. The infected organs and the associated gross lesions recorded in the present study as an outcome of S. zooepidemicus infection in the chickens were also in general agreeing with the previous reports (Edwards & Hull, Citation1937; Buxton, Citation1952; Sato et al., Citation1960; Peckham, Citation1966).
To understand the pathogenesis of the WT and SCV strains better, the experimental infected birds were inoculated via different routes. From them a proportionately higher number of chickens showed severe clinical signs during the entire period following inoculation with the WT strain by the i.v. route. Also, manifestation of macroscopic and microscopic lesions appeared earlier following inoculation with the WT strain by either the i.v. or i.t. route (from day 1 p.i. onwards). All tested samples were also found culture-positive in the WT inoculated groups at day 1 p.i., indicating the rapid development of septicaemia following the WT strain infection. The findings clearly indicate that the WT strain induces septicaemia and lesions in organs beyond its route of introduction, which could explain why the highest number of birds demonstrating gross lesions was observed in the WT inoculated groups. However, the locations of gross lesions caused by the SCV strain were seemingly route-specific. Valvular endocarditis was only seen in the SCV-IV group, whereas bronchopneumonia was only seen in the SCV-IT and WT-IT groups—both examples of route-specific lesions in this study. Although bronchopneumonia was common in the SCV-IT group, other clinico-pathological findings were insignificantly stated in the chickens of that group, confirming the i.t. route as the apparently less pathogenic route for the SCV infection. Possibly for the same reason, the number of chickens displaying oophoritis/peritonitis in the WT-infected groups was higher compared with the SCV-infected ones. Thus, the present findings indicate that both the i.v. and i.t. routes are part of the natural pathogenesis for the WT infection, whereas the SCV generally seems to disseminate internally in the birds by the i.v. route.
Death of birds was recorded when the bacteria were injected via the i.v. route. As possible important causes of death for the chickens, liver necrosis and valvular endocarditis were noted in the SCV-IV group and liver necrosis in the WT-IV group. The chronic type of microscopic lesions was dominant in the liver sections of the dead birds. The high mortality in chickens, caused by an SCV strain relative to the WT, has not been reported before, but has been recorded for a different organism, Enterococcus faecalis (Petersen et al., Citation2008). The intracellular adaptability of SCVs, the mechanism of which was not known from this study, and macrophageal functional insufficiency to remove them from the cellular microenvironment might result in a higher mortality by the strain (Sendi & Proctor, Citation2009). An SCV strain may lodge in the mitral valves by a chemotactic affinity, causing valvular verrucous endocarditis. The findings therefore underline that SCV also may play a role as a cause of death in chickens, and mortality can even exceed the rates induced by WT through the i.v. route of infection.
Because more birds had gross liver lesions as opposed to the frequency of spleen lesions, and also because the liver often is selected for histopathology irrespective of the disease under consideration, the liver was only chosen for histopathology to, for example, evaluate whether generalization had occurred. The amorphous eosinophilic material found in the liver sections (type C lesion) could be a plasma protein derivative (Riddell, Citation1996). Type A histological lesions were only seen at days 9 to 14 p.i. This is in contrast to the obvious acute nature of the lesions, and the lesion type is thus interpreted as being not directly linked to the infection, but occurring secondary to a manifestation of changed liver-cell function. In agreement with a late-onset infection to SCV, but also late onset related to the i.t. inoculation route, acute histological lesions were primarily found in these chickens. Some birds of the SCV-IV group at day 14 p.i. had microscopic lesions in the liver associated with the culture-negative samples along with the absence of clinical signs and macroscopic lesions; again inferring the dormancy of the SCV in intra-cellular milieu and entailing the recurrence of infection. Possibly for the same reason, a proportionately higher number of chickens in the SCV-IV group were found with microscopic lesions in their livers, and the hepatic tissues were also associated with all categories of lesions defined in the present study.
Overall, the experimental study proved a different pathogenic potential of WT and SCV strains in the chickens infected by both i.v. and i.t. routes of inoculation, where the i.v. route was found more prone to cause the disease especially by the SCV. Based on the previous findings (Bisgaard et al., Citation2012), it was also evident that SCV led to a more chronic phase of infection mainly due to the development of valvular endocarditis, whereas WT-infected birds experienced a more acute phase of infection.
In conclusion, the recently isolated SCV strain of S. zooepidemicus (Bisgaard et al., Citation2012) clearly has pathogenic potential in adult layer chickens, comparable with the WT strain. When inoculated intravenously, the SCV strain caused a mortality of about 36%, and might induce a recurring infection, unlike WT. The present study implies the importance of investigation on SCVs of S. zooepidemicus infecting chicken at the cellular level to explore specific mechanisms of pathogenesis. It is also necessary to investigate the source of transmission, the natural route of infection—for example, oral and footpad—and cross-transmission of WT and SCV, and to compare the pathogenicity of SCVs of different bacterial pathogens before a more complete picture of this type of infection is evident.
Acknowledgements
The authors would like to thank the Danish Poultry Council and University of Copenhagen for funding. Laboratory technicians Pia Mortensen, Lisbet Kiørboe, and Betina Andersen, Department of Veterinary Disease Biology, Faculty of Health and Medical Sciences, University of Copenhagen are thanked for excellent technical assistance. Thanks to professor Nils Toft, Department of Large Animal Sciences, Faculty of Health and Medical Sciences, University of Copenhagen for advice on statistical matters.
References
- Allegrucci , M. & Sauer , K. 2007 . Characterization of colony morphology variants isolated from Streptococcus pneumoniae biofilms . The Journal of Bacteriology , 189 , 2030 – 2038 . doi: 10.1128/JB.01369-06
- Bancroft , J.D. & Gamble , M. 2007 . A Textbook on Theory and Practice of Histological Techniques (.) , 6th edn . New York : Churchill Livingston .
- Bisgaard , M. , Bojesen , A.M. , Petersen , M.R. & Christensen , H. 2012 . A major outbreak of Streptococcus equi subsp. zooepidemicus infections in free range chickens is linked to horses . Avian Diseases , 56 , 561 – 566 . doi: 10.1637/10123-030712-Reg.1
- Buxton , J.C. 1952 . Disease in poultry associated with Streptococcus zooepidemicus . The Veterinary Record , 64 , 221 – 223 .
- Edwards , P.R. & Hull , F.E. 1937 . Hemolytic streptococci in chronic peritonitis and salpingitis of hens . Journal of the American Veterinary Medical Association , 44 , 656 – 660 .
- Eiff , C.V. , Peters , G. & Becker , K. 2006 . The small colony variant (SCV) concept—the role of staphylococcal SCVs in persistent infections , Injury , 37 , S26 – S33 .
- Genest , P. & Nadeau , J.D. 1944 . Observation, chez la poule, d'une epizootie due a Streptococcus zooepidemicus . Canadian Journal of the Comparative Medicine and Veterinary Science , 8 , 342 – 349 .
- Kaase , M. , Anders , A. & Gatermann , S.G. 2004 . First description of small-colony variants of Enterococcus faecalis isolated from an endocarditis patient . International Journal of Medical Microbiology , 294 , 146 .
- Motulsky , H.J. 2010 . In Chapter 13: Multiple Comparison, Intuitive Biostatistics: A Nonmathematical Guide to Statistical Thinking , (2nd edn.) . New York , US : Oxford University Press .
- Peckham , M.C. 1966 . An outbreak of Streptococcosis (Apoplectiform septicemia) in white rock chickens . Avian Diseases , 10 , 413 – 421 . doi: 10.2307/1588248
- Petersen , A. , Chadfield , M.S. , Christensen , J.P. , Christensen , H. & Bisgaard , M. 2008 . Characterization of small-colony variants of Enterococcus feacalis isolated from chickens with amyloid arthropathy . Journal of Clinical Microbiology , 46 , 2686 – 2691 . doi: 10.1128/JCM.00343-08
- Proctor , R.A. , Eiff , C.V. , Kahl , B.C. , Becker , K. , McNamara , P. , Herrmann , M. & Peters , G. 2006 . Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections . Nature Reviews: Microbiology , 4 , 295 – 305 . doi: 10.1038/nrmicro1384
- Riddell , C 1996 . Liver . In H.J. Barnes , T.P. Brown , M.D. Ficken , M.A. Goodwin , F.J. Hoerr , R.J. Julian , D.A. Pass , C.R. Pope , C. Riddell , & D.E. Swayne . Avian Histopathology , 2nd edn (p. 147 ). The American Association of Avian Pathologists. Rose Printing , Tallahassee , Florida .
- Sato , G. , Miura , S. & Ushijima , J. 1960 . An outbreak of hemolytic-streptococcal infection among chickens of a flock. II. Characters of the isolated streptococci . Japanese Journal of Veterinary Research , 8 , 285 .
- Sendi , P. & Proctor , R.A. 2009 . Staphylococcus aureus as an intracellular pathogen: the role of small colony variants . Trends in Microbiology , 17 , 54 – 58 . doi: 10.1016/j.tim.2008.11.004
- Thayer , S.G. , Waltman , W.D. & Wages , D.P. 2008 . Streptococcus and Enterococcus . In : Y.M. Saif A.M. Fadly J.R. Glisson L.R. McDougald L.K. Nolan & D.E. Swayne (Eds.). Diseases of Poultry , 12th edn (pp. 900 – 908 ). Ames , IA : Blackwell .