Abstract
Riemerella anatipestifer is the causative agent of polyserositis and septicaemia in waterfowl. Twenty-one serotypes have been reported, and there is a strong variation in virulence between strains according to serotype or strain. However, little information is available to assess virulence, such as virulence-associated genes; thus, it is difficult to estimate the risk from field strains. Hence, we established a chicken embryo lethality assay (ELA) model to determine the virulence of R. anatipestifer strains. Three virulent strains (RA T1, RA T7, and V-1) and three avirulent strains (Av-1, Av-2, and Av-3), which were confirmed by duck challenge, were used to perform the ELA. Inoculating 102 to 104 colony-forming units into the allantoic cavity of 10-day-old embryos discriminated between virulent and avirulent strains based on mortality. Differences in invasion rates into embryonic tissues were found between the RA T1 and Av-1 strains. The maximum colony-forming units of the RA T1 strain were about 1000 times higher than those of the Av-1 strain in the tissue invasion rate for 4 days. We found that the virulent strains killed embryos at mortality rates ≥50% during the first 3 days after inoculation and that the avirulent strains had death rates of ≤20% over 5 days. These results obtained by repeated testing suggest that the ELA could be used as a first-line screening method to determine the virulence of R. anatipestifer strains.
Introduction
Riemerella anatipestifer infection is the most important infectious disease among waterfowl, particularly domestic ducks and geese, with a 5 to 75% mortality rate according to strain virulence. Twenty-one serotypes have been identified, and the serotypes vary in virulence (Pathanasophon et al., Citation1995). Several serotypes including 1, 2, and 5 are highly virulent and commonly found in the USA, Denmark, and South Asia such as China and Singapore (Sandhu, Citation1991; Sandhu & Leister, Citation1991; Andreasen & Sandhu, Citation1993). In addition, differences in strain virulence are also observed within a given serotype (Brogden, Citation1989). Although serotype 1 has a high mortality rate in most countries, there is an avirulent vaccine strain of this serotype in the USA (Sandhu, Citation1991). Additionally, R. anatipestifer serotype 4 is commonly distributed in duck groups with 70 to 100% mortality rates in China (Wang et al., Citation2002). However, Ryll et al. suggested that serotypes such as serotype 4 are not associated with disease and might possibly represent a part of the pharyngeal flora of healthy Pekin ducks (Ryll et al., Citation2001).
Chinese duck farms in 2012 showed high positive rates for R. anatipestifer, as 46% (26/56) of ducks had R. anatipestifer based on a clinical test and 11% (10/85) was observed in clinically healthy ducks in China (Wang et al., Citation2012). In 1996, 80% (39/49) of clinically healthy ducklings in Denmark had R. anatipestifer in their pharynx (Ryll et al., Citation2001). Virulent R. anatipestifer strains associated with high mortality are found in many countries, whereas potentially avirulent R. anatipestifer strains have also has been isolated from healthy ducks because of the co-existence of virulent and avirulent strains. Despite these variations and the necessity to assess virulence, there is no indicator or simplified method to determine virulence.
The chicken embryo lethality assay (ELA) is a relatively simple and inexpensive test to predict virulence from death of embryos. The ELA has been used to discriminate between virulent and avirulent avian Escherichia coli, Campylobacter jejuni, and Staphylococcus aureus isolates and is regarded as a sensitive and specific tool in diagnostic laboratories (Nolan et al., Citation1992; Stewart-Tull et al., Citation2009; Oh et al., Citation2012; Polakowska et al., Citation2012).
In this study, we established a chicken embryo model for evaluating the virulence of virulent and avirulent strains of R. anatipestifer and applied the assay to field strains from clinical cases of riemerellosis.
Materials and Methods
Bacterial strains and growth conditions
Eighteen field strains isolated from the liver or a pharyngeal swab from ducks at domestic farms during 2011 and 2012 in South Korea and two strains, RA T1 and RA T7, obtained from the Animal, Plant, and Fisheries Quarantine and Inspection Agency in South Korea were used in the ELA (). Six field strains (V-1, FI-10, FI-11, FI-12, FI-13 and FI-14) originated from clinical cases of riemerellosis at domestic farms in Korea. Ten strains (Av-1, Av-2, FI-1, FI-2, FI-3, FI-4, FI-5, FI-6, FI-7 and FI-9) were isolated from the pharynx of healthy ducks, and two strains (Av-3 and FI-8) were obtained from cloacal swabs of clinically normal ducks. These R. anatipestifer strains were cultured on 5% sheep's blood agar (Hanil Komed, Seung-nam, South Korea) in a 5% CO2 incubator at 37°C for 48 h. Single colonies were subcultured in tryptic soy broth in a 37°C shaking incubator, and the culture broth was used for assay. R. anatipestifer strains were confirmed via the API-20NE test kit (bioMerieux, Marcy l'Etoile, France) and 16S rRNA polymerase chain reaction (PCR) according to a protocol described previously (Qu et al., Citation2006).
Table 1. Strains of R. anatipestifer used for the ELA in this study.
Duck inoculation
Six R. anatipestifer strains were used to challenge ducks to confirm their pathogenicity. Five 3-week-old Pekin ducks (Anas platyrhynchos) of each group were used to evaluate the pathogenicity of the R. anatipestifer strains. Each of the six R. anatipestifer strains was injected intramuscularly into ducks with a dose of 1×109 colony-forming units (CFU). The ducks were monitored daily for 7 days after challenge, and virulence was assessed by clinical signs and mortality. Bacterial cultures were prepared from the liver and brain of dead or live ducklings to isolate R. anatipestifer. Dead birds were necropsied immediately, and live ducks were sacrificed at day 7 post inoculation (p.i.) for bacterial isolation (). All experimental and animal management procedures were undertaken in accordance with the requirements of the Animal Care and Ethics Committee of Chonbuk National University. The animal facility at Chonbuk National University is fully accredited by the National Association of Laboratory Animal Care (approval number: CBU 2012-0050).
Table 2. Experimental infection of 3-week-old ducks with virulent and avirulent strains of R. anatipestifer.
Chicken embryo lethality assay
Specific pathogen free chicken eggs (Hy-Vac Laboratory, Redfield, Iowa, USA) were inoculated via the allantoic cavity (AC) or on the chorioallantoic membrane (CAM) based on a previous procedure (Powell & Finkelstein, Citation1966). Groups of 10 eggs containing 10-day-old embryos were inoculated with a dose of 101 to 109 CFU of virulent (RA T1) or avirulent (Av-1) strains or with phosphate-buffered saline (PBS) (pH 7.2) alone via the AC to determine the appropriate inoculation titre for the ELA.
Ten 10-day-old embryos per group were inoculated in the AC or on the CAM with cells with a dose of 102 or 104 CFU of RA T1 or Av-1 strains to compare the AC and CAM inoculations.
Groups of 10 seven-day-old, 10-day-old, 13-day-old, and 15-day-old embryos were also inoculated via the AC with a dose of 102 or 103 CFU of the RA T1 or Av-1 strains to determine the effect of age. The ELA was conducted with six strains confirmed for virulence by duck lethality in 10 embryos, each 10 days old, of each strain with a dose of 102 CFU via the AC to assess the relationship between ELA and duck lethality.
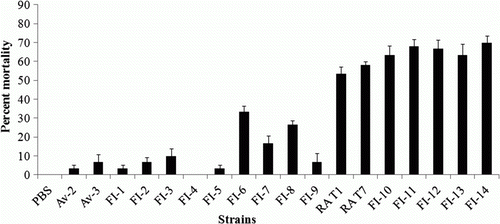
To test virulence of the field strains, 16 field strains from domestic duck farms (Av-2, Av-3, and FI-1 to F-14) and two reference strains (RA T1 and RA T7) were applied for ELA conducted in 10 embryos, each 10 days old, per each strain with a dose of 102 CFU via the AC.
The eggs for ELA were candled every 24 h after inoculation for 5 days to detect dead embryos. The embryo mortality rate was calculated as the mean percentage of embryonic deaths. The ELAs for determining titration, the inoculation route, and the effect of age were repeated five times. The experiment to test the virulence of the field strains was repeated three times.
In ovo growth curve
Mean viable counts obtained from five embryos inoculated with virulent (RA T1) or with avirulent (Av-1) strain were recorded. A group inoculated with PBS was also assessed. Forty-five eggs inoculated with 102 CFU per strain via the AC were sacrificed at 0, 6, 12, 18, 24, and 36 h and daily thereafter for 4 days. AC fluid of five eggs was collected aseptically. Bacterial titres were determined by plating 0.1 ml of undiluted and diluted samples of AC fluid onto blood agar. Viable counts were also determined by plating dilutions of homogenized heart and liver from the embryos onto blood agar to confirm the bacterial invasion rate into the embryos.
Statistical analyses
All statistical analyses were performed using SPSS version 12.0 (SPSS Inc., Chicago, IL, USA). Fisher's exact test was used. P<0.05 was considered statistically significant.
Results
Duck lethality assay
shows the ducks inoculated with the six R. anatipestifer strains divided into two groups. The virulent strains (RA T1, RA T7, and V-1) showed 80 to 100% mortality over 7 days (n = 5). No clinical signs and no mortality were observed for the three avirulent strains (Av-1, Av-2 and Av-3), except for Av-3. Three ducks in the Av-3 group showed mild clinical signs such as lameness or depression; however, they recovered 4 days after the challenge. Three virulent strains were recovered from both the liver and brain of 14 dead ducks by day 7 p.i. and one live duck sacrificed at day 7 p.i. However, the three avirulent strains were recovered only from the livers of the 15 ducks examined.
Virulence titration in chicken embryos with representative strains
PBS or 101 to 109 CFU diluted bacteria of the RA T1 and Av-1 strains were inoculated into 10-day-old chicken embryos to confirm dose dependence and to determine the appropriate titre to discriminate the virulent and avirulent strains (). No significant differences were observed between the virulent and avirulent strains, demonstrating >60% fatality with the high dose (108 and 109 CFU). The most evident difference in embryo mortality between the virulent and avirulent strains was confirmed for embryos inoculated with 102 CFU. The greatest difference in embryo mortality between the virulent and avirulent strains was confirmed in the inoculum containing 102 CFU (P = 0.003). The median lethal dose for each representative strain could not be determined as there was no clear relationship between dose and mortality rate. Embryos inoculated with a virulent strain died within the first 3 days. None of the embryos inoculated with PBS died during the 5 days.
Table 3. Embryo lethality assay of two representative R. anatipestifer strains for each titration following inoculation into the allantoic cavity.
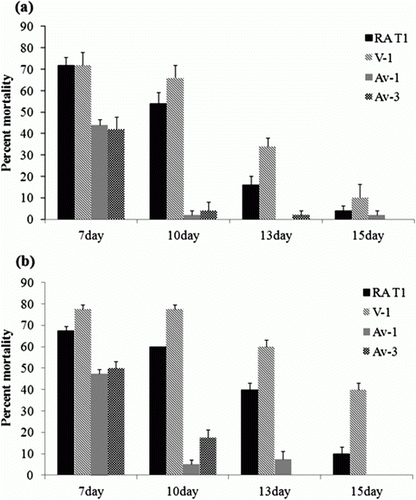
Effect of embryo age at inoculation on virulence
The age of the chicken embryos significantly influenced susceptibility to lethal infection with the four representative strains (). Seven-day-old embryos were more susceptible and showed the highest mortality to the virulent and avirulent strains among the 7-day-old, 10-day-old, 13-day-old, and 15-day-old embryos. In contrast, 13-day-old and 15-day-old embryos were less susceptible, exhibiting 20% mortality to RA T1 despite its virulence. Differences between the virulence of the virulent and avirulent strains were most evident in 10-day-old chicken embryos in the 102 and 103 CFU inoculated embryos (P = 0.003 and P = 0.002, respectively).
Figure 1. Effect of chicken embryo age on susceptibility to virulent and avirulent strains of R. anatipestifer following inoculation into the AC (n = 10). The mean mortality rate of embryos represents results from five assays. Error bars represent the standard error. 1a: a dose of 102 CFU was inoculated in 7-day-old, 10-day-old, 13-day-old, and 15-day-old chicken embryos. 1b: a dose of 103 CFU was inoculated in 7-day-old, 10-day-old, 13-day-old, and 15-day-old chicken embryos.
Effect of inoculation route on virulence
Ten-day-old chicken embryos were inoculated with 102 and 104 CFU in the AC or on the CAM to confirm the influence of various routes on virulence. A comparison of ELA results by both routes is shown in . Embryo death rates due to CAM inoculation of the two strains were higher than those inoculated through the AC. Both the RA T1 and Av-1 strains showed ≥50% mortality for the CAM route, whereas RA T1 showed ≥50% embryo mortality and Av-1 showed no mortality via the AC route. Differences between the virulence of the virulent and avirulent strains were clearer for the AC inoculated embryos than those for the CAM route with a dose of 102 CFU (P = 0.011).
Table 4. Effect of inoculation route on virulence of R. anatipestifer for 10-day-old chicken embryos.
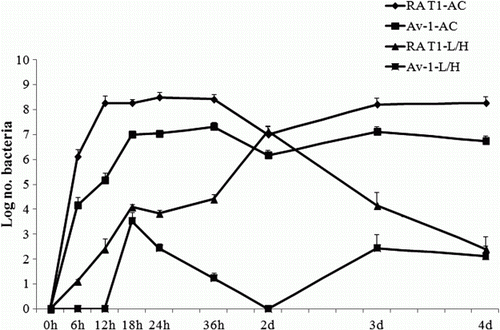
Relationships between growth in ovo and virulence
In ovo growth curves were determined in both the AC fluid and the embryonic tissues of specific pathogen free eggs to investigate the correlation between replication or invasion capability of R. anatipestifer and strain virulence (). Comparison of the growth rate of bacteria in allantoic fluid, indicated that RA T1 reached 108 to 109 CFU/ml as a maximum titre at 12 h p.i. and maintained 107 to 109 CFU/ml up to day 4 p.i. However, Av-1, an avirulent strain, reached 107 to 108 CFU/ml at 18 h p.i. and maintained 107 to 108 CFU/ml up to day 4 p.i. The maximum titre of the RA T1 strain was up to 10 times higher than that of the Av-1 strain. The invasion rate of bacteria into the liver and heart were different between the RA T1 and Av-1 strains. The highest titre (107 CFU/ml) of the RA T1 strain was confirmed at day 2 p.i. in liver and heart tissues and then decreased to day 4 p.i., whereas the highest titre (104 CFU/ml) of the Av-1 strain was identified at 18 h p.i.
Figure 2. Growth curve of R. anatipestifer RA T1 and Av-1 strains after inoculation with 102 CFU into the AC of 10-day-old chicken embryos (n = 5). The mean CFU present in AC fluid and embryonic liver and heart (L/H) was followed over time. Each point represents the mean±standard deviation of five embryos per group. None of the strains were recovered from control eggs inoculated with PBS alone.
Relationships between duck lethality and ELA
The virulent strains, V-1, RA T1, and RA T7 with duck mortality rates ≥80%, had embryo death rates of 50 to 80% in three repeated ELA tests (). The avirulent strains, Av-1, Av-2, and Av-3 with mild clinical signs and no duck deaths, revealed embryo death rates ≤20%. A classification based on the bird experiments and ELA results of these strains was developed. The virulent strains lead to embryo deaths rates of ≥50% during the first 3 days after inoculation, whereas the avirulent strains lead to embryo death rates of ≤20%, and the deaths were spread out over 5 days.
Table 5. Comparison of the duck lethality assay and the ELA in 10-day-old chicken embryos inoculated into the AC with R. anatipestifer isolates.
Virulence assessment of field strains by ELA
Two reference strains and 16 field strains isolated from diagnostic laboratories including four R. anatipestifer strains challenged in ducks were inoculated into AC to confirm virulence of the field strains (). The ELA results of 16 field strains showed that five strains (31.3%) were virulent, two were intermediate (12.5%), and nine were avirulent (56.2%).
Discussion
Although various genes such as OmpA, VapD, and CAMP cohemolysin have been associated with virulence, no method is available to determine virulence except for the duck lethality assay (Chang et al., Citation1998; Crasta et al., Citation2002; Hu et al., Citation2011). The duck lethality assay is time consuming, labour intensive, and a non-ethical method, therefore evaluation of R. anatipestifer virulence is required as a first step for the development of practical and laboratory-based alternatives. In this study, we established an ELA that can be applied as a diagnostic tool for R. anatipestifer.
The best discrimination between virulence and avirulence of the R. anatipestifer strains was achieved in 10-day-old embryonated chicken eggs (). It is important to determine the age of inoculation for the ELA because strain pathogenicity appears to differ based on embryonic development. One of the avirulent strains killed a number of ≤7-day-old embryos in the present study, although the virulence was low in ducks (). In addition, embryos ≥13 days old do not appear appropriate to evaluate strain pathogenicity, because resistance to virulent strains seemed to develop as the embryos reached full development. These findings were similar to the results of McCabe, who demonstrated that virulence differences in pathogenic and non-pathogenic staphylococci are most evident in embryos 10 days of age or older (McCabe, Citation1964). We found differences in the resistance of two strains in 10-day-old embryos. This result suggests that 10-day-old embryos are more resistant to avirulent strains than 7-day-old embryos but are more susceptible to virulent strains than 13-day-old and 15-day-old embryos.
The CAM route of inoculation did not distinguish between virulent and avirulent R. anatipestifer strains because the two strains were lethal regardless of virulence (≥50% mortality) (). Powell & Finkelstein (Citation1966) emphasized the inoculation route in studies of chicken embryo bacterial virulence. They suggested the ability of small inocula of E. coli strains to kill both 13-day-old and 16-day-old embryos when inoculated via the CAM despite resistance as development proceeded, indicating that other pathogenic mechanisms may be involved in embryos with a systemic infection. Similarly, in this study, mortality was 50% in embryos inoculated via the CAM even with the avirulent strain. Other pathogenic mechanisms of the CAM route, which are different from the AC route, are likely to cause death of embryos regardless of virulence. Thus, inoculation via the CAM is not appropriate for use in the ELA to estimate virulence of R. anatipestifer strains.
Differences in invasion rates between virulent and avirulent strains were observed in the chicken egg embryonic tissues (). This pattern of virulent strain invasion into embryonic tissues was similar to the 5-day mortality pattern of the virulent strain. For example, most embryonic deaths occurred within 2 to 3 days after inoculation when the amount of invasive bacteria was maximum in the liver and heart. This observation suggests that invasion of virulent R. anatipestifer strains into embryonic tissues is related to systemic infection of the embryo and death.
According to the criteria presented in this study, the 16 R. anatipestifer field strains were separated into three groups, suggesting that there are various R. anatipestifer strains with a variety of virulence characteristics (). FI-10, FI-11, FI-12, FI-13, and FI-14 are virulent strains isolated from commercial Pekin ducks that show clinical signs of lameness, anorexia, or ataxia on farms and pathological gross lesions such as airsacculitis, pericarditis or perihepatitis at the time of necropsy. Avirulent strains, Av-2, FI-1, FI-2, FI-3, FI-4, FI-5, FI-7, and FI-9 have been isolated from the pharynx of healthy ducks and are likely to exist as part of the normal flora (Ryll et al., Citation2001). Additionally, Av-3 has been isolated from cloacas of clinically normal ducks. However, the mean lethality of the intermediate strains FI-6 and FI-8 in embryos was 33.3% and 26.6% respectively, which was slightly higher than the standard for avirulent strains. These intermediate strains should be evaluated by the duck lethality assay to confirm their exact virulence.
These results verify that the ELA in this study could be applied to classify field strains by virulence. Although this method can be reproduced reliably, a mean value of at least three repeated experiments is required to obtain more accurate results.
The ELA is a meaningful and valuable method, as it does not require the use of ducklings and was designed based on the 3R concept (replace, reduce, and refine). This valid alternative will reduce the number of ducklings subjected to testing.
In conclusion, we developed a reproducible and reliable method to discriminate virulent and avirulent R. anatipestifer strains without animal testing. Our results suggest that the chicken ELA could be used as a first-line in vivo experimental approach to assess the virulence capacity of R. anatipestifer strains. This method will allow diagnostic laboratories to provide domestic farms with proper diagnoses, and could be used for virulence-associated research on R. anatipestifer.
Acknowledgements
This study was supported by the Animal, Plant, and Fisheries Quarantine Inspection Agency (Z-AD21-2011-12-02), and the Cooperative Research Program for Agriculture Science & Technology Development (PJ009314, PJ006336, PJ009278), Rural Development Administration, Republic of Korea.
Additional information
Notes on contributors
Se-Yeoun Cha
Hye-Suk Seo and Se-Yeoun Cha contributed equally to this studyReferences
- Andreasen , J.R. Jr & Sandhu , T. 1993 . Pasteurella anatipestifer-like bacteria associated with respiratory disease in pigeons . Avian Diseases , 37 , 908 – 911 . doi: 10.2307/1592053
- Brogden , K.A. 1989 . Pasteurella anatipestifer infection . In C. Adlam , & J.M. Rutter , Pasteurella and Pasteurellosis (pp. 115 – 129 ). London : Academic Press .
- Chang , C.F. , Hung , P.E. & Chang , Y.F. 1998 . Molecular characterization of a plasmid isolated from Riemerella anatipestifer . Avian Pathology , 27 , 339 – 345 . doi: 10.1080/03079459808419349
- Crasta , K.C. , Chua , K.L. , Subramaniam , S. , Frey , J. , Loh , H. & Tan , H.M. 2002 . Identification and characterization of CAMP cohemolysin as a potential virulence factor of Riemerella anatipestifer . Journal of Bacteriology , 184 , 1932 – 1939 . doi: 10.1128/JB.184.7.1932-1939.2002
- Hu , Q. , Han , X. , Zhou , X. , Ding , C. , Zhu , Y. & Yu , S. 2011 . OmpA is a virulence factor of Riemerella anatipestifer . Veterinary Microbiology , 150 , 278 – 283 . doi: 10.1016/j.vetmic.2011.01.022
- McCabe , W.R. 1964 . Studies of staphylococcal infections. I. Virulence of staphylococci and characteristics of infections in embryonated eggs . Journal of Clinical Investigation , 43 , 2146 – 2157 . doi: 10.1172/JCI105088
- Nolan , L.K. , Wooley , R.E. , Brown , J. , Spears , K.R. , Dickerson , H.W. & Dekich , M. 1992 . Comparison of a complement resistance test, a chicken embryo lethality test, and the chicken lethality test for determining virulence of avian Escherichia coli . Avian Diseases , 36 , 395 – 397 . doi: 10.2307/1591518
- Oh , J.Y. , Kang , M.S. , Yoon , H. , Choi , H.W. , An , B.K. , Shin , E.G. , Kim , Y.J. , Kim , M.J. , Kwon , J.H. & Kwon , Y.K. 2012 . The embryo lethality of Escherichia coli isolates and its relationship to the presence of virulence-associated genes . Poultry Science , 91 , 370 – 375 . doi: 10.3382/ps.2011-01807
- Pathanasophon , P. , Sawada , T. & Tanticharoenyos , T. 1995 . New serotypes of Riemerella anatipestifer isolated from ducks in Thailand . Avian Pathology , 24 , 195 – 199 . doi: 10.1080/03079459508419059
- Polakowska , K. , Lis , M.W. , Helbin , W.M. , Dubin , G. , Dubin , A. , Niedziolka , J.W. , Miedzobrodzki , J. & Wladyka , B. 2012 . The virulence of Staphylococcus aureus correlates with strain genotype in a chicken embryo model but not a nematode model . Microbes and Infection , 14 , 1352 – 1362 . doi: 10.1016/j.micinf.2012.09.006
- Powell , C.J. Jr & Finkelstein , R.A. 1966 . Virulence of Escherichia coli strains for chick embryos . Journal of Bacteriology , 91 , 1410 – 1417 .
- Qu , F. , Cai , C. , Zheng , X. & Zhang , D. 2006 . Rapid identification of Riemerella anatipestifer on the basis of specific PCR amplifying 16S rDNA . Acta microbiologica Sinica , 46 , 13 – 17 .
- Ryll , M. , Christensen , H. , Bisgaard , M. , Christensen , J.P. , Hinz , K.H. & Kohler , B. 2001 . Studies on the prevalence of Riemerella anatipestifer in the upper respiratory tract of clinically healthy ducklings and characterization of untypable strains . Journal of Veterinary Medicine, Series B , 48 , 537 – 546 . doi: 10.1046/j.1439-0450.2001.00471.x
- Sandhu , T.S. 1991 . Immunogenicity and safety of a live Pasteurella anatipestifer vaccine in White Pekin ducklings: laboratory and field trials . Avian Pathology , 20 , 423 – 432 . doi: 10.1080/03079459108418780
- Sandhu , T.S. & Leister , M.L. 1991 . Serotypes of ‘Pasteurella’ anatipestifer isolates from poultry in different countries . Avian Pathology , 20 , 233 – 239 . doi: 10.1080/03079459108418760
- Stewart-Tull , D.E. , Coote , J.G. , Thompson , D.H. , Candlish , D. , Wardlaw , A.C. & Candlish , A. 2009 . Virulence spectra of typed strains of Campylobacter jejuni from different sources: a blinded in vivo study . Journal of Medical Microbiology , 58 , 546 – 553 . doi: 10.1099/jmm.0.005611-0
- Wang , M.S. , Cheng , A.C. , Chen , X.Y. , Liu , Z.Y. , Fang , P.F. & Guo , Y.F. 2002 . Studies on the isolation and pathogenicity of Riemerella anatipestifer serotype 4 in China . Chinese Journal of Veterinary Medicine , 38 , 5 – 9 .
- Wang , X.P. , Zhu , D.K. , Wang , M.S. , Cheng , A.C. , Jia , R.Y. , Chen , S. , Chen , X.Y. & Tang , T. 2012 . Development and application of specific polymerase chain reaction assay targeting the gyrB gene for rapid detection of Riemerella anatipestifer . Poultry Science , 91 , 2450 – 2453 . doi: 10.3382/ps.2012-02375