Abstract
Outbreaks of infectious bursal disease in vaccinated chicken flocks are frequent in Nigeria. For the control of infectious bursal disease, live vaccines based on foreign infectious bursal disease virus (IBDV) strains are used. The present study investigated the phylogenetic relationship between field and vaccine IBDV strains from northwestern Nigeria. Thirty field IBDV strains and three commercial vaccines strains were characterized through sequencing the VP2 hypervariable region. In addition, the complete genome segment A coding region for two vaccines and two field strains was sequenced. The deduced amino acid sequences (position 212 to 331) of IBDV strains from Nigeria and other regions of the world were aligned and possible regional and virulence markers were identified associated with VP2 minor hydrophilic peaks. Reversion to virulence of a vaccine strain with a Q to L mutation at position 253 was observed. Phylogenetic analyses revealed a unique cluster of northwest Nigerian field IBDV strains alone or related to imported characterized classical and very virulent IBDV vaccines. The results suggest that when IBDV strains spread from their region of origin to a different region they mutate alongside indigenous field strains but may retain their identity on the VP2 region.
Introduction
Infectious bursal disease (IBD), discovered by Cosgrove (Citation1962) in the USA, is an acute, highly contagious viral disease of great economic importance affecting young chickens. The aetiologic agent is a bisegmented (segments A and B) double-stranded RNA Avibirnavirus. Classical serotype 1 infectious bursal disease virus (IBDV) strains have been of great concern to the poultry industry for a long time, while all known serotype 2 IBDV strains are avirulent for chickens (Ismail et al., Citation1988). In the late 1980s, classical serotype 1 IBDV evolved into variant forms, in the USA (Synder, Citation1990), or very virulent (vvIBDV) forms in Europe, Asia and other major parts of the world (van den Berg, Citation2000). However, all three major subtypes of IBDV are commonly found in a single country (Kim et al., Citation2010). Segment A of the IBDV genome encodes a polyprotein from its larger open reading frame, which is processed into two structural proteins (VP2, VP3) and a viral protease (VP4) (Hudson et al., Citation1986). The smaller partially overlapping segment A open reading frame encodes a non-structural protein (VP5) (Mundt et al., Citation1995). The IBDV segment B encodes VP1, which has RNA-dependent RNA polymerase activity (Spies et al., Citation1987). The antigenic site responsible for the induction of neutralizing antibodies against IBDV is located on VP2 structural protein (Azad et al., Citation1987). The genetic basis for the antigenicity and/or virulence of the VP2 protein of IBDV has been studied extensively (Schnitzler et al., Citation1993; Brandt et al., Citation2001; van den Berg et al., Citation2004; Letzel et al., Citation2007; Durairaj et al., Citation2011).
Phylogenetic analysis placed vvIBDV strains from many different countries and geographic regions in a single clade with some minor non-significant branching (Jackwood & Sommer-Wagner, Citation2007). A hypothesis suggested that the origin of vvIBDV was through possible genetic re-assortment of IBDV segment A from an unidentified reservoir (Lasher & Shane, Citation1994). Hon et al. (Citation2006) alternatively argued that the phylogeny of segment B suggested a possible re-assortment event estimated to have taken place around the mid-1980s and initiated vvIBDV expansion in the late 1980s. Owoade et al. (Citation2004) proposed that vvIBDV may have a West African origin, however, as suggested by the high genetic variability observed.
The economic impact of clinical and sub-clinical IBD warrants the search and use of efficient vaccines and vaccination strategies to match changes in the field situation for effective control of IBDV (Whitfill et al., Citation1995; Pitcovski et al., Citation2003; Yong-Chang et al., Citation2005; Lazarus et al., Citation2008; Le Gros et al., Citation2009). In Nigeria, most vaccines used for the control of IBDV as well as the virus seed for local vaccine production are imported (Okeke & Tanimu, Citation1982), leading to frequent vaccination failures in the field (Abdu et al., Citation2001) due to antigenic differences between local strains and vaccine strains (Owoade et al., Citation2004; Adamu et al., Citation2011a). In a preliminary study on a killed vaccine developed from a Nigerian vvIBDV, vaccinated commercial chickens were protected against challenge with other field vvIBDV strains (Adamu et al., Citation2011b). The aim of this study was to characterize field and vaccine IBDV strains from northwestern Nigeria including the vaccine candidate under trial and to compare them with published IBDV sequences to furnish a strong basis for using local IBDV strains as vaccine candidates.
Materials and Methods
Bursa samples and vaccines
Fresh bursae were collected during 23 reported outbreaks of IBD in Kano (n =3), Kaduna (n=8), Katsina (n=11) and Zamfara (n=1) States in northwest Nigeria between May 2010 and June 2011 to produce a 50% w/v bursa suspension in phosphate-buffered saline (pH 7.4). Fifty-four homogenates of bursae from cases of IBD diagnosed at the Veterinary Teaching Hospital Ahmadu Bello University, Zaria from 1985 to 2011 were also included. Three commercial live IBDV vaccines—ABIC (ABIC Biological Laboratories Ltd, Netanya, Israel), Georgia (INDOVAX Private Ltd, Hisar, India) and Vom (National Veterinary Research Institute, Vom, Nigeria)–were purchased in Nigeria and reconstituted into 200 doses/ml phosphate-buffered saline (pH 7.4).
RNA extraction, reverse transcription-polymerase chain reaction and nested polymerase chain reaction
Viral RNA was extracted from the samples using the QIAamp Viral RNA Mini kit (Qiagen, Venlo, the Netherlands). Reverse transcription was performed using Superscript III (Life Technologies, Merelbeke, Belgium). IBDV was detected by a semi-nested polymerase chain reaction (PCR) targeting the VP2 hypervariable region (HVR). PCR fragments were also sequenced using PCR primers as sequencing primers (). Different primer pair combinations were used to amplify the complete segment A by amplifying overlapping PCR fragments (). The cDNA was amplified in a 25 µl reaction mixture containing 2.5 µl buffer (10×), 25 mM MgCl2, 5 mM dNTP, 25 µM IBDV forward primer, 25 µM IBDV reverse primer, 0.2 or 0.4 u (semi-nested) Platinum® Taq (Life Technologies) and the equivalent of 0.5 µl template (cDNA or first-round product). The PCR protocol was as follows. Initial denaturation at 95°C for 3 min followed by 40 amplification cycles at 95°C for 30 sec, 58°C (F4-R1 PCR) or 53°C (others) for 30 sec and 72°C for 1 or 2 min with final extension at 72°C for 7 min.
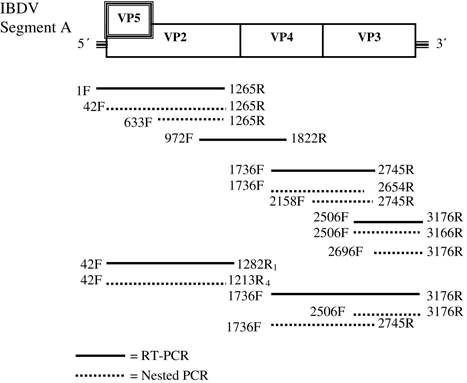
Table 1. Primers used to amplify and sequence IBDV VP2 and genome segment A.
Nucleotide sequencing and phylogenetic analysis
PCR products were purified with the JetQuick DNA purification kit (Genomed, Loehne, Germany) following the manufacturer's instructions. When multiple bands were observed from a PCR product, they were separated on a 1% agarose gel. The band of interest was excised and the DNA purified with QIAquick Gel Extraction Kit (Qiagen) following the gel extraction procedure. The purified products were sequenced using an ABI 3100 Capillary sequencer with BigDye terminator v3.1 cycle sequencing kit (Applied Biosystems Foster City, California, USA). Nucleotide sequences were aligned with ClustalW (Thompson et al., Citation1994) and analysed using the BioEdit 5.0 package (Hall, Citation1999). Phylogenetic analyses were performed by the Kimura two-parameter model neighbour-joining method with 1000 bootstrap replicates with the software MEGA version 5 (Tamura et al., Citation2011). Amino acid positions revealing possible regional identity for IBDV strains were categorized as indigenous to the region where they were predominantly isolated or foreign when they were isolated in other regions. The likelihood of countries having significant differences between the total number of IBDV strains that are indigenous or foreign was analysed with the Fisher's exact test. Based on the global distribution pattern of the proposed IBDV regional markers, countries with distinct indigenous markers were selected for comparison with the Nigerian indigenous and foreign markers. The test was repeated five times with different combinations of countries cross-tabulated in 5×2 contingency tables using SPSS 16.0 software (SPSS Inc. Chicago, Illinois, USA). The sequences used for comparison and the phylogenetic analysis were obtained from GenBank, and when presented the accession number is written alongside each sequence.
GenBank accession numbers
The GenBank accession numbers JX424047 to JX424079 are for the IBDV sequences obtained from the present study in the following order: IBDV1, IBDV2, IBDV4, IBDV8, IBDV10, IBDV11, IBDV12, IBDV13, IBDV17, IBDV22, IBDV25, IBDV27, IBDV33, IBDV34, IBDV36, IBDV37, IBDV38, IBDV43, IBDV49, IBDV51, IBDV58, IBDV59, IBDV61, IBDV63, IBDV64, IBDV65, IBDV71, IBDV73, IBDV76, IBDV77, IBDV78, IBDV79 and IBDV80.
Results
Extraction and amplification of the IBDV gene from samples
Twenty-nine samples obtained between 2009 and 2011 and a sample obtained in 1998 when tested with PCR yielded reliable amplicons of IBDV VP2 HVR gene (). The amplification of gene fragments for sequencing complete IBDV segment A succeeded with two field IBDV strains (IBDV2 and IBDV80) and two vaccine strains (Georgia and ABIC). Gene fragments amplification for partial segment A sequencing was successful with IBDV10, IBDV34, IBDV49 and IBDV79.
Table 2. Origin of field and vaccine IBDV-positive samples as tested with PCR.
Sequence analysis
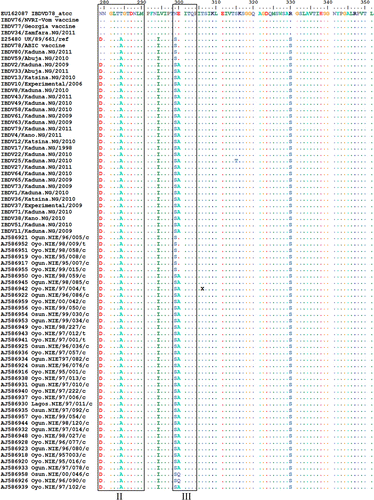
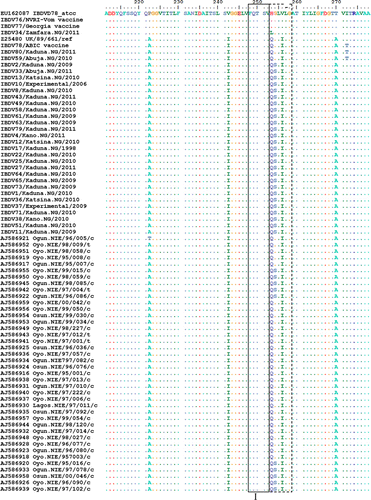
Amino acid sequences (211 to 351) of VP2 HVR from 30 field strains and three vaccine strains in this study were aligned with 40 isolates reported as being unique to southwestern Nigeria (Owoade et al., Citation2004), a classical IBDV strain D78 and a vvIBDV reference strain UK/89/661 (). Two field strains (IBDV59 and IBDV80) were similar to the ABIC vaccine strain and differed by two amino acid mutations (I272T and H279D) from other Nigerian strains. Field strain IBDV34 differed only by one amino acid mutation H253L from Vom and Georgia vaccine strains. Amino acid differences between field and vaccine-related strains were in the region adjacent to minor hydrophilic peak I and inside minor hydrophilic peaks II and III. Two mutations, G254S and E300A, were in all northwestern Nigerian strains sequenced except the vaccine-related strains and in some of the southwest Nigerian strains reported earlier (Owoade et al., Citation2004). Two southwestern Nigerian strains had E300Q and another had P222T. The H253L mutation in IBDV34 was due to a single non-synonymous A to T mutation at nucleotide position 889. An outbreak caused by IBDV34 occurred in a farm 2 weeks following vaccination with the Georgia vaccine. The outbreak lasted for 27 days and several bursae collected on the sixth day had very thin walls and atrophied with a mean weight of 0.16 g.
Comparison of amino acid sequences deduced from the sequenced nucleotides of Georgia and ABIC vaccines with six field strains in this study revealed differences at 50 (VP5=12; VP2=17; VP4=14 and VP3=7) positions in the coding region of Georgia vaccine strain segment A gene (). The Georgia and ABIC strains showed 94.8% and 97.3% nucleotide similarity to IBDV2 with 35 and 16 amino acid mutations, respectively. The amino acid sequences of ABIC and IBDV80 were 100% identical in the VP2 region, while those determined for IBDV34 were similar to Georgia except for H254L mutation. Apart from IBDV34, close relationship was apparent between all of the field strains. However, IBDV49 showed four unique mutations (I563M, L575V, R623G and N654K) in the VP4 region.
Table 3. Comparison of segment A amino acids for vaccine and field IBDV strains in northwestern Nigeria.
Alignment of amino acid sequences 212 to 331 in the VP2 HVR from available IBDV sequences published in GenBank with the Nigerian IBDV2 strain revealed different amino acid types (A, E, G, V, D, K and Q) at position 300. The global distribution of these IBDV isolates showed a distinct regional pattern of amino acid types at positions 299 and 300 (). Canadian, North American, Mexican and South American variant IBDV strains had predominantly the proposed NE regional marker, while European and Asian vvIBDV had the SE marker. Australian variant and vaccine IBDV strains also contained the proposed SE regional marker. The proposed SA regional marker was associated with vvIBDV strains from tropical regions in Africa, India and Caribbean Islands. Isolates of IBDV with proposed ND, NK and NG regional markers were predominant in South Africa, Venezuela and China but probably spread to Canada, Spain and the USA, respectively. There were significant differences (P<0.0001) between the total proposed indigenous and foreign regional markers for the five different combinations of countries cross-tabulated in 5×2 contingency tables and analysed with the Fisher's exact test in each case (). For example, 26, 156, 77, 41 and 291 were compared as indigenous while 2, 44, 12, 66 and 114 were compared as foreign IBDV strains for Australia, China, Nigeria, Spain and the USA, respectively (). The IBDV sequences were sorted according to amino acid type at position 300 and were examined visually. To enable the presentation of these amino acid differences, IBDV strains with similar sequences from the same geographical region or countries were not included (). Synonymous (data not shown) and non-synonymous mutations at amino acid positions 299 and 300 in the minor hydrophilic peak III (vertical arrows) in this comparison for the classical, variant and vvIBDV subtypes differ between some countries. Differences between IBDV subtypes were more apparent in the minor hydrophilic peaks I and II than all other amino acid changes in the VP2 region. Ten amino acids from 249 to 258 (double-headed arrow) could serve as major key for marking virulence on VP2 as presented by vvIBDV (QTSVQS[G/D]LILG), variant IBDV (KTSVQS[N]LVLG), classical vaccine IBDV (QTSVHGLVLG), variant vaccine IBDV (HTSVHGLALD) and Australian variant IBDV (QTSVQS[G]LV[A]LN[D]). Similarly, eight amino acids from 279 to 286 could serve as minor virulence markers for these IBDV subtypes, as represented by DNGLTAGT, NNGLTAGI, NNGLTTGT, DNGLTTGI and G[D]NGLTAGI[T], respectively.
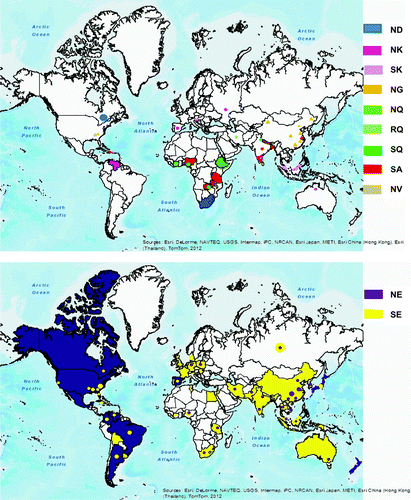
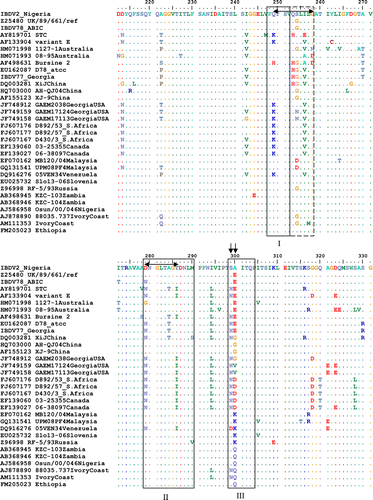
Table 4. Comparison of IBDV VP2 amino acid positions 299 and 300 as proposed regional markers for different countries.
Phylogenetic analysis
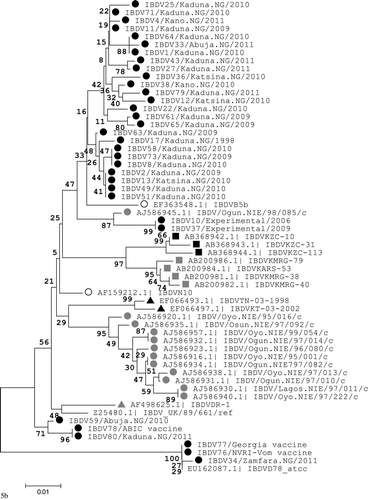
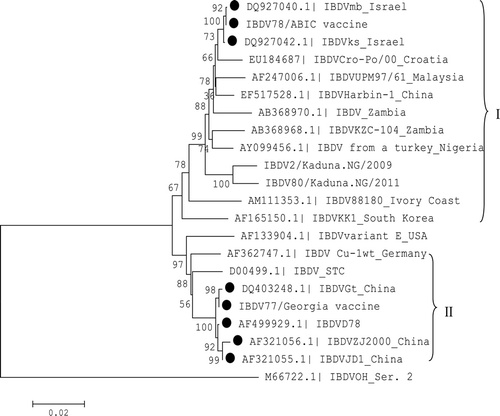
A phylogenetic tree was constructed based upon nucleotide positions 765 to 1127 of the IBDV VP2 region for sequences obtained in this study and other IBDV strains available in GenBank with alanine at amino acid sequence position 300 (A300), the proposed regional marker for IBDV strains in the tropical region. A classical IBDV strain (D78) and a vvIBDV reference strain (UK/89/661) were included. The IBDV strains analysed fell into two distinct genetic groups. The first group with 48% bootstrap value comprised vvIBDV strains with A300 from different countries in the tropics that formed a single clade with smaller branches (). The second group with 100% bootstrap value formed three distinct branches (). The largest of these branches contained vvIBDV with A300 that clustered (55% bootstrap value) with the UK/89/661 strain. The other two clusters contained vvIBDV ABIC vaccine strain (71% bootstrap value) and classical IBDV strain D78 (100% bootstrap value), respectively. In addition, field IBDV strains with A300 from Nigeria, Tanzania, Zambia, and India formed different sub-clusters with significant bootstrap values (≥85%). Field IBDV strains from northwestern Nigeria formed two different sub-clusters that each contained several identical isolates.
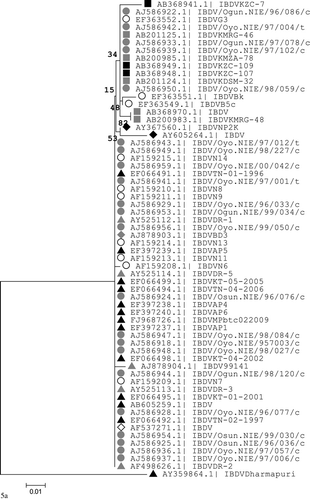
The phylogenetic tree based upon nucleotides of the entire coding region of segment A showed the ABIC strain to be closely related (100% bootstrap value) with both the attenuated virus (IBDVmb) and the vvIBDV field virus (IBDVks) from which it was derived. However, the mutant strain IBDV80 was more associated with another field strain IBDV2 in contrast to its closer association with the ABIC strain on the VP2 region phylogenetic tree. The Georgia strain was closely related (100% bootstrap value) to the classical IBDV strain D78 and other attenuated or virulent classical field strains ().
Discussion
The epidemiology of variant and vvIBDV in different regions of the world is important for understanding the trend in the evolution, spread and field status of IBDV for effective control of IBD in chickens. Earlier reports indicated Europe as the region of origin for vvIBDV, from where the strains spread rapidly to other regions (van den Berg, Citation2000). However, results of the present study suggest that widespread use of live vaccines from Europe or other sources might have contributed to the spread of IBDV strains from one region to another with the escape mutant vaccine strains adapting to the new region alongside indigenous field strains. In fact, Tong et al. (Citation1993) showed that IBD was more likely to occur in vaccinated chickens than in non-vaccinated chickens. There have been earlier reports of field isolates having sequences similar to IBDV vaccine strains (Ignjatovic & Sapats, Citation2002; Dolz et al., Citation2005; Jackwood et al., Citation2008; Hon et al., Citation2008). In the present study, the segment A region of the Georgia vaccine and other virulent classical IBDV strains isolated from the field and attenuated showed identical origin with classical IBDV strain D78, suggesting that the virulent field strains could be escaped mutants of vaccine strains.
There is a strong relationship between the degree of exposure to regional climate change and the species’ environmental niche (Thuiller et al., Citation2005). Recently, a study indicated a strong association between geography and IBDV viral diversity (Cortey et al., Citation2012). Non-synonymous mutations at amino acid position 254 and other synonymous mutations (data not shown) affected phylogenetic clustering of Nigerian and other IBDV strains in the tropical region. Different clusters of IBDV strains with identical VP2 amino acid sequences were formed based on a specific pattern of nucleotide mutations. The differences between strains in a sub-cluster did not exceed five nucleotides. The first sub-cluster of northwest Nigerian IBDV that contained IBDV79 showed unique nucleotide mutations C893T (S254) and C957T (L282).
The failure to sequence the field IBDV strain used to develop a killed IBDV vaccine in Nigeria was probably due to a decrease in quality of the generated cDNA. However, all of the other northwest Nigerian IBDV strains showed similar VP2 region amino acid sequences and presumably similar antigenicity. The developed vaccine was able to protect chickens against a natural exposure to IBDV2, whereas the Georgia IBDV vaccine did not. Vaccines derived from vvIBDV strains, especially the adapted local strains, would probably confer more protection against Nigerian vvIBDV field strains.
The present study revealed a regional divide among IBDV strains with amino acid positions 299 and 300 as possible markers for regional identity of IBDV. These markers could be used to trace the origin and spread of IBDV strains. Canadian IBDV strains similar to the South African strain 05SA8 (Ojkic et al., Citation2007) were isolated from Ontario (2) and Quebec (14) initially, but are now the predominant IBDV strains in the two districts (personal communication with the author). There is the possibility that the Arctic tern known to migrate yearly between the Arctic and Antarctic regions (Arctic Tern, Citation2012) or other long-distance migratory birds might be responsible for spreading a South African IBDV strain to Canada. Natural IBDV infections in different free-living and migratory bird species (Wilcox et al., Citation1983; Gilchrist, Citation2005; Wang et al., Citation2007; Kasanga et al., Citation2008), including the Sooty Tern (Wilcox et al., Citation1983), have been documented. Another IBDV strain isolated from a sparrow in China (Wang et al., Citation2007) was the only strain from GenBank with the same proposed regional marker as obtained on IBDV strains in the tropical regions of Africa and India.
The antigenicity of IBDV is dependent on the structural conformation of the major hydrophilic peaks A and B of the VP2 HVR variable region (Schnitzler et al., Citation1993). In addition, minor hydrophilic peaks 1 and 2 also contributed to IBDV antigenicity (van den Berg et al., Citation1996). Mutations in some specific amino acids have been associated with differences in virus antigenicity (254D or N) and in virus attenuation (Q253H, N279T and A284T) (Vakharia et al., Citation1994; Kwon & Kim, Citation2004). However, following comparison of the VP2 HVR sequences from different IBDV subtypes in the present study, it was hypothesized that minor hydrophilic peak 1 and 2 could be involved with virulence more than antigenicity of IBDV. The non-synonymous mutation at 253 that might have contributed to reversion of a vaccine IBDV strain further strengthens this hypothesis. This is also supported by an earlier study that compared full sequences of virulent and attenuated IBDV strains, which indicated that virulence was reduced without affecting antigenicity in all reported studies (Lazarus et al., Citation2008). A recent study indicated lack of correlation between amino acid changes in the minor hydrophilic peaks and antigenicity of IBDV (Durairaj et al., Citation2011). In addition, we observed similar minor hydrophilic peak 1 and 2 sequences for the different IBDV subtypes even when there were differences in the major peaks A or B.
Overall, the results suggested that amino acid positions under high selection pressure in peaks A or B, I or II and III may be essential for maintaining VP2 structural conformations in relation to antigenicity, virulence and identity of IBDV, respectively. Further studies are necessary to test and confirm whether the proposed virulence marker could be used for the classification of IBDV strains into pathotypes. Further experimental and field trials of the killed IBDV vaccine developed are essential to confirm its effectiveness for control against field IBDV strains without priming using live IBDV vaccines.
cavp_a_822055_sm2206.pdf
Download PDF (28 KB)Acknowledgements
The authors wish to thank C.J. Snoeck and Dr C.P. Muller for supporting this work. This work was supported by a fellowship grant on the project “Recherche microbiologique pour le developpement III, MAE” financed by the Luxembourg Ministry of External Affairs and Cooperation, and the Tertiary Education Trust Fund, Nigeria. Reagents and facilities were available at the University of Ibadan for the molecular aspect of this work through collaboration with the Institute of Immunology, Centre de Recherche Public de la Sante, Luxembourg.
References
- Abdu, P.A., Umoh, J.U., Abdullahi, S.U. & Sa'idu, L. (2001). Infectious bursal disease (Gumboro) of chickens in Nigeria. Tropical Veterinarian, 19, 216–236.
- Adamu, J., Abdu, P.A. & Ngbede, E.O. (2011a). The antigenic relationship between infectious bursal disease vaccine and field viruses with improvement in sensitivity of agar gel immunodiffusion test using triton X-100. Zariya Veterinarian, 8, 29–34.
- Adamu, J., Abdu, P.A., Owoade, A.A., Kazeem, H.M. & Fatihu, M.Y. (2011b). Preliminary trial of a killed infectious bursal disease vaccine developed using Nigerian field vvIBDV isolate. Zariya Veterinarian, 8, 15–20.
- Arctic Tern. (2012). The arctic tern migration project. Retrieved from http://www.arctictern.info/
- Azad, A.A., Jagadish, M.N., Brown, M.A. & Hudson, P.J. (1987). Deletion mapping and expression in Escherichia coli of the large genomic segment of a birnavirus. Virology, 161, 145–152.
- Bayliss, C.D., Spies, U., Shaw, K., Peters, R.W., Papageorgiou, A., Muller, H. & Boursnell, M.E.G. (1990). A comparison of the sequences of segment A of four infectious bursal disease virus strains and identification of a variable region in VP2. Journal of General Virology, 71, 1303–1312.
- Brandt, M., Yao, K., Liu, M., Heckert, R.A. & Vakharia, V.N. (2001). Molecular determinants of virulence, cell tropism, and pathogenic phenotype of infectious bursal disease virus. Journal of Virology, 75, 11974–11982.
- Cortey, M., Bertran, K., Toskano, J., Majo, N. & Dolz, R. (2012). Phylogeographic distribution of very virulent infectious bursal disease virus isolates in the Iberian Peninsula. Avian Pathology, 41, 277–284.
- Cosgrove, A.S. (1962). An apparently new disease of chickens: avian nephrosis. Avian Diseases, 6, 385–389.
- Dolz, R., Majo, N., Ordonez, G. & Porta, R. (2005). Viral genotyping of infectious bursal disease viruses isolated from the 2002 acute outbreak in Spain and comparison with previous isolates. Avian Diseases, 49, 332–339.
- Durairaj, V., Sellers, H.S., Linnemann, E.G., Icard, A.H. & Mundt, E. (2011). Investigation of the antigenic evolution of field isolates using the reverse genetics system of infectious bursal disease virus (IBDV). Archives of Virology, 156, 1717–1728.
- Gilchrist, P. (2005). Involvement of free-living wild birds in the spread of the viruses of avian influenza, Newcastle disease and infectious bursal disease from poultry products to commercial poultry. World's Poultry Science Journal, 61, 198–210.
- Hall, T.A. (1999). BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series, 41, 95–98.
- Hon, C.-C., Lam, T.-Y., Drummond, A., Rambaut, A., Lee, Y.-F., Yip, C.-W., Zeng, F., Lam, P.-Y., Ng, P.T.-W. & Leung, F.C.-C. (2006). Phylogenetic analysis reveals a correlation between the expansion of very virulent infectious bursal disease virus and reassortment of its genome segment B. Journal of Virology, 80, 8503–8509.
- Hon, C.-C., Lam, T.T.-Y., Yip, C.-W., Wong, R.T.-Y., Shi, M., Jiang, J., Zeng, F. & Leung, F.C.-C. (2008). Phylogenetic evidence for homologous recombination within the family Birnaviridae. Journal of General Virology, 89, 3156–3164.
- Hudson, P.J., Mckern, N.M., Power, B.E. & Azad, A.A. (1986). Genomic structure of the large RNA segment of infectious bursal disease virus. Nucleic Acids Research, 14, 5001–5012.
- Ignjatovic, J. & Sapats, S. (2002). Confirmation of the existence of two distinct genetic groups of infectious bursal disease virus in Australia. Australian Veterinary Journal, 80, 689–694.
- Ismail, N.M., Saif, Y.M. & Moorhead, P.D. (1988). Lack of pathogenicity of five serotype 2 infectious bursal disease viruses in chickens. Avian Diseases, 32, 757–759.
- Jackwood, D.J. & Sommer-Wagner, S. (2007). Genetic characteristics of infectious bursal disease viruses from four continents. Virology, 365, 369–375.
- Jackwood, D.J., Sreedevi, B., LeFever, L.J. & Sommer-Wagner, S.E. (2008). Studies on naturally occurring infectious bursal disease viruses suggest that a single amino acid substitution at position 253 in VP2 increases pathogenicity. Virology, 377, 110–116.
- Kasanga, C.J., Yamaguchi, T., Wambura, P.N., Munang'andu, H.M., Ohya, K. & Fukushi H. (2008). Detection of infectious bursal disease virus (IBDV) genome in free-living pigeon and guinea fowl in Africa suggests involvement of wild birds in the epidemiology of IBDV. Virus Genes, 36, 521–529.
- Kibenge, F.S.B., Jackwood, D.J. & Mercado, C.C. (1990). Nucleotide sequence analysis of genome segment A of infectious bursal disease virus. Journal of General Virology, 71, 569–577.
- Kim, H.-R., Kwon, Y.-K., Bae, Y.-C., Oem, J.-K. & Lee, O.-S. (2010). Genetic characteristics of virion protein 2 genes of infectious bursal disease viruses isolated from commercial chickens with clinical disease in South Korea. Poultry Science, 89, 1642–1646.
- Kwon, H.M. & Kim, S.J. (2004). Sequence analysis of the variable VP2 gene of infectious bursal disease viruses passaged in Vero cells. Virus Genes, 28, 285–291.
- Lasher, H.N. & Shane, S.M. (1994). Infectious bursal disease. World's Poultry Science Journal, 50, 133–166.
- Lazarus, D., Pasmanik-Chor, M., Gutter, B., Gallili, G., Barbakov, M., Krispel, S. & Pitcovski, J. (2008). Attenuation of very virulent infectious bursal disease virus and comparison of full sequences of virulent and attenuated strains. Avian Pathology, 37, 151–159.
- Le Gros, F.X., Dancer, A., Giacomini, C., Pizzoni, L., Bublot, M., Graziani, M. & Prandini, F. (2009). Field efficacy trial of a novel HVT-IBD vector vaccine for 1-day-old broilers. Vaccine, 27, 592–596.
- Letzel, T., Coulibaly, F., Rey, F.A., Delmas, B., Jagt, E., van Loon, A.A.M.W. & Mundt, E. (2007). Molecular and structural bases for the antigenicity of VP2 of infectious bursal disease virus. Journal of Virology, 81, 12827–12835.
- Lojkic, I., Bidin, Z. & Pokric, B. (2008). Sequence analysis of both genome segments of three Croatian infectious bursal disease field viruses. Avian Diseases, 52, 513–519.
- Mundt, E., Beyer, J. & Muller, H. (1995). Identification of a novel viral protein in infectious bursal disease virus-infected cells. Journal of General Virology, 76, 437–443.
- Ojkic, D., Martin, E., Swinton, J., Binnington, B. & Brash, M. (2007). Genotyping of Canadian field strains of infectious bursal disease virus. Avian Pathology, 36, 427–433.
- Okeke, E.N. & Tanimu, T. (1982). Development and production of infectious bursal disease (Gumboro) vaccine in Nigeria. Nigerian Journal of Animal Production, 9, 80–89.
- Owoade, A.A., Mulders, M.N., Kohnen, J., Ammeriaan, W. & Muller, C.P. (2004). High sequence diversity in infectious bursal disease virus serotype 1 in poultry and turkey suggests West-African origin of very virulent strains. Archives of Virology, 149, 653–672.
- Pitcovski, J., Gutter, B., Gallili, G., Goldway, M., Perelman, B., Gross, G., Krispel, S., Barbakov, M. & Michael, A. (2003). Development and large-scale use of recombinant VP2 vaccine for the prevention of infectious bursal disease of chickens. Vaccine, 21, 4736–4743.
- Schnitzler, D., Bernstein, F., Muller, H. & Becht, H. (1993). The genetic basis for the antigenicity of the VP2 protein of the infectious bursal disease virus. Journal of General Virology, 74, 1563–1571.
- Spies, U., Muller, H. & Becht, H. (1987). Polymerase activity associated with infectious bursal disease virus and characterization of its reaction products. Virus Research, 8, 127–140.
- Synder, D.B. (1990). Changes in the field status of infectious bursal disease virus. Avian Pathology, 19, 419–423.
- Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M. & Kumar, S. (2011). MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution, 28, 2731–2739.
- Thompson, J.D., Higgins, D.G. & Gibson, T.J. (1994). CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acids Research, 22, 4673–4680.
- Thuiller, W., Lavorel, S. & Araujo, M.B. (2005). Niche properties and geographical extent as predictors of species sensitivity to climate change. Global Ecology and Biogeography, 14, 347–357.
- Tong, J.C., Umoh, J.U., Abdu, P.A. & Sa'idu, L. (1993). Retrospective studies of Gumboro disease seen in Ahmadu Bello University Veterinary Teaching Hospital Zaria, Nigeria (1985–1990). Bulletin of Animal Health and Production in Africa, 41, 173–179.
- Vakharia, V.N., He, J., Ahamed, B. & Snyder, D.B. (1994). Molecular basis of antigenic variation in infectious bursal disease virus. Virus Research, 31, 265–273.
- van den Berg, T.P. (2000). Acute infectious bursal disease in poultry: a review. Avian Pathology, 29, 175–194.
- van den Berg, T.P., Gonze, M., Morales, D. & Meulemans, G. (1996). Acute infectious bursal disease in poultry: immunological and molecular basis of antigenicity of a highly virulent strain. Avian Pathology, 25, 751–768.
- van den Berg, T.P., Morales, D., Eterradossi, N., Rivallan, G., Toquin, D., Raue, R., Zierenberg, K., Zhang, M.F., Zhu, Y.P., Wang, C.Q., Zheng, H.J., Wang, X., Chen, G.C., Lim, B.L. & Muller, H. (2004). Assessment of genetic, antigenic and pathotypic criteria for the characterization of IBDV strains. Avian Pathology, 33, 470–476.
- Wang, Y.S., Wang, Z.C., Tang, Y.D., Shi, Z.L., He, K.W., Li, Y., Hou, J.B., Yao, H.C., Fan, H.J. & Lu, C.P. (2007). Comparison of four infectious bursal disease viruses isolated from different bird species. Archives of Virology, 152, 1787–1797.
- Whitfill, C.E., Haddad, E.E., Ricks, C.A., Skeeles, J.K., Newberry, L.A., Beasley, J.N., Andrews, P.D., Thoma, J.A. & Wakenell, P.S. (1995). Determination of optimum formulation of a novel infectious bursal disease virus (IBDV) vaccine constructed by mixing bursal disease antibody with IBDV. Avian Diseases, 39, 687–699.
- Wilcox, G.E., Flower, R.L.P., Baxendale, W. & Mackenzie, J.S. (1983). Serological survey of wild birds in Australia for the prevalence of antibodies to egg drop syndrome 1976 (EDS-76) and infectious bursal disease viruses. Avian Pathology, 12, 135–139.
- Yong-Chang, C.A.O., Quan-Cheng, S.H.I., Jing-Yun, M.A., Qing-Mei, X.I.E. & Ying-Zuo, B.I. (2005). Vaccination against very virulent infectious bursal disease virus using recombinant T4 bacteriophage displaying viral protein VP2. Acta Biochimica et Biophysica Sinica, 37, 657–664.