Abstract
Aspergillosis is the most common fungal disease of the avian respiratory tract and is caused primarily by Aspergillus fumigatus. The respiratory macrophages provide important defence against aspergillosis. T-2 toxin (T-2), a trichothecene mycotoxin produced by Fusarium spp. in improperly stored agricultural products, has immunomodulatory effects. We studied the impact of T-2 on the antifungal response of the chicken macrophage cell line HD-11 against A. fumigatus infection. The macrophages were first exposed to 0.5 to 10 ng/ml T-2 for 24 h, and then their viability, antifungal activity, and cytokine expression in response to A. fumigatus conidial infection were determined. The viability of macrophages decreased when exposed to T-2 at concentrations higher than 1 ng/ml. One hour after conidial infection, phagocytosed conidia were observed in 30% of the non-T-2-exposed macrophages, but in only 5% of the macrophages exposed to 5 ng/ml T-2. Seven hours after infection, 24% of the conidia associated with non-T-2-exposed macrophages germinated, in contrast to 75% of those with macrophages exposed to 5 ng/ml T-2. A. fumigatus infection induced upregulation of interleukin (IL)-1β, CXCLi1, CXCLi2 and IL-12β, and downregulation of transforming growth factor-β4 in macrophages. Exposure of A. fumigatus-infected macrophages to T-2 at 1 to 5 ng/ml further upregulated the expression of IL-1β, IL-6, CCLi2, CXCLi1, CXCLi2, IL-18 (at 1 and 2 ng/ml) and IL-12β, and further downregulated that of transforming growth factor-β4 (at 5 ng/ml). In conclusion, T-2 impaired the antifungal activities of chicken macrophages against A. fumigatus conidia, but might stimulate immune response by upregulating the expression of pro-inflammatory cytokines, chemokines and T-helper 1 cytokines.
Introduction
Aspergillosis is the most common fungal disease of the avian respiratory tract and is caused primarily by Aspergillus fumigatus (Beernaert et al., Citation2009). A. fumigatus produces air-borne conidia in huge amounts into the environment, and these conidia are easily inhaled by a bird and then colonize the lower respiratory tract (Tell, Citation2005; Bain et al., Citation2007). As the first line of defence against the inhaled conidia, the respiratory macrophages are responsible for phagocytosing and killing the inhaled fungal conidia (Luther et al., Citation2008; Dagenais & Keller, Citation2009; Van Waeyenberghe et al., Citation2012). The development of aspergillosis depends on the number of conidia inhaled and the host immune status (Alley et al., Citation1999; Beernaert et al., Citation2010).
T-2 toxin (T-2) is a trichothecene mycotoxin produced by Fusarium spp. and is usually detected in improperly stored agricultural products such as maize, wheat, barley, oat and rye, and the detected concentrations can reach up to thousands of micrograms per kilogram (European Food Safety Authority, Citation2011). This mycotoxin is reported to influence the immune system with a time-dependent and dose-dependent mode of action. Specifically, T-2 induces immunosuppression at high doses and immunostimulation at low doses in poultry (Sokolović et al., Citation2008), despite a short half-life of elimination in broiler chicken plasma (Osselaere et al., Citation2013). The physiological concentrations of T-2 in the respiratory tract of chickens have never been reported in the literature.
Treatments of mammalian macrophages with T-2 ranging from 0.47 to 46.7 ng/ml in earlier studies showed suppressed phagocytosis of bacterial and fungal pathogens by mammalian macrophages (Gerberick et al., Citation1984; Vidal & Mavet, Citation1989). On the other hand, treatments with T-2 up to 10 ng/ml did not affect the viability or microscopic morphology of A. fumigatus K24 (Li et al., unpublished data). However, there is no information about the effect of T-2 on the antifungal activities of avian macrophages against A. fumigatus conidia. We hypothesize that T-2 modulates the interaction of avian macrophages with A. fumigatus conidia, thus influencing the course of infection.
The inflammatory response against pathogens involves the regulation of multiple cytokines. Transcription levels of pro-inflammatory cytokines interleukin (IL)-1β, IL-6, CCLi2, CXCLi1, CXCLi2, IL-12β and IL-18 as well as anti-inflammatory cytokine transforming growth factor (TGF)-β4 in chickens were found to be altered in bacterial and mycoplasma infections (Hong et al., Citation2006; Mohammed et al., Citation2007; Beeckman et al., Citation2010), but the regulation of these cytokines in A. fumigatus infection, whether or not exposed to T-2, has never been studied before.
The chicken HD-11 cell line is a macrophage-like cell line transformed with avian myelocytomatosis virus (MC29). The HD-11 cell line demonstrates the properties of primary macrophage, such as phagocytic capacity, cell surface antigens, and Fc receptor expression (Beug et al., Citation1979), despite its nitric oxide production being reported to be different from that of chicken primary peripheral blood leukocyte-derived macrophages upon stimulation (Lillehoj & Li, Citation2004). Chicken HD-11 cell line has been used extensively as an in vitro model to study pathogenic interactions with chicken macrophages, including those in the respiratory system (Lyon & Hinshaw, Citation1991; Beeckman et al., Citation2010; Hartley et al., Citation2012).
The objective of this study was to evaluate the effects of T-2 on the antifungal responses of HD-11 cells against A. fumigatus conidial infection. For this purpose, we examined the effect of exposure of HD-11 cells to T-2 on their antifungal activity and cytokine expression in response to A. fumigatus conidial infection.
Materials and Methods
Aspergillus fumigatus conidia
The A. fumigatus isolate, K24, used in this study was obtained from a racing pigeon, which died from pulmonary aspergillosis (Beernaert et al., Citation2008). Five-day-old cultures of this isolate on Sabouraud dextrose agar (CM0041; Oxoid Ltd, Basingstoke, UK) were washed with 5 ml of 0.01% Tween 20 in Dulbecco's modified Eagle's medium (DMEM) to harvest A. fumigatus conidia. The conidia were washed three times in DMEM containing 0.01% Tween 20 and the suspension was adjusted by haemocytometer count to the desired concentrations in DMEM supplemented with 10% foetal bovine serum, 1% glutamine and 1% pyruvate (DMEM +).
Macrophage cell line
The avian MC29 virus-transformed macrophage cell line from chicken, HD-11 (Beug et al., Citation1979), was maintained in DMEM containing 10% foetal bovine serum, 1% l-glutamine, 1% penicillin/streptomycin, and 1% kanamycin and was incubated at 37°C, 5% CO2, with passage every 3 days. For the experiments, 3-day-old HD-11 cells were adjusted to the desired concentrations in DMEM+ by haemocytometer count.
Cytotoxicity of T-2 toxin on HD-11 macrophages
The effect of T-2 on the viability of HD-11 macrophages was determined using the neutral red (3-amino-7-dimethylamino-2-methyl-phenazine hydrochloride) uptake assay as previously described (Borenfreund & Puernen, Citation1985). Two hundred microlitres of a HD-11 cell suspension of 5×105 cells/ml were seeded per well in a 96-well plate. After incubation at 37°C, 5% CO2 for 4 h, the cells in each well were exposed to 200 µl T-2 solution (Sigma-Aldrich, Steinheim, Germany) at different final concentrations (0, 0.5, 1, 2, 5 or 10 ng/ml) in DMEM+, with cells exposed to 0 ng/ml T-2 serving as negative control. After 24 h of incubation at 37°C, 5% CO2, the medium was removed and the wells were gently rinsed with Hank's Buffered Salt Solution+Ca2+ and Mg2+ (HBSS+). The cells in each well were incubated with 200 µl of a 33 µg/ml neutral red solution for 2 h at 37°C, 5% CO2. Subsequently, the cells were gently rinsed with HBSS+ and the neutral red dye was eluted with 200 µl elution mixture (acetic acid/ethanol/water, 1/50/49 v/v/v) by shaking at 550 rpm in a MTS 2/4 digital microtitre shaker (IKA, Staufen, Germany) for 10 min. One hundred and fifty microlitres of the eluate from each well was transferred into a new 96-well plate (IWAKI, Chiba, Japan) and absorbance at 540 nm was read on an enzyme-linked immunosorbent assay reader. This test was performed in sextuplicate. Viability of HD-11 cells was calculated using the following formula:
Phagocytosis of A. fumigatus conidia by T-2-exposed HD-11 macrophages
Phagocytic ability of HD-11 macrophages either exposed or not to T-2 was assessed by fluorescence microscopy as previously described (Van Waeyenberghe et al., Citation2012). One millilitre of a HD-11 cell suspension of 105 cells/ml was seeded per well on a glass coverslip in a 24-well plate and exposed to 1 ml T-2 solution at a final concentration of 0, 0.5, 1, 2, or 5 ng/ml respectively for 24 h as described above. The HD-11 cells in each well were then exposed to 2×105 K24 conidia in DMEM+ with the same concentration of T-2, and conidial exposure was synchronized by centrifugation at 365×g at 37 °C for 10 min. Subsequently, the cells were allowed to phagocytose the conidia for 1 h at 37 °C, 5% CO2. Medium containing the free conidia was removed, and wells were rinsed using HBSS+. Each well was then incubated with 1 ml of 25 µM Calcofluor White M2R (Life Technologies Europe BV, Ghent, Belgium) for 30 min at 37 °C to stain the extracellular conidia, but not the intracellular conidia. After washing with HBSS+ and fixing with 4% paraformaldehyde, the coverslip was mounted on a microscope slide with DABCO glycerol. The numbers of macrophages with intracellular and extracellular conidia in 100 randomly observed cells from each treatment were counted with a fluorescence microscope. This test was performed in quadruplicate.
Germination rate of A. fumigatus conidia associated with T-2-exposed HD-11 macrophages
One millilitre of a HD-11 macrophage suspension of 105 cells/ml was seeded per well in a 24-well plate and exposed to 1 ml T-2 at a final concentration of 0, 0.5, 1, 2 or 5 ng/ml respectively for 24 h as described above. The HD-11 cells in each well were then exposed to 2×105 K24 conidia in DMEM+ with the same concentration of T-2, and the conidial exposure was synchronized by centrifugation at 365×g at 37°C for 10 min. After 1 h of incubation, the free conidia were removed by washing with HBSS+, and the HD-11 cells with conidia were incubated in DMEM+ containing T-2 at the same concentrations for another 6 h. After washing with HBSS+ and fixed with 4% paraformaldehyde, the cells were covered by a glass coverslip with DABCO glycerol. One hundred macrophages with conidia in each treatment were randomly observed with a light microscope, and the germination rate of conidia was calculated by dividing the number of cell-associated conidia that had germinated by the total number of cell-associated conidia. This test was performed in quadruplicate.
Cytokine mRNA expression in HD-11 macrophages in response to A. fumigatus conidial infection
Cytokine mRNA expression was examined by quantitative reverse transcriptase polymerase chain reaction (qRT-PCR), and the fold change in cytokine expression per unit amount of macrophages (CEUAM) between a treatment and a control was then calculated by the 2−ΔΔCt method (Livak & Schmittgen, Citation2001). First, the kinetic change of cytokine expression in HD-11 cells was established during 20 h post A. fumigatus conidial infection. Then, to evaluate the effect of T-2 on cytokine expression in A. fumigatus-infected macrophages, cytokine expression was examined in A. fumigatus-infected HD-11 cells either exposed or not to T-2.
To establish the kinetic cytokine expression, 1 ml HD-11 cell suspension of 5×105 cells/ml was seeded per well in a 24-well plate as described above. The macrophages were then exposed to 106 K24 conidia in 1 ml DMEM+, and the conidial exposure was synchronized by centrifugation at 365×g at 37 °C for 10 min. Macrophages not exposed to conidia served as a negative control. At 1, 4, 6, 12 and 20 h post conidial exposure, total RNA from the macrophages was isolated using RNeasy Mini Kit (Qiagen, Valencia, CA, USA), and was reverse transcribed using the iScript cDNA Synthesis kit (Bio-Rad Laboratories, Hercules, CA, USA) according to the manufacturer's instructions. Information for the analysed genes and their primers for qRT-PCR analysis is presented in . GAPDH and β-actin were used as reference genes. qRT-PCR reactions were carried out using SensiMix SYBR No-ROX Kit (Bioline, Taunton, MA, USA) in a C1000 Thermal Cycler coupled with a CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories). The cycle profile was as follows: one cycle of 95 °C for 10 min and 45 cycles of 95 °C for 10 sec and 60 °C for 30 sec. The threshold cycle values (Ct) were first normalized to the geometric means of reference mRNAs and the fold changes in CEUAM between infected macrophages and uninfected macrophages (control) were calculated by the 2−ΔΔCt method (Livak & Schmittgen, Citation2001).
Table 1. Genes and sequences of the primers used for qRT-PCR analysis.
To compare cytokine mRNA expression in response to conidial infection between HD-11 macrophages either exposed or not to T-2, the same assay was used, but the macrophages were exposed to T-2 at a final concentration of 0, 1, 2 or 5 ng/ml during 24 h prior to conidial exposure as described above. The cells were then inoculated with K24 conidia as described above and incubated in medium containing the respective concentration of T-2. Total mRNA was isolated at 6 h after conidial exposure. By the 2−ΔΔCt method (Livak & Schmittgen, Citation2001), the fold changes in CEUAM between T-2-exposed macrophages and non-T-2-exposed macrophages (control) in response to conidial infection were calculated. Both tests were performed in triplicate.
Data analysis
The differences in cell viability, phagocytic activity, conidial germination and cytokine expression among different treatments were assessed by performing one-way analysis of variance after determination of normality and variance homogeneity. The significance level was set at 0.05.
Results
Cytotoxicity of T-2 toxin on HD-11 macrophages
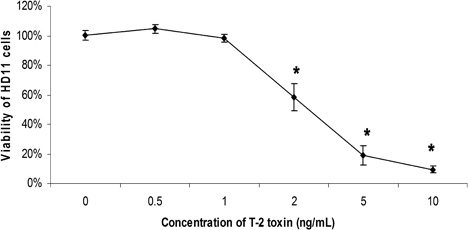
The viability of the HD-11 macrophages significantly decreased when exposed to T-2 at concentrations higher than 1 ng/ml, but the cell viability was not affected by T-2 at 0.5 and 1 ng/ml (). No morphological changes were noted at 0.5 and 1 ng/ml, but the cells with T-2 higher than 5 ng/ml seemed to have shrunk somewhat.
Phagocytosis and germination of A. fumigatus in T-2-exposed HD-11 macrophages
In both phagocytosis and germination assays, the same number (n=100) of macrophages was counted in different treatments (0, 1, 2, and 5 ng/ml) to calculate the phagocytosis and germination rates of conidia. One hour after conidial exposure, phagocytosed (intracellular) and cell-associated (intracellular+extracellular) conidia were observed in 30% and 59% of the non-T-2-exposed macrophages (0 ng/ml) respectively, while in macrophages exposed to 5 ng/ml T-2 these percentages decreased to 5% and 26% respectively (). Six hours later, 24% of the conidia associated with non-T-2-exposed macrophages germinated, in contrast to 75% in macrophages exposed to 5 ng/ml T-2 (). Phagocytosis and the germination rate at high T-2 concentrations (2 and 5 ng/ml) were significantly lower and higher than the controls, respectively.
Kinetic cytokine mRNA expression in HD-11 macrophages in response to A. fumigatus conidial infection
Cytokine expression in A. fumigatus-infected HD-11 macrophages was compared with that in uninfected controls at 1, 4, 6, 12 and 20 h post infection (p.i.). Significant upregulation of IL-1β, CXCLi1, CXCLi2 and IL-12β was noted from 6, 4, 4 and 12 h p.i., and finally increased to 248-fold, 727-fold, 371-fold and 387-fold of that in uninfected controls at 20 h p.i. respectively. Significant downregulation of TGF-β4 was noticed from 12 h p.i., and finally decreased to 0.4-fold of that in uninfected controls at 20 h p.i. A. fumigatus conidial infection did not induce any significant change in IL-6 and IL-18 expression throughout the 20 h incubation period ().
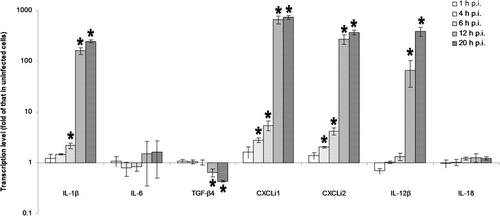
Cytokine mRNA expression in T-2-exposed HD-11 macrophages in response to A. fumigatus conidial infection
Compared with the non-T-2-exposed controls, the macrophages exposed to 1 to 5 ng/ml T-2 showed significantly upregulated expression of IL-1β, IL-6, CCLi2, CXCLi1, CXCLi2, IL-12β and IL-18 (at 1 and 2 ng/ml), and significantly downregulated expression of TGF-β4 (at 5 ng/ml) 6 h after exposure to A. fumigatus conidia. Expression patterns were dose dependent from 1 to 5 ng/ml except for IL-18 ().
Discussion
T-2 was shown to be highly toxic at very low concentrations (>1 ng/ml) for chicken macrophages. A similar result was observed in a study by Jaradat et al. (Citation2006), where T-2 inhibited mitogen-stimulated chicken lymphocyte proliferation in vitro at concentrations of 1 ng/ml or higher, and proliferation was completely abolished at 10 ng/ml. In a study by Kidd et al. (Citation1997), 1 h exposure of chicken macrophages to 10 µg/ml and 160 µg/ml T-2 tetraol, a T-2 derivative, resulted in respectively 83% and 63% viability. The half inhibition concentration (IC50) in this study was approximately 2 ng/ml, while that of human macrophages was demonstrated at 10 ng/ml (Hymery et al., Citation2009). Exposure to similar levels of T-2 in vivo may thus be expected to negatively affect macrophage viability and as a consequence be the first-line defence mechanism against aspergillosis.
Further evidence for impaired macrophage function was the decreased phagocytic capacity of the macrophages after exposure to T-2. This result is in agreement with those obtained in mammalian macrophages. The phagocytosis of Pseudomonas aeruginosa by mouse peritoneal macrophages exposed to 0.47 to 46.7 ng/ml T-2 decreased dose dependently (Vidal & Mavet, Citation1989). T-2 at 4.7 and 23.4 ng/ml decreased the phagocytic capacity of rat alveolar macrophages for yeast cells to respectively 76.9% and 16.8% of the controls (Gerberick et al., Citation1984), and serum from rabbits treated with 0.5 mg T-2 per kilogram of body weight per day suppressed the phagocytosis of A. fumigatus conidia by rabbit alveolar macrophages (Niyo et al., Citation1988). Impaired macrophage function by T-2 was further supported by the increased germination rate of macrophage-associated A. fumigatus conidia in this study. This result is compatible with a report by Verbrugghe et al. (Citation2012) that T-2 at 1 ng/ml increased the susceptibility of porcine macrophages to Salmonella Typhimurium invasion. Phagocytosis and germination of A. fumigatus conidia in chicken HD-11 cells were similar to those in primary respiratory macrophages of pigeons (Van Waeyenberghe et al., Citation2012), but the current study is the first to address the effect of T-2 on the antifungal response of chicken macrophages. In conclusion, pronounced cytotoxicity of T-2 for avian macrophages coincided with impaired antifungal activity, which would thus facilitate conidial infection in the avian respiratory tract.
In response to A. fumigatus infection, the expression of pro-inflammatory cytokines IL-1β, IL-12β (T-helper [Th] 1 cytokine), CXCLi1 (chemokine) and CXCLi2 (chemokine) in HD-11 macrophages was upregulated, and that of TGF-β4 (anti-inflammatory cytokine) was downregulated. These results suggest that A. fumigatus infection promotes inflammatory response in chicken macrophages, induces migration of other immunocytes to the infection sites, and stimulates Th1 immune responses (Beeckman et al., Citation2010). The upregulated expression of IL-1 and IL-12 in A. fumigatus-infected HD11 cells was compatible with the A. fumigatus-induced production of these cytokines in mouse alveolar and peritoneal macrophage (Taramelli et al., Citation1996), equine alveolar macrophages (Laan et al., Citation2005), and mouse bronchoalveolar lavage fluid (Cenci et al., Citation1998). Aspergillosis also induced upregulation of IL-6 and IL-18 in dog mucosal tissue (Day, Citation2009) and mouse bronchoalveolar lavage fluid and lung tissue (Brieland et al., Citation2001), but upregulation of IL-6 and IL-18 did not happen to the chicken HD-11 cells upon conidial infection. Significant changes in cytokine expression occurred from around 6 h after exposure to A. fumigatus conidia, which corresponded to the time of conidial germination. The effect of T-2 exposure on cytokine expression in A. fumigatus-infected macrophages was therefore subsequently determined at 6 h p.i.
In response to A. fumigatus infection, the expression of pro-inflammatory cytokines IL-1β, IL-6, CCLi2 (chemokine), CXCLi1, CXCLi2, IL-12β and IL-18 (Th1 cytokine) was upregulated and that of TGF-β4 was downregulated in T-2-exposed HD-11 macrophages as compared with the non-T-2-exposed macrophages (control). This result suggests that exposure of chicken macrophages to T-2 further promotes the pro-inflammatory response, immunocyte migration, and Th1 immune response induced solely by A. fumigatus conidia. It is interesting that A. fumigatus infection did not induce the expressions of IL-6 and IL-18 when the HD-11 cells were not exposed to T-2, but both IL-6 and IL-18 were upregulated upon A. fumigatus infection when the HD-11 cells were exposed to T-2. These finding supports the notion that T-2 enhanced the immune response by increasing the production of pro-inflammatory cytokines (Kankkunen et al., Citation2009). No previous study has been performed on how T-2 affects cytokine expression in fungus-infected macrophages. Production of IL-1β, IL-6 and IL-12 in mammalian macrophages was reduced by T-2 exposure when the macrophages were uninfected (T-2 at ≤1 ng/ml) (Ahmadi & Riazipour, Citation2008a) or co-stimulated with bacterial lipopolysaccharide (Dugyala & Sharma, Citation1997), lingzhi (Ganoderma lucidum) extract (Ahmadi & Riazipour, Citation2008b), or Toll-like receptor agonists (Seeboth et al., Citation2012), but upregulation of IL-1β, IL-6 and IL-18 was also reported in macrophages stimulated solely with T-2 (Kankkunen et al., Citation2009) or co-stimulated with bacterial lipopolysaccharide (Wang et al., Citation2012). With these diverse effects of T-2, the co-effects of T-2 and different pathogens or antigens on macrophages have to been examined specifically. T-2 has been reported to have both suppressive and stimulatory effects on immunity, and the enhanced immune response was suggested to be associated with the increased production of pro-inflammatory cytokines and migration of macrophages (Sokolović et al., Citation2008; Kankkunen et al., Citation2009; European Food Safety Authority, Citation2011). The changes in cytokine expression in T-2-exposed HD-11 cells seemed to be the result of the immunostimulatory effect of T-2.
Another study by our group showed that K24 conidia did not secrete any proteins or mycotoxins in the non-germination condition (Li et al., unpublished data). The assays on phagocytosis, conidial germination, and cytokine expression involving T-2 were performed in the non-germination condition or during the early germination stage to avoid potential interferences of other K24 metabolites with the effects of T-2. In conclusion, T-2 impaired the antifungal activity of HD-11 cells against A. fumigatus conidial infection, but promoted a pro-inflammatory response in infected macrophages, which might compensate for the observed macrophage functional impairment.
Acknowledgements
The authors hereby express the most sincere and hearty gratitude to Dr Virginie Vandenbroucke, Mr Guangzhi Zhang, Ms Pascale Van Rooij, Ms Mojdeh Sharifian-Fard, Ms Miet Vermoote, Ms Myrthe Joosten, Dr Connie Adriaensen, Ms Venessa Eeckhaut, Mr Gunther Antonissen and Dr David Hermans for their technical advice and assistance.
References
- Ahmadi, K. & Riazipour, M. (2008a). Effects of T-2 toxin on cytokine production by mice peritoneal macrophages and lymph node T-Cells. Iranian Journal of Immunology, 5, 177–180.
- Ahmadi, K. & Riazipour, M. (2008b). T-2 toxin regulated Ganoderma lucidum induced cytokine release. American Journal of Immunology, 4, 8–13.
- Alley, M.R., Castro, I. & Hunter, J.E.B. (1999). Aspergillosis in hihi (Nofiomystis cincta) on Mokoia Island. New Zealand Veterinary Journal, 47, 88–91.
- Bain, J.M., Tavanti, A., Davidson, A.D., Jacobsen, M.D., Shaw, D., Gow, N.A.R. & Odds, F.C. (2007). Multilocus sequence typing of the pathogenic fungus Aspergillus fumigatus. Journal of Clinical Microbiology, 45, 1469–1477.
- Beeckman, D.S.A., Rothwell, L., Kaiser, P. & Vanrompay, D.C.G. (2010). Differential cytokine expression in Chlamydophila psittaci genotype A-, B- or D-infected chicken macrophages after exposure to Escherichia coli O2:K1 LPS. Developmental and Comparative Immunology, 34, 812–820.
- Beernaert, L.A., Pasmans, F., Baert, K., Van Waeyenberghe, L., Chiers, K., Haesebrouck, F. & Martel, A. (2009). Designing a treatment protocol with voriconazole to eliminate Aspergillus fumigatus from experimentally inoculated pigeons. Veterinary Microbiology, 139, 393–397.
- Beernaert, L.A., Pasmans, F., Haesebrouck, F. & Martel, A. (2008). Modelling Aspergillus fumigatus infections in racing pigeons (Columba livia domestica). Avian Pathology, 37, 545–549.
- Beernaert, L.A., Pasmans, F., Van Waeyenberghe, L., Haesebrouck, F. & Martel, A. (2010). Aspergillus infections in birds: a review. Avian Pathology, 39, 325–331.
- Beug, H., von Kirchbach, A., Doderlein, G., Conscience, J.F. & Graf, T. (1979). Chicken hematopoietic cells transformed by seven strains of defective avian leukemia viruses display three distinct phenotypes of differentiation. Cell, 18, 375–390.
- Borenfreund, G. & Puernen, A. (1985). Toxicity determined in vitro by morphological alternation and neutral red absorption. Toxicology Letters, 24, 119–124.
- Brieland, J.K., Jackson, C., Menzel, F., Loebenberg, D., Cacciapuoti, A., Halpern, J., Hurst, S., Muchamuel, T., Debets, R., Kastelein, R., Churakova, T., Abrams, J., Hare, R. & O'Garra, A. (2001). Cytokine networking in lungs of immunocompetent mice in response to inhaled Aspergillus fumigatus. Infection and Immunity, 69, 1554–1560.
- Cenci, E., Mencacci, A., Fè d'Ostiani, C., Del Sero, G., Mosci, P., Montagnoli, C., Bacci, A. & Romani, L. (1998). Cytokine- and T helper-dependent lung mucosal immunity in mice with invasive pulmonary aspergillosis. Journal of Infectious Diseases, 178, 1750–1760.
- Dagenais, T.R.T. & Keller, N.P. (2009). Pathogenesis of Aspergillus fumigatus in invasive aspergillosis. Clinical Microbiology Reviews, 22, 447–465.
- Day, M.J. (2009). Canine sino-nasal aspergillosis: parallels with human disease. Medical Mycology, 47, 315–323.
- Dugyala, R.R. & Sharma, R.P. (1997). Alteration of major cytokines produced by mitogen-activated peritoneal macrophages and splenocytes in T-2 toxin-treated male CD-1 mice. Environmental Toxicology and Pharmacology, 3, 73–81.
- European Food Safety Authority. (2011). Scientific opinion on the risks for animal and public health related to the presence of T-2 and HT-2 toxin in food and feed, EFSA Journal, 9, 2481–2668.
- Gerberick, G.F., Sorenson, W.G. & Lewis, D.M. (1984). The effects of T-2 toxin on alveolar macrophage function in vitro. Environmental Research, 33, 246–260.
- Hartley, C., Salisbury, A.M. & Wigley, P. (2012). CpG oligonucleotides and recombinant interferon-γ in combination improve protection in chickens to Salmonella enterica serovar Enteritidis challenge as an adjuvant component, but have no effect in reducing Salmonella carriage in infected chickens. Avian Pathology, 41, 77–82.
- Hong, Y.H., Lillehoj, H.S., Lee, S.H., Dalloul, R.A. & Lillehoj, E.P. (2006). Analysis of chicken cytokine and chemokine gene expression following Eimeria acervulina and Eimeria tenella infections. Veterinary Immunology and Immunopathology, 114, 209–223.
- Hymery, N., Léon, K., Carpentier, F.G., Jung, J.L. & Parent-Massin, D. (2009). T-2 toxin inhibits the differentiation of human monocytes into dendritic cells and macrophages. Toxicology in Vitro, 23, 509–519.
- Jaradat, Z.W., Vila, B. & Marquardt, R.R. (2006). Adverse effects of T-2 toxin on chicken lymphocytes blastogenesis and its protection with Vitamin E. Toxicology, 225, 90–96.
- Kankkunen, P., Rintahaka, J., Aalto, A., Leino, M., Majuri, M.L., Alenius, H., Wolff, H. & Matikainen, S. (2009). Trichothecene mycotoxins activate inflammatory response in human macrophages. The Journal of Immunology, 182, 6418–6425.
- Kidd, M.T., Qureshi, M.A., Hagler, W.M., Jr. & Ali, R. (1997). T-2 tetraol is cytotoxic to a chicken macrophage cell line. Poultry Science, 76, 311–313.
- Laan, T.T., Bull, S., Pirie, R.S. & Fink-Gremmels, J. (2005). Evaluation of cytokine production by equine alveolar macrophages exposed to lipopolysaccharide, Aspergillus fumigatus, and a suspension of hay dust. The American Journal of Veterinary Research, 66, 1584–1589.
- Lillehoj, H.S. & Li, G. (2004). Nitric oxide production by macrophages stimulated with coccidia sporozoites, lipopolysaccharide, or interferon-γ, and its dynamic changes in SC and TK strains of chickens infected with Eimeria tenella. Avian Diseases, 48, 244–253.
- Livak, K.J. & Schmittgen, T.D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt method. Methods, 25, 402–408.
- Lu, Y., Sarson, A.J., Gong, J., Zhou, H., Zhu, W., Kang, Z., Yu, H., Sharif, S. & Han, Y. (2009). Expression profiles of genes in toll-like receptor-mediated signaling of broilers infected with Clostridium perfringens. Clinical and Vaccine Immunology, 16, 1639–1647.
- Luther, K., Rohde, M., Sturm, K., Kotz, A., Heesemann, J. & Ebel, F. (2008). Characterisation of the phagocytic uptake of Aspergillus fumigatus conidia by macrophages. Microbes and Infection, 10, 175–184.
- Lyon, J.A. & Hinshaw, V.S. (1991). Replication of influenza A viruses in an avian macrophage cell line. Journal of General Virology, 72, 2011–2013.
- Mohammed, J., Frasca, S. Jr., Cecchini, K., Rood, D., Nyaoke, A.C., Geary, S.J. & Silbart, L.K. (2007). Chemokine and cytokine gene expression profiles in chickens inoculated with Mycoplasma gallisepticum strains Rlow or GT5. Vaccine, 25, 8611–8621.
- Niyo, K.A., Richard, J.L., Niyo, Y. & Tiffany, L.H. (1988). Effects of T-2 mycotoxin ingestion on phagocytosis of Aspergillus fumigatus conidia by rabbit alveolar macrophages and on hematologic, serum biochemical, and pathologic changes in rabbits. The American Journal of Veterinary Research, 49, 1766–1773.
- Osselaere, A., Devreese, M., Goossens, J., Vandenbroucke, V., De Baere, S., De Backer, P. & Croubels, S. (2013). Toxicokinetic study and absolute oral bioavailability of deoxynivalenol, T-2 toxin and zearalenone in broiler chickens. Food and Chemical Toxicology, 51, 350–355.
- Seeboth, J., Solinhac, R., Oswald, I.P. & Guzylack-Piriou, L. (2012). The fungal T-2 toxin alters the activation of primary macrophages induced by TLR-agonists resulting in a decrease of the inflammatory response in the pig. Veterinary Research, 43, 35.
- Sokolović, M., Garaj-Vrhovac, V. & Simpraga, B. (2008). T-2 toxin: incidence and toxicity in poultry. Archives of Industrial Hygiene and Toxicology, 59, 43–52.
- Taramelli, D., Malabarba, M.G., Sala, G., Basilico, N. & Cocuzza, G. (1996). Production of cytokines by alveolar and peritoneal macrophages stimulated by Aspergillus fumigatus conidia or hyphae. Journal of Medical and Veterinary Mycology, 34, 49–56.
- Tell, L.A. (2005). Aspergillosis in mammals and birds: impact on veterinary medicine. Medical Mycology, 43, 71–73.
- Van Waeyenberghe, L., Pasmans, F., D'Herde, K., Ducatelle, R., Favoreel, H., Li, S.J., Haesebrouck, F. & Martel, A. (2012). Germination of Aspergillus fumigatus inside avian respiratory macrophages is associated with cytotoxicity. Veterinary Research, 43, 32.
- Verbrugghe, E., Vandenbroucke, V., Dhaenens, M., Shearer, N., Goossens, J., De Saeger, S., Eeckhout, M., D'Herde, K., Thompson, A., Deforce, D., Boyen, F., Leyman, B., Van Parys, A., De Backer, P., Haesebrouck, F., Croubels, S. & Pasmans, F. (2012). T-2 toxin induced Salmonella Typhimurium intoxication results in decreased Salmonella numbers in the cecum contents of pigs, despite marked effects on Salmonella-host cell interactions. Veterinary Research, 43, 22.
- Vidal, D. & Mavet, S. (1989). In vitro and in vivo toxicity of T-2 toxin, a Fusarium mycotoxin, to mouse peritoneal macrophages. Infection and Immunity, 57, 2260–2264.
- Wang, X., Liu, Q., Ihsan, A., Huang, L., Dai, M., Hao, H., Cheng, G., Liu, Z., Wang, Y. & Yuan, Z. (2012). JAK/STAT pathway plays a critical role in the proinflammatory gene expression and apoptosis of RAW264.7 cells induced by trichothecenes as DON and T-2 toxin. Toxicological Sciences, 127, 412–424.