Abstract
A study was carried out in French breeder duck flocks in 2008 and 2009 to identify practices and events related to the introduction of avian influenza viruses (AIVs). The status of flocks was assessed using serological methods for all subtypes of AIV without typing. Flocks managed with both natural mating and artificial insemination were investigated every 4 weeks from the beginning of the laying period up to seroconversion or for a maximum of 6 months. A questionnaire was completed with the farmer during each visit and 20 female ducks were randomly sampled for blood testing. Only flocks that tested seronegative at the first visit were included in the study (n =151 flocks managed with natural mating or artificial insemination). Data were analysed using survival analysis to identify factors influencing the time to seroconversion. Three separate models were constructed: one for the whole sample, one for natural mating flocks, and one for artificial insemination flocks. Factors related to the time to introduction of AIV included the type of production system linked to artificial insemination practices, the neighbourhood, poor disinfection practices, liquid manure management, presence of wildlife, and vehicles entering the building. No clear relationship could be observed in the serological status of male and female ducks in farms keeping male ducks separately from female ducks for artificial insemination. By respecting carefully biosecurity measures, it should be possible to decrease AIV infection of breeder duck flocks.
Introduction
Avian influenza epidemics in the poultry industry have caused severe economic losses worldwide due to bird deaths, mass slaughter, and national and international trade restrictions (Davison et al., Citation1999; Akey, Citation2003; Stegeman et al., Citation2004; Brown, Citation2010). These epidemics are caused by avian influenza viruses (AIVs), classified as low pathogenic (LP) or highly pathogenic (HP). In each category, pathobiological characteristics differ according to the virus strain and species susceptibility. For example, HPAIVs cause severe disease and high mortality in some species such as chickens and turkeys but can be clinically unapparent or very mild in ducks (Alexander et al., Citation1986; Harder et al., Citation2009). It has been shown that certain strains of HPAIV can lead to clinical signs in ducks (Capua & Mutenelli, Citation2001; Löndt et al., Citation2008) but historically ducks are considered to be a reservoir of AIV. Several studies have shown that ducks inoculated with different strains of LPAIV or HPAIV can shed large quantities of the virus up to 17 or 21 days post infection, by faecal or oral routes, with mild or no clinical signs (Wood et al., Citation1995; Jeong et al., Citation2009; Mundt et al., Citation2009; Jourdain et al., Citation2010). There is therefore a need for awareness of AIV contamination in poultry flocks, particularly in duck populations. Since the emergence in Asia in 1997 of the H5N1 HPAIV, official surveillance has been set up in the European Union to monitor the circulation of LPAIV subtypes H5 and H7. In France, breeder duck flocks have been shown to be more frequently seropositive for H5 subtype than other poultry (19 holdings out of 112 sampled holdings tested positive for H5 subtype) (European Commission, Citation2011). Therefore a study was implemented in France to identify risk factors associated with the introduction of AIV in breeder duck flocks and to propose measures to prevent flock infection. The avian influenza epizootics that occurred in different countries in recent years enabled one to identify risk factors for secondary spread of AIV in poultry farms (Mc Quinston et al., Citation2005; Thomas et al., Citation2005; Sharkey et al., Citation2008; Busani et al., Citation2009). On the contrary, our study addresses the identification of risk factors for the introduction of AIV in breeder duck farms in an epidemiological context outside an outbreak situation.
Materials and Methods
Study design
In France, two methods are used for the production of duck hatching eggs: natural mating (for meat ducks) and artificial insemination (for force-feeding ducks). Natural mating generally concerns males and females of the same species, either Muscovy (Cairina moschata) or Pekin (Anas platyrhynchos). Artificial insemination is used to cross male Muscovy and female Pekin ducks. This study is a longitudinal survey on incident cases, where data collection was done during a prospective investigation. The target population included both types of breeding (natural mating and artificial insemination). Duck hatcheries agreeing to take part in the study provided their planning, indicating date of onset of lay of their breeder flocks between February 2008 and April 2009. A list was obtained of approximately 240 farms working with 90% of the hatcheries that produce breeder ducks in France. The epidemiological unit of the study was one breeder flock per farm. If a selected farm had more than one layer building on a site, either the first flock introduced was selected or one was selected at random if ducks had been introduced on the same day.
Description of monitoring procedure
The flocks were monitored from the introduction of ducks into the breeding house (Pekin ducks at 18 to 20 weeks of age, Muscovy ducks at 24 to 25 weeks of age) and for a maximum of 6 months; this period corresponds to the average time before moulting of Muscovy ducks. The monitoring began preferentially within the 5 days following the transfer of ducks from their rearing house to their laying house. Blood samples were taken from the occipital sinus of 20 females randomly selected in the whole building: the poultry house was divided into quarters, and five ducks per quarter were randomly sampled. A flock was included in the study if all sera tested negative. The flock status was checked monthly on 20 randomly selected ducks. The monitoring ended as soon as the flock seroconverted, or 6 months after its inclusion. For naturally mating flocks, males and females had been reared together for several weeks before transfer to the laying building. Males were therefore considered to have the same serological status as females and only females were tested. For artificially inseminated flocks, some breeder duck sites contained males and others did not. For the former, males were bred elsewhere before being transferred to the laying site, which often took place after the arrival of females (either in cages in the same building as females or in another building). In addition, males were replaced about every 15 weeks (“spiking”). Consequently, 20 male Muscovy ducks were randomly blood sampled at different times: after their transfer, when they were replaced, at the end of the monitoring of the site and/or when females had seroconverted in case where males were previously seronegative.
Questionnaire design
Questionnaires were completed by four trained staff from Anses during an interview with farmers at the same time as blood sampling. To avoid bias, training sessions were organized prior to the farm visits to standardize interviewing techniques and data input. The questionnaires comprised mostly closed questions. The first questionnaire was administered during the first visit to collect information about the farm, flock management and installation of the new flock (). Another questionnaire was completed during each monthly visit concerning events that had occurred during the intervening time. The two questionnaires (in French) are available upon request. The questionnaires had been tested at the beginning of the study and validated by the steering committee composed of persons from Anses, veterinarians, and professionals of breeder duck production.
Table 1. Summary of items in the questionnaires used to identify risk factors for avian influenza contamination (151 breeder flocks, France, 2008 and 2009).
Serological analysis
After collection, sera were stored between 4 and 8°C and sent to one of the four selected departmental veterinary laboratories officially accredited for avian influenza analysis (laboratories in Nantes, La Roche-sur-Yon, Angers and Mont de Marsan). After centrifugation (10 min, 3000×g), an enzyme-linked immunosorbent assay (ELISA) for nucleoprotein (NP) method (Pourquier® ELISA competition avian influenza A kit; Institut Pourquier, Montepellier, France) was used to identify antibodies against all types of AIV; the sensitivity as reported by the manufacturer was equal to 99.7% on vaccinal strains for all poultry species with a specificity of 95.4%. The sera were considered negative when the inhibition percentage was below 35%, positive when the inhibition percentage was above 45%, and doubtful when the inhibition percentage was between 35 and 45%. When more than two sera out of the 20 blood samples were positive, the flock was considered seropositive; and if no serum was positive, the flock was considered seronegative. When there were only one or two positive sera out of 20, the serological status of the flock was confirmed either by another visit to collect more samples or by retesting the 20 sera with another ELISA test when no visit could be done (Idexx® FlockChek AI Multi-Screen Antibody ELISA kit, sensitivity=99.7%, specificity=95.4%; IDEXX Inc, Westbrook, ME, USA). If more than two sera tested positive with the second test, then the flock was considered positive. Otherwise the flock was excluded from the study.
Statistical analysis
Data from the questionnaires were entered into a database (Sphinx® version 5.1.0.5; Le Sphinx Développement, cChavanod, France). All variables were coded by category and the number of categories per variable was limited so that the frequencies of categories were above 10%. A three-step statistical procedure was used to assess the relationships between explanatory variables and the serological status of flocks. A survival analysis was performed with the number of days between two visits as the time unit. In the first step, survival distributions were compared between categories of each explanatory variable using the Kaplan–Meier method (LIFETEST procedure, SAS 9.1; SAS Institute Inc., Cary, NC, USA). The difference in survival between levels of each variable was tested using the log-rank test. During visits, similarities in practices and/or infrastructures were observed between farms selling hatching eggs to the same hatchery. A Cox model (TPHREG procedure, SAS 9.1) was then used to relate each variable with P<0.30 on the log-rank test to the time to seroconversion, stratifying on the hatchery to control for any hatchery-level confounding factor. The “hatchery effect” was taken into account stating the hatchery variable as a repeated statement and using a robust sandwich covariance matrix estimate (Allison, Citation2001). Variables related to the outcome (P≤0.20) of the Cox model and biological plausibility were selected. Pairwise comparisons were performed between selected variables using a chi-square test to detect variables showing strong statistical association (P≤0.05): the variable most closely related to the serological status was chosen. In the last step, a multivariate Cox proportional hazard model was set up with selected variables, using a backwards elimination procedure (P≤0.05).Variables were only removed if they did not affect the coefficients of other variables included in the model by >30%. Univariate and multivariate survival analyses were carried out for all flocks and for artificial insemination flocks. The positive/negative ratio did not enable a multivariate model to be developed for the natural mating flocks.
Results
Descriptive results
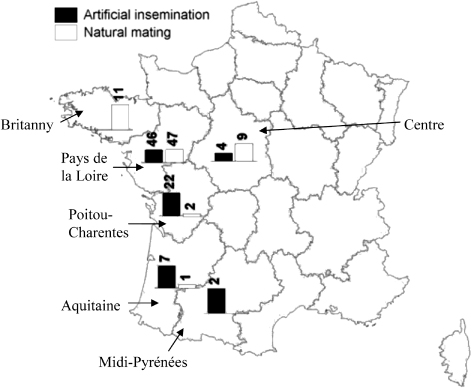
Two hundred flocks were visited between February 2008 and October 2009. Among this sample, 49 flocks were excluded: 41 were positive at the first visit (30 using artificial insemination and 11 using natural mating), and in eight the serological status was doubtful and could not be confirmed. The final sample therefore consisted of 151 flocks, comprising 70 flocks subject to natural mating (46.4%) and 81 flocks using artificial insemination (53.6%). Natural mating flocks were predominantly composed of Muscovy ducks (87%). This sample enabled one to detect a risk factor with a hazard ratio (HR) superior to or equal to 1.75, with a power of 80% when found in one-half of the flocks (Machin et al., Citation2009). Breeder duck flocks were located in the west of France, mainly in the Pays de la Loire region and the department of the Deux Sèvres (in Poitou-Charentes region). Several regions were “specialized” in a particular method of production; for example, natural mating in Brittany, and artificial insemination in the south-west of France and Deux Sèvres (). Among the 151 flocks in the study, 56 seroconverted (37%): 16 natural mating flocks (23% of all the natural mating flocks), and 40 artificial insemination flocks (49% of the artificial insemination flocks). During the study, six flocks that did not seroconvert were slaughtered for economic reasons before 6 months of lay and data for these flocks were censored at the time of slaughter. The Kaplan–Meier survivor functions are shown in for flocks in artificial insemination and for flocks in natural mating. Among the 81 farms using artificial insemination, 63 bred Muscovy males for semen collection. Those were kept in the female building or in another one. On almost all the farms that were, practicing spiking, blood samples were obtained from successive batches of males. No clear relationship was observed in the serological status of males and females () and no conclusion could be drawn regarding the role of males as a risk factor for AIV introduction in breeder duck flocks using artificial insemination.
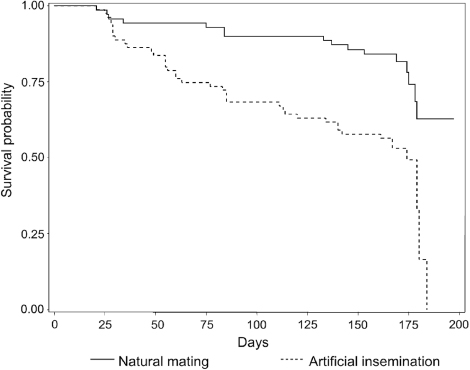
Table 2. Serological results of males and females on the 63 farms using artificial insemination that had male Muscovy ducks.
Factors associated with time to seroconversion in all flocks
After univariate analysis, 104 variables were selected and compared pairwise to eliminate inter-related variables. A large number of these variables were linked to the reproduction method used. A final set of 17 independent variables with P≤0.20 were selected as candidates for the multivariate Cox proportional hazard model. Six variables were found to be associated with time to seroconversion in the final model (). The risk of AIV infection was higher on farms using artificial insemination than those using natural mating (HR=3.53, 95% confidence interval [CI]=1.53 to 8.14). Likewise, the existence of poultry farms (ducks, hens, turkeys, etc.) within a 1-km radius of the farm increased the risk of infection (HR=3.3, 95% CI=2.4 to 4.53). The presence of wild animals in or around the building was associated with time to seroconversion of flocks. For instance, when farmers reported large numbers of wild birds (gulls, pigeons or starlings) on the roof or near the laying building, the risk of AIV introduction increased (HR=2.16, 95% CI=1.40 to 3.33). Similarly, when rodents were observed in the building, mainly during the depopulated period, the risk increased by 2.29 (95% CI=1.05 to 5.00). The storage of liquid manure near the building (100 m) was shown to be associated with infection (HR=1.42, 95% CI=1.07 to 1.90). A higher risk was also observed when the ground around the building was not disinfected during the depopulated period (HR=1.78, 95% CI=1.22 to 2.56).
Table 3. Results of univariate and multivariate Cox models performed to assess risk factors of AIV introduction in breeder duck flocks, for all types of production in France.
Factors associated with time to seroconversion in flocks subject to artificial insemination
For flocks using artificial insemination, 119 variables were selected in the univariate step and 15 were selected for the multivariate model after checking for independence (). Five variables remained in the final model. Two variables that were strongly linked to time to seroconversion concerned disinfection practices: no regular disinfection of the ante-room (HR=1.97, 95% CI=1.16 to 3.37), and disinfection of the building during the depopulated period carried out by the farmer and not by a hygiene specialist (HR=2.46, 95% CI=1.31 to 4.63). Moreover, when the ante-room was not disinfected, neither was the egg storeroom or the ground around the building (P<0.01). Vehicles entering the building to spread new litter was demonstrated to be a risk factor (HR=2.50, 95% CI=1.52 to 4.10). Moreover, the use of straw pellets for litter represents a protective factor (HR=0.37, 95% CI=0.18 to 0.76). The presence of many wild birds around the building was again strongly linked to time to seroconversion (HR=2.92, 95% CI=1.44 to 5.93).
Table 4. Results of univariate and multivariate Cox models performed to assess risk factors of AIV introduction in breeder duck flocks using artificial insemination in France.
Factors associated with time to seroconversion in flocks subject to natural mating
A univariate Cox model was used, and 14 out of 69 variables associated with the serological status of flocks were selected after pairwise comparisons at P≤0.10 (). The presence of other poultry farms within a 1-km radius of the farm was linked to seroconversion particularly when these farms produced ducks (HR=4.40, 95% CI=1.98 to 9.84) rather than other species (HR=3.49, 95% CI=1 to 12.23). Several variables related to disinfection increased the risk of flock contamination: the lack of regular disinfection of the egg storeroom (HR=4.86, 95% CI=1.21 to 19.55), of the ground around the building during the depopulated period (HR=4.07, 95% CI=1.42 to 11.66) and of the ante-room (HR=3.43, 95% CI=1.24 to 9.53). Disinfection of the building by the farmer rather than a hygiene specialist and no specific disinfection of the floor of the building during the depopulated period were also linked to the serological status at a threshold of 10% (HR=3.83, 95% CI=0.78 to 19, and HR=2.43, 95% CI=0.85 to 7, respectively). Manure spreading by the farmer or by a neighbour in the vicinity of the building during the previous month also emerged as a potential risk factor (HR=3.78, 95%, CI=1.48 to 9.68). Moreover, laying farms with multiple batches of ducks on the same site had a higher risk of the avian influenza virus being introduced than sites with a single batch (HR=3.54, 95% CI=2 to 6.24).
Table 5. Results of univariate Cox model performed to assess risk factors of AIV introduction in breeder duck flocks using natural mating in France at P≤0.10.
Discussion
Sampling and serological results
Two hundred of the 240 breeder duck farms in France were visited at least once during the study, which was almost exhaustive. Forty-one flocks were seropositive at the first visit showing that AIV infection can occur as early as during the rearing period. Flocks that were positive at first visit were excluded from the survey. Nevertheless some flocks, infected at the end of the rearing period but showing no seroconversion at first visit, might have been included in the study. However, experimental infection trials with HPAIV on ducks showed that the seroconversion could be detected from 7 days post infection (Maughan et al., Citation2013; Wibawa et al., Citation2013). As the first visit occurred on average 5 days after the transfer of ducks to the laying farm, only flocks infected during the last 3 days of the rearing period may have been included in the study; this risk could thus be considered very low. Over the 151 flocks included in the study, 56 flocks were seropositive. This result is higher than those obtained in previous surveillance programmes in France. Indeed, the latter investigated only subtypes H5 and H7 whereas the present study covered all subtypes of AIV. The epidemiological situation turned out to be different in the two kinds of breeder duck production; but the housing conditions and the duck management methods were also very different. Survival analyses were therefore carried out separately for the two production methods to identify specific risk factors in each production system. A survival model for all flocks had still been produced but it dealt only with general factors like the poultry house's environment that were not linked to the production method. The number of positive flocks was sufficient to produce multivariate models for all flocks and for flocks using artificial insemination, but not for natural mating flocks. While the results for the natural mating flocks are less significant than those obtained with multivariate models and should be interpreted with caution, they raise some interesting points.
Production method
Serological results indicate that flocks subject to artificial insemination were more at risk of infection with AIV than those using natural mating. The main difference between these productions is the species of ducks used, suggesting a difference in resistance to AIV infection between Pekin and Muscovy ducks. Recent experimental infection trials showed that the pathogenicity of H5N1 AIV strains may differ in Pekin and Muscovy ducks but the infection rates observed in the both species was similar (Phong et al., Citation2011; Cagle et al., Citation2012). In addition, these observations were carried out on HPAI viruses only, and to the best of our knowledge no comparative study on the infection by LPAIV viruses of different duck species has been carried out. The higher prevalence of the infection in flocks subject to artificial insemination could also be explained by insemination practices. The Pekin female ducks are inseminated twice a week, requiring staff to enter in the building several times. Thus insufficient compliance with biosecurity measures may allow the virus to be introduced into the building via people or materials. Moreover, on 78% of sites using artificial insemination, Muscovy males were kept either in the same building as females or in another one nearby. As males are regularly replaced, the introduction of new stock could contribute to AIV transmission, either directly or indirectly (via vehicles and/or staff). It has been shown that LPAIV H1N1 can infect turkeys via the intra-uterine or intra-cloacal route during artificial insemination (Pantin-Jackwood et al., Citation2010). Routine insemination (individual handling and manual eversion of the cloaca to locate the vagina to insert the insemination straw) could initiate infection through direct inoculation from infectious fomites on contaminated hands, or bird-to-bird transmission through mechanical fomite inoculation to the cloaca or reproductive tract by the inseminators. Moreover, twice-weekly insemination can cause stress, thereby increasing the females’ susceptibility to infection.
Disinfection practices
In our study, disinfection practices emerged as a critical factor in the introduction of AIV into buildings. Viruses shed via nasal secretions or faeces are relatively unstable in the environment and are easily destroyed by organic solvents and detergents. However, in field situations AIVs are protected by organic material such as nasal secretions or faeces, increasing their resistance to physical and chemical inactivation; according to types of viruses, they can survive 30 to 35 days at 4°C and to 7 days at 20°C in faeces, and 105 days in liquid manure during winter (Swayne & Halvorson, Citation2003). Experimental studies have also shown that LPAI H13N7 viruses can persist on feathers for up to 6 days at room temperature, and that the infectivity of the HPAI H5N1 virus persists in duck feather tissue for 120 days at 4°C and 15 days at 20°C (Tiwari et al., Citation2006; Yamamoto et al., Citation2010). Thus, material such as feathers, litter or the faeces of wild birds may continue to contaminate the environment for several days after the building has been emptied (Campitelli et al., Citation2004; Alexander, Citation2007). Disinfection of the ground around the building helps eliminate these sources of contamination and may decrease the risk of AIV entering buildings. Our data indicate that materials and/or weeds around the building decrease the efficacy of disinfection and that good up-keep and cleanliness of the site reduce the risk of infection. Good practice therefore involves clearing and disinfecting the area around buildings. Regular disinfection of the ante-room and egg-store also emerged as critical factors. Contamination could be introduced and spread between farms due to inadequate precautions when farm staff and carriers enter the building to remove egg trolleys or when clean and dirty trolleys are transported together in hatchery trucks. Thus regular disinfection of the ante-room and egg-store after introduction of new material is essential to avoid the introduction of the virus via boots, clothes and/or fomites. For example, the LP H13N7 influenza virus can persist up to 72 h on tiles, steel, gumboots and plastic at room temperature (Tiwari et al., Citation2006). Moreover, a significant association between layer-type poultry and the presence of HPAI virus was found during the highly pathogenic H7N7 infection in the Netherlands in 2003, and the underlying causal factor could have been inadequate hygiene measures taken by carriers between farm visits (Thomas et al., Citation2005). The univariate model for natural mating flocks shows that disinfecting the floor of the building with lime or caustic soda during the depopulated period decreased the risk of virus entry, certainly by eliminating all residual contaminated material. The multivariate model for artificial insemination flocks and the univariate model for natural mating flocks show that the risk of infection of the new flock decreased when the building was disinfected by a specialized firm, probably due to the use of more appropriate techniques.
Farming practices
We observed that vehicles entering the building occurred sufficiently frequently to emerge in the multivariate model for flocks using artificial insemination. Tractors driving only a few metres into the building to bring in litter multiplied the risk of flock contamination by 2.5 (95% CI=1.5 to 4.1). Moreover, the risk of infection was reduced when straw pellets were used; these were stocked in a silo, protected from external contamination sources, and were distributed via a pipe, removing the need for vehicles to enter the building. Multiple batches were shown to be significantly linked to serological status in the univariate model for natural mating flocks. On 74% of the sites in our study, the other buildings essentially contained breeder duck flocks, and the remainder housed pre-force-feeding ducks or pre-laying ducks. The presence of several buildings on a site may increase infective pressure and thus the risk of AIV introduction by animals, vehicles or people, as shown in the H7N7 outbreak in the Netherlands in 2003 (Thomas et al., Citation2005).
Wildlife
Evidence suggests that LPAIV could be primarily introduced into poultry populations by the activity of wild birds, usually waterfowl, although gulls and shorebirds have also been implicated. This can be through mechanical transfer of the virus via infective faeces by humans, other animals or material (Campitelli et al., Citation2004; Alexander, Citation2007). In our study, farmers often reported the presence of wild birds, particularly gulls, starlings and pigeons. The orders of Anseriformes and Charadriiformes, which include gulls, are considered to be wild reservoirs of AIV (Olsen et al., Citation2006; Munster et al., Citation2007). Using an experimental infection with the HPAI H5N1 virus, Brown et al. (Citation2008) found that gulls could contribute to the geographical dissemination of the virus. However, the main strains isolated from gulls are H13 and H16, which are genetically distinct from those found in other hosts (Munster et al., Citation2007), and therefore transmission of these strains to poultry may be limited (Brown et al., Citation2012). Webster et al. (Citation1992) established experimentally that the gene pool of AIV in shorebirds and gulls was different from that in Pekin ducks. Boon et al. (Citation2007) observed that experimental infection of European starlings with HPAI H5N1 was not fatal and that the birds shed the virus for 6 days, suggesting that they may act as an intermediate host and reservoir for the influenza A virus (H5N1), although there was little evidence of transmission to contact starlings. Studies on experimental infection of pigeons with HPAIV H5N1 indicate that these birds have limited susceptibility to this virus and shed only small amounts after infection with no transmission to contact birds. Their role in the ecology of the influenza A virus (H5N1) may therefore be minor (Panigrahy et al., Citation1996; Perkins & Swayne, Citation2002, Citation2003a, Citationb). However, Kopfleisch et al. (Citation2006) observed that pigeons could be more susceptible with higher doses and with other strains of H5N1. All these studies were carried out with the H5N1 virus, whereas our study investigated all subtypes of AIV. Nevertheless, gulls, starlings and pigeons may all play a role in introducing AI virus onto farms, particularly starlings, by shedding contaminated faeces. The presence of rodents in the building also emerged as a risk factor in our model, although their existence as feral reservoirs for AIV has never been demonstrated (Cardona et al., Citation2009). However, rodents could act as mechanical vectors. In natural mating flocks, the use of outside pest-control firms, which was more common on farms where rodents had been seen during the first visit, emerged as a risk factor; indeed, we observed that pest control (rodent bait checking) is carried out more regularly by farmers than by outside firms (on average 12 and five times a year, respectively). In addition, the staff from pest-control firms may also introduce AIV in the poultry house if proper biosecurity measures are not taken.
Effluents
In the multivariate model including all flocks, the existence of a liquid manure pit near the building increased the risk of AIV infection. Although excretion of the virus can be greater via the respiratory than the faecal route (Alexander & Gough, Citation1986), faeces are an important source of AIV. Moreover, the duration of this faecal excretion varies with the type of virus and host species, but it has been shown in the study that ducks inoculated with the H5N1 virus and those in direct contact with them could shed the virus for 11 to 17 days post infection (Hulse-Post et al., Citation2005). A slurry pit on site could thus be a physical reservoir of AIV for several days if a flock has shed the virus. When the pit is emptied, contaminated aerosols could be generated and infect or re-infect the flock in the building. Likewise, new flocks could be contaminated if the pit remains full between batches. Manure spreading also emerged as a risk factor in the univariate model for natural mating flocks. Spreading contaminated manure could contaminate the environment and production site.
Neighbourhood
The neighbourhood turned out to be an important risk factor in the models. The presence of other poultry farms within a 1-km radius of the farm could increase infective pressure in regions with high poultry density. Busani et al. (Citation2009) also suggested that a high density of poultry farms was a major factor in the highly pathogenic H7N1 epidemic in Italy in 1999 and 2000. Likewise, during the highly pathogenic H7N7 epidemic in the Netherlands in 2003, the chance of becoming infected was greatest in regions with a high density of poultry farms (Boender et al., Citation2007). Movement of staff and/or material between farms may contribute to spreading viruses if adequate biosecurity measures are not taken: the spread of viruses by vehicles was observed during epidemics in Pennsylvania in the 1980s and in Italy in 1999 and 2000 (Capua et al., Citation2003; Alexander, Citation2007). This hypothesis could not be confirmed in our study, but it is supported by the finding that the presence of poultry-processing industries within a 2-km radius was a risk factor in the model for flocks subject to natural mating.
Conclusion
To our knowledge, this is the first study that has investigated all AIV strains in duck flocks not during an outbreak. The prevalence of seroconversion observed might appear high but all AIV subtypes were targeted, and this finding could thus be considered consistent with those of the national surveillance programme. The number of infected flocks has to be reduced to avoid the potential spread and/or mutation of LPAIV to HPAIV. Our study shows that the external environment is probably regularly contaminated by AIVs shed by wild birds, or by human activity. However, for a duck flock to become infected, there must be a sufficient quantity of the virus persisting in the environment and transferred into buildings. Various precautions therefore have to be systematically respected, including correct cleaning and disinfection of the building surroundings and of the ante-room. The ante-room should also be divided into three zones (dirty, intermediate and clean) that must be systematically respected; the fact that artificial insemination emerged as a risk factor is probably due to the passage of people and/or materials. Likewise, the egg storeroom should be cleaned and disinfected after each delivery of new material. The study also shows that AIV can be introduced into the building by vehicles bringing in new litter. There are efficient alternative systems; for example, hanging trolleys that can be pushed to the edge of the building. The presence of rodents in the building and of wild birds near the building was also shown to be a risk factor. It is important to eradicate rodents from buildings in order to remove this potential route of mechanical transfer of AIV, and to avoid attracting birds on the site (without food under the silo, for example). By respecting all these simple measures, it should be possible to decrease AIV infection of breeder duck flocks.
Acknowledgements
The authors would like to thank Mr Geleon and Mr Le Doeuff and the hatcheries and breeders involved for their support that made this large-scale study possible. The project was funded by the Food Safety Department of the French Ministry of Agriculture.
References
- Akey, B.L. (2003). Low-pathogenicity H7N2 avian influenza outbreak in Virginia during 2002. Avian Diseases, 47, 1099–1103.
- Alexander, D.J. (2007). An overview of the epidemiology of avian influenza. Vaccine, 25, 5367–5644.
- Alexander, D.J. & Gough, R.E. (1986). Isolations of avian influenza virus from birds in Great Britain. The Veterinary Record, 118, 537–538.
- Alexander, D.J., Parsons, G. & Manvell, R.J. (1986). Experimental assessment of the pathogenicity of eight avian influenza A viruses of H5 subtype for chickens, turkeys, ducks and quails. Avian Pathology, 15, 647–662.
- Allison, P.D. (2001). Survival Analysis Using the SAS System: A Practical Guide 2nd ed. Cary, USA: SAS Publishing.
- Boender, G.J., Hagenaars, T.J., Bouma, T.J., Nodeljijk, G., Elbers, A.R., de Jong, M.C. & van Boven, M. (2007). Risk maps for the spread of highly pathogenic avian influenza in poultry. PLoS Computer Biology, 3, e71.
- Boon, A.C., Sandbulte, M.R., Seiler, P., Webby, R.J., Songserm, T., Guan, Y. & Webster, R.G. (2007). Role of terrestrial wild birds in ecology of influenza A virus (H5N1). Emerging Infectious Diseases, 13, 1720–1724.
- Brown, I.H. (2010). Summary of avian influenza activity in Europe, Asia and Africa. Avian Diseases, 54, 187–193.
- Brown, J., Poulson, R., Carter, D., Lebarbenchon, C., Pantin-Jackwood, M., Spackman, E., Shepherd, E., Killian, M. & Stallknecht, D. (2012). Susceptibility of avian species to North American H13 low pathogenic avian influenza viruses. Avian Diseases, 56, 969–975.
- Brown, J.D., Stallknecht, D.E. & Swayne, E.D. (2008). Experimental infections of herring gulls (Larus argentus) with H5N1 highly pathogenic avian influenza viruses by intranasal inoculation of virus and ingestion of virus-infected chicken meat. Avian Pathology, 37, 393–397.
- Busani, L., Valsecchi, M.G., Rossi, E., Toson, M., Ferre, N., Pozza, M.D. & Marangon, S. (2009). Risk factors for highly pathogenic H7N1 avian influenza virus infection in poultry during the 1999–2000 epidemic in Italy. Veterinary Journal, 181, 171–177.
- Cagle, C., Wasilenko, J., Adams, S.C., Cardona, C.J., To, T.L., Nguyen, T., Spackman, E., Suarez, D.L., Smith, D., Shepherd, E., Roth, J. & Pantin-Jackwood, M.J. (2012). Differences in pathogenicity, response to vaccination, and innate immune responses in different types of ducks infected with a virulent H5N1 highly pathogenic avian influenza virus from Vietnam. Avian Diseases, 56, 479–487.
- Campitelli, L., Mogavero, E., De Marco, M.A., Delogu, M., Puzelli, S., Frezza, F., Facchini, M., Chiapponi, C., Fioni, E., Cordioli, P., Webby, R.J., Barigazzi, G., Webster, R.G. & Donatelli, I. (2004). Interspecies transmission of an H3N7 avian influenza virus from wild birds to intensively reared domestic poultry in Italy. Virology, 323, 24–36.
- Capua, I., Marangon, S. & Cancellotti, F.M. (2003). The 1999–2000 avian influenza (H7N1) epidemic in Italy. Veterinary Research Communications, 27 Suppl. 1, 123–127.
- Capua, I. & Mutenelli, F. (2001). Mortality in Muscovy ducks (Cairina moschata) and domestic geese (Anser anser var. domestica) associated with natural infection with a highly pathogenic avian influenza virus of H7N1 subtype. Avian Pathology, 30, 179–183.
- Cardona, C.J., Xing, Z., Sandrock, C.E. & Davies, C.E. (2009). Avian influenza in birds and mammals. Comparative Immunology, Microbiology and Infectious Diseases, 32, 255–273.
- Davison, S., Galligan, D., Eckert, T.E., Ziegler, A.F. & Eckroade, R.J. (1999). Economic analysis of an outbreak of avian influenza, 1997–1998. Journal of American Veterinary Medical Association, 214, 1164–1167.
- European Commission. (2011). Annual Report for Avian Influenza Surveillance in Poultry in Member States of the European Union in 2010. Retrieved from http://ec.europa.eu/food/animal/diseases/controlmeasures/avian/annual_report_2010_poultry_en.pdf
- Harder, T., Teuffert, J., Starick, E., Gethmann, J., Grund, C., Fereidouni, S., Durban, M., Bogner, K.-H., Neubauer-Juric, A., Repper, R., Hlinak, A., Engelhardt, A., Nöckler, A., Smietanka, K., Minta, Z., Kramer, M., Globig, A., Mettenleiter, T.C., Conraths, F.J. & Beer, M. (2009). Highly pathogenic avian influenza virus (H5N1) in frozen duck carcasses, Germany, 2007. Emerging Infectious Diseases, 15, 272–279.
- Hulse-Post, D.J., Sturm-Ramirez, K.M., Humberd, J., Seiler, P., Govorkova, E. A., Krauss, S., Scholtissek, C., Puthavathana, P., Buranathi, C., Nguyen, T.D., Long, H.T., Baipospos, T.S., Chen, H., Ellis, T.M., Guan, Y., Peiris, J.S. & Webster, R.G. (2005). Role of domestic ducks in the propagation and biological evolution of highly pathogenic H5N1 influenza viruses in Asia. Proceedings of the National Academy of Sciences of the USA, 102, 10682–10687.
- Jeong, O.M., Kim, M.C., Kim, M.J., Kang, H.M., Kim, H.R., Kim, Y.J., Joh, S.J., Kwon, J.H. & Lee, Y.J. (2009). Experimental infection of chickens, ducks and quails with highly pathogenic H5N1 avian influenza virus. Journal of Veterinary Science, 10, 53–60.
- Jourdain, E., Gurnasson, G., Wahlgren, J., Latorre-Margelef, N., Brojer, C., Sahlin, S., Svensson, L., Waldenstrom, J., Lundkvist, A., & Olsen, B. (2010). Influenza virus in a natural host, the mallard: experimental infection data. PLoS One, 5, e8935.
- Kopfleisch, R., Werner, O., Mundt, E., Harder, T. & Teikfe, J.P. (2006). Neurotropism of highly pathogenic avian influenza virus A/chicken/Indonesia/2003 (H5N1) in experimentally infected pigeon (Columbia livia f. domestica). Veterinary Pathology, 43, 463–470.
- Löndt, B.Z., Nunez, A., Banks, J., Nili, H., Johnson, L.K. & Alexander, D.J. (2008). Pathogenesis of highly pathogenic avian influenza A/turkey/Turkey.1/2005 H5N1 in Pekin ducks (Anas platyrhynchos) infected experimentally. Avian Pathology, 37, 619–627.
- Machin, D., Campbell, M.J., Tan, S.B., Tan, S.H. (2009). Comparing survival curves. In Sample size tables for clinical studies 3rd edn. (pp. 84–101). Chichester, UK: Wiley-Blackwell.
- Maughan, M.N., Dougherty, L.S., Preskenis, L.A., Ladman, B.S., Gelb, J., Spackman, E.V. & Keeler, C.L. (2013). Transcriptional analysis of the innate immune response of ducks to different species-of-origin low pathogenic H7 avian influenza viruses. Virology Journal, 10, 94.
- McQuiston, J.H., Garber, L.P., Porter-Spalding, B.A., Hahn, J.W., Pierson, F.W., Wainwright, S.H., Senne, D.A., Brignole, T.J., Akey, B.L. & Holt, T.J. (2005). Evaluation of risk factors for the spread of low pathogenicity H7N2 avian influenza virus among commercial poultry farms. Journal of the American Veterinary Medical Association, 226, 767–772.
- Mundt, E., Gay, L., Jones, L., Saavedra, G., Tompkins, S.M. & Tripp, R.A. (2009). Replication and pathogenesis associated with H5N1, H5N2 and H5N3 low-pathogenic avian influenza virus infection in chickens and ducks. Archives of Virology, 154, 1241–1248.
- Munster, V.J., Baas, C., Lexmond, P., Waldenstrom, J., Wallensten, A., Fransson, T., Rimmelzwaan, G.F., Beyer, W.E., Schutten, M., Olsen, B., Osterhaus, A.D. & Fouchier, R.A. (2007). Spatial, temporal and species variation in prevalence of influenza A viruses in wild migratory birds. PLoS Pathogens, 3, e61.
- Olsen, B., Munster, V.J., Wallensten, A., Osterhaus, A.D. & Fouchier, R.A. (2006). Global patterns of influenza A virus in wild birds. Science, 312, 384–388.
- Panigrahy, B., Senne, D.A., Pedersen, J.C., Shafer, A.L. & Pearson, J.E. (1996). Susceptibility of pigeons to avian influenza. Avian Diseases, 40, 600–604.
- Pantin-Jackwood, M., Wasilenko, J.L., Spackman, E., Suarez, D.L. & Swayne, D.E. (2010). Susceptibility of turkeys to pandemic-H1N1 virus by reproductive tract insemination. Virology Journal, 7, 27.
- Perkins, L.E. & Swayne, D.E. (2002). Pathogenicity of a Hong Kong-origin H5N1 highly pathogenic avian influenza virus for emus, geese, ducks and pigeons. Avian Diseases, 46, 53–63.
- Perkins, L.E. & Swayne, D.E. (2003a). Comparative susceptibility of selected avian and mammalian species to a Hong Kong-origin H5N1 high-pathogenicity avian influenza virus. Avian Diseases, 47, 956–967.
- Perkins, L.E. & Swayne, D.E. (2003b). Varied pathogenicity of a Hong Kong-origin H5N1 avian influenza virus in four passerine species and budgerigars. Veterinary Pathology, 40, 14–24.
- Phong, D.Q., Dung, N.T., Jorgensen, P.H., Handberg, K.J., Vinh, N.T. & Christensen, J.P. (2011). Susceptibility of Muscovy (Cairina moschata) and mallard ducks (Anas plathyrynchos) to experimental infections by different genotypes of H5N1 avian influenza viruses. Veterinary Microbiology, 148, 168–174.
- Sharkey, K.J., Bowers, R.G., Morgan, K.L., Robinson, S.E. & Christley, R.M. (2008). Epidemiological consequences of an incursion of highly pathogenic H5N1 avian influenza into the British poultry flock. Proceedings of the Royal Society B Biological Sciences, 275, 19–28.
- Stegeman, A., Bouma, A., Elbers, A.R., De Jong, M.C., Nodeljijk, G., de Klerk, F., Koch, G. & Van Boven, M. (2004). Avian Influenza A virus (H7N7) epidemic in the Netherlands in 2003: course of the epidemic and effectiveness of control measures. Journal of Infectious Diseases, 190, 2088–2095.
- Swayne, D.E. & Halvorson, D.A. (2003). Influenza. In Y.M. Saif, H.J. Barnes, J.R. Glisson, A.M. Fadly, L.R. McDougald, D.E. Swayne, (Eds.) Diseases of Poultry 11th edn (pp. 135–160). Ames: Iowa State University Press.
- Thomas, M.E., Bouma, A., Ekker, H.M., Fonken, A.J., Stegeman, J.A. & Nielen, M. (2005). Risk factors for the introduction of high pathogenicity Avian Influenza virus into poultry farms during the epidemic in the Netherlands in 2003. Preventive Veterinary Medicine, 69, 1–11.
- Tiwari, A., Patnayak, D.P., Chander, Y., Parsad, M. & Goyal, S.M. (2006). Survival of two avian respiratory viruses on porous and nonporous surfaces. Avian Diseases, 50, 284–287.
- Webster, R.G., Bean, W.J., Gorman, O.T., Chambers, T.M. & Kawaoka, Y. (1992). Evolution and ecology of influenza A viruses. Microbiology Reviews, 56, 152–179.
- Wibawa, H., Bingham, J., Nuradji, H., Lowther, S., Payne, J., Harper, J., Wong, F., Lunt, R., Junaidi, A., Middleton & D., Meers, J. (2013). The pathobiology of two Indonesian H5N1 avian influenza viruses representing different clade 2.1 sublineages in chickens and ducks. Comparative Immunology, Microbiology and Infectious Diseases, 36, 175–191.
- Wood, G.W., Parsons, G. & Alexander, D.J. (1995). Replication of avian A viruses of high and low pathogenicity for chickens at different sites in chickens and ducks following intranasal inoculation. Avian Pathology, 24, 545–551.
- Yamamoto, Y., Nakalura, K., Yamada, M. & Mase, M. (2010). Persistence of avian influenza virus (H5N1) in feathers detached from bodies of infected domestic ducks. Applied and Environmental Microbiology, 76, 5496–5499.