Abstract
Newcastle disease, which is caused by Newcastle disease virus (NDV), is a highly contagious viral disease of poultry and other bird species. The mucosa is the first line of defence to invading pathogens, including NDV, and it has been confirmed that the mucosa can contribute to host protection. This study was conducted to evaluate the intestinal mucosal immunology in NDV infection. Forty specific-pathogen-free chickens were divided into two groups, 20 birds in each group. Group 1 was inoculated with NDV by the intravenous route. Group 2 was used as the control group and was given sterile phosphate-buffered saline by the same route. At 24, 48, 72, and 96 h post infection (h.p.i.), five chickens from each treatment were killed. Samples of the duodenum, jejunum, and ileum were collected to quantify intestinal intraepithelial lymphocytes (IEL), goblet cells and secretory IgA (sIgA) by cytochemistry and immunohistochemistry analysis. The results indicated that IEL were increased from 24 to 72 h.p.i. in the infected tissues, and were significantly higher than in the control group at 48 h.p.i. (P < 0.01). In contrast to IEL, goblet cell numbers were reduced dramatically from 24 to 96 h.p.i. in the infected birds (P < 0.01) Furthermore, the content of sIgA was significantly higher at 48 and 72 h.p.i. in the infected tissues (P < 0.01). sIgA positivity was observed in the epithelial lining of the intestinal mucosa. These data suggest that IEL, goblet cells, and sIgA were involved in the intestinal mucosal immunity against NDV infection.
Introduction
Newcastle disease, defined as a list A disease by the Office International des Epizooties, is a highly contagious avian disease, which causes severe economic losses in domestic poultry, especially in chickens (Alexander, Citation1995; Seal et al., Citation2000; Moncada et al., Citation2003). In China, Newcastle disease has been endemic since it was first reported in 1946. Since the 1980s, strict vaccination programmes have been practiced on poultry farms, so the presentation and prevalence of Newcastle disease has been both mild and sporadic. However, Newcastle disease virus (NDV) still causes a high mortality rate in some regions of China (Liu et al., Citation2007).
The mucosa has long been regarded as a physical barrier to invading pathogens, and studies over the last decade have shown that the mucosa contributes to host protection more than initially thought. Since the mucosal surface is the site of initial replication of a variety of viruses including NDV, induction of a local immune response against those viruses is expected to protect the animals from systemic infection, subsequent to the local infection (Bienenstock & Befus, Citation1980; Kagnoff, Citation1996). Evidence gathered from several independent studies suggests that the mucosa may directly influence adaptive immune responses (Neutra et al., Citation1996; Mowat & Viney, Citation1997).
As the largest mucosal tissue, the intestinal tract has been suggested to have the most important role in the immunologic homeostasis of the body (MacDonald & Monteleone, Citation2005). The main site of the mucosal immune system in the intestine is referred to as the gut-associated lymphoid tissue, with the immune-associated cells that include mast cells, goblet cells, secretory IgA (sIgA)-positive cells and intestinal intraepithelial lymphocytes (IEL) being involved in many processes to prevent pathogen invasion. The collaboration of those immuno-competent cells protects the animals from various infectious pathogens (Kagnoff, Citation1996; DeWitt & Kudsk, Citation1999). Recently, we found mast cells were increased significantly in NDV-infected birds and demonstrated that these cells were crucial in tissue damage induced by NDV (Sun et al., Citation2008, Citation2009). The present study was conducted to investigate the role of IEL, goblet cells, and sIgA in intestinal mucosal immunology during NDV infection.
Materials and Methods
Birds and experimental treatments
Forty 4-week-old specific pathogen free chickens (White Avian, Merial Co. Ltd, Beijing, China) were divided into two groups: 20 chickens in the control group, and 20 chickens in the NDV-infected group. NDV F48E8 strain was obtained from the China Institute of Veterinary Drug Control (Beijing, China). The virus was propagated in 9-day-old embryonated chicken eggs inoculated by the chorioallantoic route, and a dilution of approximately 105.0 embryo infectious doses was prepared. Each chicken in the infected group was inoculated intravenously with 0.1 ml NDV dilution. In the control group, chickens received the same volume of sterile phosphate-buffered saline (PBS) by the same route. All the chickens were provided feed and water ad libitum and this study was approved by the Institutional Animal Care and Use Committee of China Agricultural University.
Sampling
Five chickens from each group were randomly sampled at 24, 48, 72, 96 h post infection (h.p.i.). Tissues including the duodenum, jejunum, and ileum were collected and fixed in 2.5% glutaraldehyde–polyoxymethylene solution for 72 h for further analysis. Tissues were routinely processed into paraffin wax, and 4 µm sections were prepared for histological staining and immunohistochemical analysis.
Histological examination for intestinal intraepithelial lymphocytes
Sections were stained with haematoxylin and eosin and then sealed with a cover slip. The number of IEL in five different fields of an intestinal villus in each bird was counted for statistical analysis.
Measurement of goblet cells in the intestine with PAS staining
Goblet cells are the major source of mucin and are usually demonstrated using a PAS stain (Shatos et al., Citation2003). Serial histological sections (4 µm) were prepared using the same protocol described previously (Wang et al., Citation2009). After dewaxing and immediately washing with distilled water for 1 min, the specimens were immersed in 0.5% periodate solution (Sigma, Co., Beijing, China) for 5 min at room temperature in the dark. Sections were then immediately washed (30 sec × 2) and soaked in Schiff's solution at 37°C. After 60 min of incubation the sections were washed twice with a sulphurous acid solution, and then quickly rinsed with distilled water. The subsequent steps followed the laboratory's routine protocols. The number of goblet cells in different intestinal sections was counted using an Olympus light microscope (Olympus Optical Co, Beijing, China). Glycogen appeared as a prune colour and glucoprotein as pink.
Expression of secretory IgA with immunohistochemistry staining
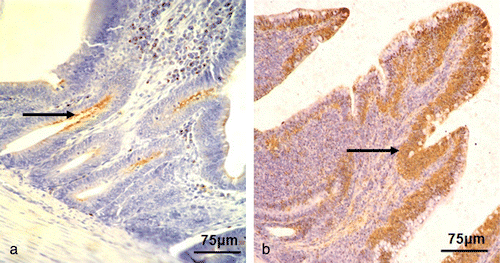
Expression of sIgA in the tissue samples was performed by immunohistochemical analysis. The histological sections (4 µm) were prepared using the same protocol as described above. Firstly, the sections were heated at 120°C for 10 min, naturally cooled for 30 min, and then incubated with 3% aqueous hydrogen peroxide for endogenous peroxidase ablation at room temperature for 30 min. The following steps were executed in a moist chamber. Sections were incubated with blocking buffer containing 20% normal bovine serum and 80% PBS (0.01 M, pH 7.4) at 37°C for 30 min. After discarding the bovine serum, the sections were incubated with the serum of mouse anti-chicken IgA monoclonal antibody (Southern Biotechnology, Inc., Beijing, China) for 2 h at 37°C, and the antibody was used at a dilution of 1:100 in PBS (0.01 M PBS, pH 7.4), which gave optimal staining. After 2 h at 37°C, the sections were washed in PBS (3 × 5 min) and subsequently incubated for 1 h at 37°C with biotinylated goat anti-mouse IgG (Sigma, Co.). After washing in PBS (3 × 5 min), the sections were incubated with a 1:200 dilution of Extr-avidin-conjugated horseradish peroxidase (Beijing Zhong Shan Golden Bridge Biotechnology, Co., Ltd, Beijing, China) for 30 min at 37°C. Then the sections were incubated with 3,3-diaminobenzidine (1:20 in distilled water; Zymed Co., South San Francisco, CA, USA) for 12 min and kept at room temperature after washing in PBS (3 × 5 min). After rinsing (3 × 5 min) in PBS, the tissues were counterstained with haematoxylin and dehydrated, cleared and mounted with neutral gums.
In parallel, tissue specimens, in which the primary antibody was omitted, served as negative controls. Specificity was established by demonstrating the loss of immunoreactivity in matched tissue sections.
IgA-positive cells were counted separately in serial sections of normal and NDV-infected tissues using ImagePro analysis program 4.0.1 (MicroOptic Industrial Group Co., Ltd., Beijing, China). The method was conducted as described previously (Sun et al., Citation2008). Results were expressed as the area density (area of the IgA-positive granules/area of the whole field).
Statistical analysis
Experimental data were analysed by the SPSS 13.0 statistical program (statistical product and service solutions, © 1999 to 2003; SPSS Institute Inc., Armonk, NY, USA). The results were expressed as means and standard errors. The control group represents all 20 birds across the four time-points. Differences were considered significant at P < 0.05 or P < 0.01.
Results
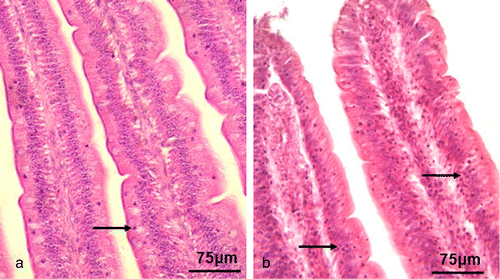
Distribution and prevalence of intestinal intraepithelial lymphocytes
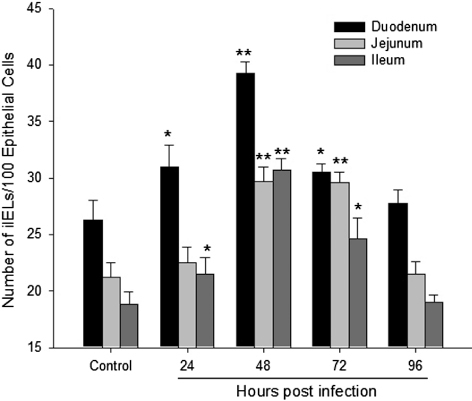
The IEL were randomly distributed among the endothelial cells of the intestinal epithelium (). As shown in , the number of IEL in the duodenum and ileum increased from 24 to 72 h.p.i. (P < 0.05), and was significantly higher than in the control tissue at 48 h.p.i. (P < 0.01). In the jejunum, the number increased dramatically from 48 to 72 h.p.i. (P < 0.01). There was no significant difference between the NDV-infected tissues and the control at 96 h.p.i.
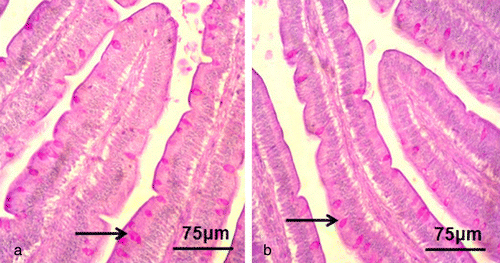
Distribution and prevalence of goblet cells
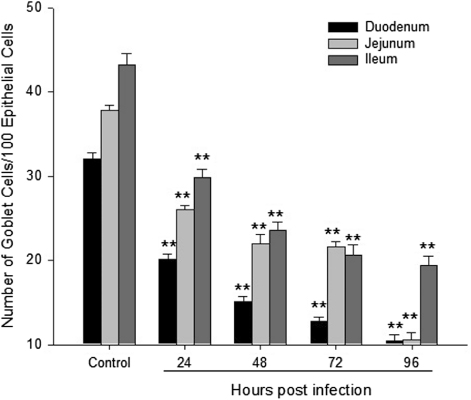
Goblet cells were mainly seen among the columnar cells and presented with the typical goblet shape (). shows that the numbers of goblet cells in the duodenum, jejunum and ileum were reduced significantly from 24 to 96 h.p.i. in the NDV-infected group compared with the control group (P < 0.01).
Distribution and prevalence of secretory IgA-positive cells
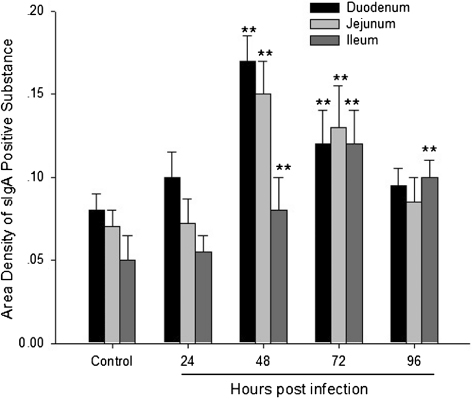
The sIgA-positive cells were mainly distributed in the epithelium of the intestinal crypts and villus of the intestine. There was significant difference in the distribution of sIgA-positive cells between the NDV-infected group and the control group ( and ). The number of sIgA-positive cells in the duodenum, jejunum, and ileum increased dramatically from 48 to 72 h.p.i. in the NDV-infected group compared with the control group (P < 0.01). Furthermore, the number of sIgA-positive cells in the ileum was significantly higher than that of the control group at 96 h.p.i. (P < 0.01) ().
Discussion
In addition to the mechanical barrier of the epithelial lining, host protection relies on a complex mucosal immune system. IEL are part of the intestinal mucosal immune system and constitute the first immune cell line of defence in the intestine. IEL have long been considered to serve a critical role in the mucosal immune system by performing a variety of regulatory functions, including cytokine production, cytotoxic activity, and induction of apoptosis in intestinal epithelial cells (Guy-Grand et al., Citation1991; Taguchi et al., Citation1991; Yamamoto et al., Citation1994; Inagaki-Ohara et al., Citation1997). It has been reported that chicken intestinal IEL and their cytokines released during a primary immune response against Eimeria parasites were primarily responsible for the protective immunity against subsequent secondary infections (Lillehoj & Trout, Citation1996). It has also been reported that the number of intestinal IEL expressing CD3, CD4, and CD8 significantly increased within 6 to 8 days following primary experimental infection with Eimeria parasites (Hong et al., Citation2006). The present study focused on IEL as a defender of the host against NDV infection. The results indicated that IEL were prevalent among endothelial cells of the intestinal epithelium and the number of IEL was significantly increased in NDV-infected duodenum, jejunum and ileum compared with that in the control group. Although IEL are increased during NDV infection, the exact role of IEL is unclear. It would be very interesting to determine the cell surface phenotype of the IEL. Further investigation is needed to examine the cytokine production by IEL and their role in maintaining the epithelial barrier during NDV challenge.
Considering the critical role that goblet cells appear to play in host defence against enteric pathogens, we evaluated the response of these cells to NDV infection. Intestinal goblet cells are highly polarized secretory cells that are present throughout the intestinal tract (Specian & Oliver, Citation1991). These specialized epithelial cells are thought to play an important protective role in the intestine by synthesizing and secreting several mediators, including mucin (Moncada et al., Citation2003) and the small peptide trefoil factor 3 (Taupin & Podolsky, Citation2003). Goblet cell depletion has been observed in infections with several enteric pathogens (Luperchio & Schauer, Citation2001). However, goblet cell depletion has not really been shown in response to enteric pathogens in the chicken. We observed that goblet cells were reduced significantly after NDV infection. The observation that these cells were depleted during NDV infection may have important implications with regards to the pathogenesis of this disease, as well as infection by other clinically important pathogens in other species, including Shigella (Steinberg et al., Citation1975; Sachdev et al., Citation1993) and Campylobacter (Lambert et al., Citation1979), where goblet cell depletion is also observed. However, whether the goblet cell depletion seen during NDV infection reflects the death or functional alteration of goblet cells is not clear. Moreover, further studies are required to evaluate the release of goblet cell mediators in this process.
sIgA is a major component of the local immune barrier of the intestine and is produced by B lymphocytes in the intestinal mucosa. sIgA plays a major role in protecting mucosal surfaces against colonization and possible invasion by pathogenic microorganisms (Mestecky & McGhee, Citation1987; Kraehenbuhl & Neutra, Citation1992; Brandtzaeg, Citation1996; Corthesy & Kraehenbuhl, Citation1999). Previous studies suggested that chicken sIgA, as mammalian sIgA, has a prominent role in first line defence and contributes to the maintenance of intestinal homeostasis (Wieland et al., Citation2004; Lammers et al., Citation2010). Our results showed that the expression of sIgA was significantly increased in NDV-infected tissues compared with the control group, which suggested that sIgA participated in the host defence against NDV infection. In addition, the distribution of sIgA was mostly located at the surface of the intestinal mucosa, which was the site of initial replication of NDV (Bienenstock & Befus, Citation1980; Kagnoff, Citation1996). It is possible that sIgA plays a protective role by inhibiting virus replication, as indicated in previous reports (Mazanec et al., Citation1993; Fujioka et al., Citation1998).
The present study has shown that IEL, goblet cells, and sIgA were involved in the intestinal mucosal response against NDV infection.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 30471301). The authors are particularly grateful to Professor Liu Jinhua and Jiang Taozhen, who supplied the viruses and bird material for use in this study.
References
- Alexander, D.J. (1995). The epidemiology and control of avian influenza and Newcastle disease. Journal of Comparative Pathology, 112, 105–126. 10.1016/S0021-9975(05)80054-4
- Bienenstock, J. & Befus, A.D. (1980). Mucosal immunology. Immunology, 41, 249–270.
- Brandtzaeg, P. (1996). History of oral tolerance and mucosal immunity. Annals of the New York Academy of Sciences, 778, 1–27. 10.1111/j.1749-6632.1996.tb21110.x
- Corthesy, B. & Kraehenbuhl, J.P. (1999). Antibody-mediated protection of mucosal surfaces. Current Topics in Microbiology and Immunology, 236, 93–111.
- DeWitt, R.C. & Kudsk, K.A. (1999). The gut's role in metabolism, mucosal barrier function, and gut immunology. Infectious Disease Clinics of North America, 13, 465–481. 10.1016/S0891-5520(05)70086-6
- Fujioka, H., Emancipator, S.N., Aikawa, M., Huang, D.S., Blatnik, F., Karban, T., DeFife, K. & Mazanec, M.B. (1998). Immunocytochemical colocalization of specific immunoglobulin A with Sendai virus protein in infected polarized epithelium. Journal of Experimental Medicine, 188, 1223–1229. 10.1084/jem.188.7.1223
- Guy-Grand, D., Malassis-Seris, M., Briottet, C. & Vassalli, P. (1991). Cytotoxic differentiation of mouse gut thymodependent and independent intraepithelial T lymphocytes is induced locally. Correlation between functional assays, presence of perforin and granzyme transcripts, and cytoplasmic granules. Journal of Experimental Medicine, 173, 1549–1552. 10.1084/jem.173.6.1549
- Hong, Y.H, Lillehoj, H.S, Lillehoj, E.P. & Lee, S.H. (2006). Changes in immune-related gene expression and intestinal lymphocyte subpopulations following Eimeria maxima infection of chickens. Veterinary Immunology and Immunopathology, 114, 259–272. 10.1016/j.vetimm.2006.08.006
- Inagaki-Ohara, K., Nishimura, H., Sakai, T., Lynch, D.H. & Yoshikai, Y. (1997). Potential for involvement of Fas antigen/Fas ligand interaction in apoptosis of epithelial cells by intraepithelial lymphocytes in murine small intestine. Laboratory Investigation, 77, 421–429.
- Kagnoff, M.F. (1996). Mucosal immunology: new frontiers. Immunology Today, 17, 57–59. 10.1016/0167-5699(96)80579-2
- Kraehenbuhl, J.P. & Neutra, M.R. (1992). Molecular and cellular basis of immune protection of mucosal surfaces. Physiological Reviews, 72, 853–879.
- Lambert, M.E., Schofield, P.F., Ironside, A.G. & Mandal, B.K. (1979). Campylobacter colitis. British Medical Journal, 1, 857–859. 10.1136/bmj.1.6167.857
- Lammers, A., Wieland, W.H., Kruijt, L., Jansma, A., Straetemans, T., Schots, A., den Hartog, G. & Parmentier, H.K. (2010). Successive immunoglobulin and cytokine expression in the small intestine of juvenile chicken. Developmental and Comparative Immunology, 34, 1254–1262. 10.1016/j.dci.2010.07.001
- Lillehoj, H.S., & Trout, J.M. (1996). Avian gut-associated lymphoid tissues and intestinal immune responses to Eimeria parasites. Clinical Microbiology Reviews, 9, 349–360.
- Liu, H., Wang, Z., Wu, Y., Zheng, D., Sun, C., Bi, D., & Xu, T. (2007). Molecular epidemiological analysis of Newcastle disease virus isolated in China in 2005. Journal of Virological Methods, 140, 206–211. 10.1016/j.jviromet.2006.10.012
- Luperchio, S.A. & Schauer, D.B. (2001). Molecular pathogenesis of Citrobacter rodentium and transmissible murine colonic hyperplasia. Microbes and Infection, 3, 333–340. 10.1016/S1286-4579(01)01387-9
- MacDonald, T.T. & Monteleone, G. (2005). Immunity, inflammation, and allergy in the gut. Science, 307, 1920–1925. 10.1126/science.1106442
- Mazanec, M.B., Nedrud, J.G., Kaetzel, C.S. & Lamm, M.E. (1993). A three-tiered view of the role of IgA in mucosal defense. Immunology Today, 14, 430–435. 10.1016/0167-5699(93)90245-G
- Mestecky, J., & McGhee, J.R. (1987). Immunoglobulin A (IgA): molecular and cellular interactions involved in IgA biosynthesis and immune response. Advances in Immunology, 40, 153–245.
- Moncada, D.M., Kammanadiminti, S.J. & Chadee, K. (2003). Mucin and Toll-like receptors in host defense against intestinal parasites. Trends in Parasitology, 19, 305–311. 10.1016/S1471-4922(03)00122-3
- Mowat, A.M. & Viney, J.L. (1997). The anatomical basis of intestinal immunity. Immunological Reviews, 156, 145–166. 10.1111/j.1600-065X.1997.tb00966.x
- Neutra, M.R., Pringault, E., & Kraehenbuhl, J.P. (1996). Antigen sampling across epithelial barriers and induction of mucosal immune responses. Annual Review of Immunology, 14, 275–300. 10.1146/annurev.immunol.14.1.275
- Sachdev, H.P.S., Chadha, V., Malhotra, V., Verghese, A. & Puri, R. K. (1993). Rectal histopathology in endemic Shigella and Salmonella diarrhea. Journal of Pediatric Gastroenterology and Nutrition, 16, 33–38. 10.1097/00005176-199301000-00006
- Seal, B.S., King, D.J. & Sellers, H.S. (2000). The avian response to Newcastle disease virus. Developmental and Comparative Immunology, 24, 257–268. 10.1016/S0145-305X(99)00077-4
- Shatos, M.A., Ríos, J.D., Horikawa, Y., Hodges, R.R., Chang, E.L., Bernardino, C.R., Rubin, P.A. & Dartt, D.A. (2003). Isolation and characterization of cultured human conjunctival goblet cells. Investigative Ophthalmology and Visual Science, 44, 2477–2486. 10.1167/iovs.02-0550
- Specian, R.D. & Oliver, M.G. (1991). Functional biology of intestinal goblet cells. American Journal of Physiology – Cell Physiology, 260, C183–193.
- Steinberg, S.E., Banwell, J.G., & Yardley, J.H. (1975). Comparison of secretory and histological effects of shigella and cholera enterotoxins in rabbit jejunum. Gastroenterology, 68, 309–317.
- Sun, Q., Li, W., She, R., Wang, D., Han, D., Li, R., Ding, Y. & Yue, Z. (2009). Evidence for a role of mast cells in the mucosal injury induced by Newcastle disease virus. Poultry Science, 88, 554–561. 10.3382/ps.2008-00468
- Sun, Q., Wang, D., She, R., Li, W., Liu, S., Han, D., Wang, Y. & Ding, Y. (2008). Increased mast cell density during the infection with velogenic Newcastle disease virus in chickens. Avian Pathology, 37, 579–585. 10.1080/03079450802499092
- Taguchi, T., Aicher, W.K., Fujihashi, K., Yamamoto, M., McGhee, J. R., Bluestone, J.A. & Kiyono, H. (1991). Novel function for intestinal intraepithelial lymphocytes: murine CD3+, γ/δ TCR+ T cells produce IFN-γ and IL-5. Journal of Immunology, 147, 3736–3744.
- Taupin, D. & Podolsky, D.K. (2003). Trefoil factors: initiators of mucosal healing. Nature Reviews Molecular Cell Biology, 4, 721–732. 10.1038/nrm1203
- Wang, D., Ma, W., She, R., Sun, Q., Liu, Y., Hu, Y., Liu, L., Yang, Y. & Peng, K. (2009). Effects of swine gut antimicrobial peptides on the intestinal mucosal immunity in specific-pathogen-free chickens. Poultry Science, 88, 967–974. 10.3382/ps.2008-00533
- Wieland, W.H., Orzaez, D., Lammers, A., Parmentier, H.K., Verstegen M.W. & Schots, A. (2004). A functional polymeric immunoglobulin receptor in chicken (Gallus gallus) indicates ancient role of secretory IgA in mucosal immunity. Biochemical Journal, 380, 669–676. 10.1042/BJ20040200
- Yamamoto, M., Fujihashi, K., Amano, M., McGhee, J.R., Beagley, K.W. & Kiyono, H. (1994). Cytokine synthesis and apoptosis by intestinal intraepithelial lymphocytes: signaling of high density αβ T cell receptor+ and γδ T cell receptor+ T cells via T cell receptor-CD3 complex results in interferon-γ and interleukin-5 production, while low density T cells undergo DNA fragmentation. European Journal of Immunology, 24, 1301–1306. 10.1002/eji.1830240609