Abstract
Clinically healthy racing pigeons may harbour notifiable pathogens and serve as an unnoticed reservoir. Thus, 3480 healthy racing pigeons from 172 different lofts were monitored over a period of 2 years for the presence of avian influenza virus (AIV) and avian paramyxovirus-1 (APMV-1). Pharyngeal and cloacal swabs as well as blood samples were collected from juvenile and adult pigeons. Pools of five pharyngeal swabs per loft and age group were initially screened by real-time reverse transcriptase-polymerase chain reaction (rRT-PCR). Pharyngeal and cloacal samples from lofts that were positive or suspect in the AIV rRT-PCR or the APMV-1 rRT-PCR were inoculated into embryonated chicken eggs for virus isolation. In addition, sera were examined for antibodies against AIV by enzyme-linked immunosorbent assay. The antibody levels after vaccination against APMV-1 were determined by haemagglutination inhibition assay. Of the investigated lofts, 0.0 to 1.4% were positive by rRT-PCR for APMV-1 and 0.0 to 6.7% for AIV during this 2-year period with a total of four samplings. No sample yielded replicating virus in egg culture. No antibodies against AIV were detected. Haemagglutination inhibition test of vaccinated racing pigeons indicated age-dependent APMV-1 titres. The results suggest that the examined racing pigeons may have had contact with AIV, but virus replication may have been too low to induce detectable circulating antibody levels. Only a low percentage of samples were positive for APMV-1, but two outbreaks were observed in monitored flocks, indicating ongoing circulation of APMV-1 in the racing pigeon population. These observations highlight the relevance of APMV-1 vaccination and indicate the importance of flock immunity.
Introduction
In many European Union countries, homing pigeons are kept in large numbers and often in close contact with humans, pet animals and commercial poultry. For example, approximately three million racing pigeons are kept in Germany according to the German Federation of Racing Pigeon Breeders (“Verband Deutscher Brieftaubenzüchter e.V.”, personal communication, 2013) and they may cover distances of over 500 kilometres during racing flights. Racing pigeons may serve as an epidemiological link between wild birds and poultry (Alexander, Citation2011), but their role in the distribution of pathogens and as reservoirs is not clear. In general avian influenza virus (AIV) H7N9 infects poultry only subclinically (Office International des Epizooties [OIE], Citation2013), and especially the detection of H7N9 in pigeons in China (Han et al., Citation2013) in the context of the recent fatal human cases (Gao et al., Citation2013; Li et al., Citation2013) emphasizes the need to investigate the epidemiology of AIV in pigeons.
In general only clinically healthy racing pigeons are allowed to compete, but subclinically infected birds are difficult to identify and may also participate in races. The circulation of pathogens is facilitated by the transportation of thousands of pigeons from different lofts in the same vehicle to the starting point. Thus pigeon races increase the risk of transmission (Alexander et al., Citation1984a, Citationb; Tudor, Citation1991).
According to the regulations of the German Federation of Racing Pigeon Breeders, avian paramyxovirus-1 (APMV-1) vaccination has been mandatory for birds participating in races since 1988. Nevertheless, outbreaks are common each year. In particular, juvenile and unvaccinated pigeons are affected (Rupiper, Citation1998). Experimental studies have shown that vaccination of pigeons reduces clinical signs but cannot restrict shedding completely (Duchatel & Vindevogel, Citation1986; Duchatel et al., Citation1992).
In general, pigeons may be infected with APMV-1 (Newcastle disease virus) (Hlinak et al., Citation1998; Wakamatsu et al., Citation2006) and are particular susceptible to a specific pigeon-type avian paramyxovirus (PPMV-1), which induces paramyxovirosis in this species (Alexander et al., Citation1984a). Remarkably, pigeons infected with Newcastle disease virus do not necessarily show signs and may serve as subclinical carriers (Wakamatsu et al., Citation2006; de Oliveira Torres Carrasco et al., Citation2008).
PPMV-1 is a variant of APMV-1 and can be differentiated antigenically by monoclonal antibodies (Lana et al., Citation1988; Collins et al., Citation1989) or with molecular techniques by analysing the fusion protein gene (F-gene) (Ballagi-Pordany et al., Citation1996; Aldous et al., Citation2003). Currently PPMV-1 is assumed to be enzootic in racing pigeons and pigeons may be considered as a reservoir for the regular outbreaks of PPMV-1 infections in European poultry flocks (Alexander, Citation2011). Pigeon-specific strains may have intracerebral pathogenicity indices > 0.7 (Alexander & Parsons, Citation1984; Hlinak et al., Citation1998) or may exhibit multibasic F2/F1 cleavage sites typical of virulent strains with an intracerebral pathogenicity indices < 0.7 (Meulemans et al., Citation2002; Dortmans et al., Citation2010) and are notifiable in poultry according to the World Organisation for Animal Health (OIE). Therefore it is of interest whether clinically healthy and vaccinated pigeons harbour this pathogen.
The literature concerning the susceptibility of pigeons for AIV remains contradictory (Kaleta & Honicke, Citation2004). Many authors stated that pigeons are resistant or minimally susceptible to infection with AIV (Panigrahy et al., Citation1996; Perkins & Swayne, Citation2002), but on the other hand natural infections of pigeons with highly pathogenic H5N1 were described (Ellis et al., Citation2004; Li et al., Citation2004; Songserm et al., Citation2006). An investigation in feral pigeons in Europe revealed that 12 (24%) of the 50 pigeons examined were positive for AIVs by nested reverse transcriptase-polymerase chain reaction (RT-PCR) (Gronesova et al., Citation2009).
To our knowledge there is no recent study about the distribution of AIV and APMV-1 in the German racing pigeon population, although clinical outbreaks of paramyxovirosis in racing pigeons are reported each year to our clinic. A monitoring study of urban and free-ranging pigeons in Germany from 2006 until 2008 demonstrated the presence of AIV antibodies in sera of two out of 123 wood pigeons. Furthermore, three isolates of PPMV-1 were obtained from urban pigeons during this study (Kohls et al., Citation2011). Data about the current situation of these two viruses in the German poultry population are regularly published by the Federal Ministry of Food, Agriculture and Consumer Protection. Highly pathogenic influenza virus had last been detected in poultry in 2007 and Newcastle disease virus in 2008. Low-pathogenic AIV outbreaks in poultry of different subtypes have been repeatedly detected during the last 5 years (BMELV, Citation2013). A variety of virological and molecular methods have been used to determine the incidence of AIV and APMV-1 in pigeons (Motha et al., Citation1997; Gronesova et al., Citation2009; Kohls et al., Citation2011). For our study a very sensitive method was needed because it is expected that clinically healthy pigeons may only harbour small amounts of virus. Furthermore, routine vaccination against APMV-1 is supposed to reduce shedding of field virus (Kapczynski et al., Citation2006). Moreover, even in experimental infections with high doses of AIV, pigeons excreted only minimal amounts of virus (Werner et al., Citation2007).
Effective screening of large numbers of samples can be done by PCRs, which are used for first-line screening in wild-bird monitoring for AIV (Munster et al., Citation2007; Rabl et al., Citation2009) and APMV-1 (Camenisch et al., Citation2008; Hoque et al., Citation2012).
The aim of our study was to investigate, over a 2-year period, the APMV-1 and AIV status of clinically healthy racing pigeon flocks by serology and virus detection methods. For this purpose, pigeon lofts were sampled twice in consecutive years during the racing season in summer and during the winter. Overall, 3480 pigeons from 172 different flocks were sampled. Sera were tested for APMV-1 and AIV specific antibodies by haemagglutination inhibition (HI) assay and enzyme-linked immunosorbent assay (ELISA), respectively. The APMV-1-vaccination status, housing conditions and performance in racing flights were assessed by questionnaires. Pharyngeal swabs were screened initially for viral RNA by real-time RT-PCRs (rRT-PCRs). Samples from lofts with cycle threshold (CT) values < 38 were further examined for viral isolation (Kalhoro et al., Citation2009) by inoculation of the virus transport medium of pharyngeal as well as cloacal swabs into embryonated chicken eggs.
Materials and Methods
Sampling of clinically healthy racing pigeons
A total of 3480 clinically healthy racing pigeons from 172 different lofts were sampled during a period of 2 years (2008 to 2010). The lofts belonged to randomly chosen racing flight associations (RVs) and were located in the German federal states of North Rhine–Westphalia, Lower Saxony, Brandenburg, Berlin and Saxony–Anhalt. These states were selected to obtain a representative cross-section of states with different traditions in keeping racing pigeons. North Rhine–Westphalia is known to have a very long tradition in breeding racing pigeons and overall the highest pigeon density of the former West German states, followed by Lower Saxony. Brandenburg together with the Berlin area and Saxony–Anhalt are representatives of former East German states, with comparable low numbers of pigeon flocks. Pigeon breeders participated voluntarily and the study was approved by the Animal Welfare Committee of Lower Saxony (licence number: 33.9-42502-05-08A567).
To evaluate whether the season or the attendance of racing flights influenced the detection rate of AIV or APMV-1, two samplings per year were conducted. One sampling was performed in winter during the breeding period (February to March) and the other during the racing season in summer (July to September). The first summer sampling had to be extended until November. From the 172 different lofts examined, 77 participated in all four samplings.
Age-related differences were only investigated during summer samplings due to the natural reproductive cycle. Juvenile pigeons were defined as pigeons younger than 1 year of age according to their foot ring.
Five pigeons per age group were sampled from each loft. One pharyngeal and one cloacal swab sample were collected per pigeon as well as one blood sample. The swabs were placed immediately in 1 ml virus transport media (VTM), pH 7.2, consisting of the following reagents (Biochrom AG, Berlin, Germany): 80 µl BME-Earle, 100 µl tryptose phosphate buffer, 20 µl hepes (1 M), 8 µl penicillin–streptomycin (10,000 u/ml; 10,000 µg/ml), 4 µl amphotericin B (250 µg/ml), 10 µl l-glutamine (200 mM), 10 µl NaOH (1 M) and 768 µl Ampuwa (Fresenius Kabi AG, Bad Homburg, Germany). The samples were transported on ice, and swabs from each age group and loft were pooled for further investigations. Serum was stored at -20°C whereas swabs were stored at −80°C until processing.
Samples from clinical APMV-1 outbreaks
During the whole sampling period two participating racing pigeon lofts suffered from APMV-1 outbreaks in 2009. The first case was confirmed in July. Affected juvenile pigeons had been vaccinated 10 days before the outbreak. One month after the outbreak the loft was sampled for this study. The apparently clinically healthy pigeons from this loft were sampled according to the standard protocol described above. However, one juvenile pigeon died on the day of sampling after a history of clinical disease. This bird was necropsied but only the kidney was made available for further virological investigations and tested by rRT-PCR for APMV-1.
Another outbreak in a participating racing pigeon loft in October 2009 was also confirmed as APMV-1-positive by rRT-PCR, 3 months after the regular sampling of this loft. Both unvaccinated adult and juvenile pigeons were affected and six blood samples were collected. Additionally, pharyngeal swabs, kidneys and brains of two diseased pigeons were examined for APMV-1.
In both cases, infection with Salmonella ser. Typhimurium var. Copenhagen was excluded.
RNA extraction
A maximum of five pharyngeal swabs of the same loft and age group were pooled. RNA was extracted from swabs with peqGOLD TriFast™ (peqlab, Erlangen, Germany) whereas RNA from allantoic fluid or VTM was extracted using peqGOLD TriFast™FL (peqlab). The isolation of RNA was performed according to the manufacturer's instructions, modified by the addition of an internal control RNA (INTYPE IC-RNA; Labor Diagnostik Leipzig, Leipzig, Germany). Briefly, after sample lysis 3 µl in vitro-transcribed internal control RNA (8 × 105 copies/µl) was added to determine the efficiency of RNA extraction (elution volume 30 µl) and rRT-PCR.
As the pharyngeal swabs of the first sampling were used for the detection of other pathogens (data not shown), RNA was extracted from VTM. In contrast, swabs from samplings two to four were directly used for RNA isolation since pre-trials showed a higher RNA yield in swabs compared with the VTM (data not shown).
RNA was immediately used for AIV rRT-PCR and stored afterwards at -80°C.
Avian influenza virus real-time RT-PCR
In total, 703 pooled samples of pharyngeal swabs were screened for AIVs by targeting part of the matrix (M) gene in an AIV-specific rRT-PCR described by Spackman et al. (Citation2002), which was modified by the introduction of an internal control system (Gall et al., Citation2008). The internal control system had been reported previously (Hoffmann et al., Citation2006). The Ambion® AgPath-ID™ One-Step RT-PCR kit (Life Technologies, Carlsbad, California, USA) was used with a final volume of 25 µl reaction mix.
For one reaction, the mix contained 3.25 µl RNase-free water, 12.5 µl reaction mix (2× RT-PCR buffer), 0.5 µl M-gene specific forward and reverse primer (10 pmol/µl), 0.75 µl internal control forward and reverse primer (10 pmol/µl), 0.25 µl M-gene specific probe (FAM-labelled,10 pmol/µl), 0.5 µl internal control probe (HEX-labelled,10 pmol/µl), 1 µl enzyme mix (25× RT-PCR Enzyme Mix) and 5 µl template RNA. The AIV rRT-PCR was conducted using the Stratagene MX 3005P rRT-PCR detection system (Stratagene, La Jolla, California, USA) with the following temperature profile: one cycle at 45°C for 10 min and 95°C for 10 min, followed by 40 cycles at 95°C for 15 sec and 57°C for 45 sec. All samples but the positive and negative controls were tested in duplicate. The data are presented as mean CT values. Samples were considered as inhibited if they showed either no HEX signal or both replicates displayed a CT value > 35 for the internal control (HEX).
Based on the internal control, 25 of 703 pooled samples were inhibited. Hence, 678 pooled samples were included in the result analysis. Samples with CT values < 35 were considered positive (Rabl et al., Citation2009).
Avian paramyxovirus-1 real-time RT-PCR
Our aim was to follow the infection status of the 77 lofts that had participated in all four samplings to correlate possible positive results with vaccination and management approaches. Therefore, the isolated RNA from the pharyngeal swab pools of only these 77 lofts was tested. Samples that showed either inhibition in the previously performed AIV rRT-PCR or were suspicious for AIV were not examined (total n = 16). Since seven lofts did not provide juvenile pigeons in summer samplings, a total number of 439 pooled samples were investigated for the presence of APMV-1.
A real-time RT-PCR detecting the polymerase (L) gene of APMV-1 was performed as described previously by Fuller et al. (Citation2010). Samples were amplified in a one-step APMV-1 rRT-PCR using the same real time kit (Ambion® AgPath-ID™ One-Step RT-PCR; Life Technologies) as described for AIV detection. The reaction mix with a final volume of 25 µl for one sample contained: 3 µl RNase-free water, 12.5 µl reaction mix (2× RT-PCR buffer), 1.875 µl forward primer (NDF; 10 pmol/µl), 0.625 µl reverse primer (NDR; 10 pmol/µl), 0.5 µl each probe (NDpro1, NDpro2; 1.25 pmol/µl), 1 µl enzyme mix (25× RT-PCR Enzyme Mix) and 5 µl template RNA. The real-time thermal cycler, the number of cycles performed and the temperature profile were identical with the protocol for AIV, except for a lower annealing–elongation temperature of 55°C. Samples were tested in duplicate and data are presented as mean CT values, with positive samples exhibiting CT values < 35.
Virus isolation
The VTM of pharyngeal swabs of lofts that showed CT < 38 in the AIV or AMPV-1 rRT-PCR were examined for replicating virus (Kalhoro et al., Citation2009). Samples were supplemented to reach a final concentration of 176.5 U/ml penicillin, 176.5 µg/ml streptomycin and 3.4 µg/ml amphotericin B.
Additionally, the VTM of cloacal swabs from the same loft and age group were processed equally. Hence, two 9-day-old to 10-day-old specific pathogen free-embryonated chicken eggs (VALO BioMedia GmbH, Osterholz-Scharmbeck, Germany) were inoculated per loft with 0.1 ml sample via the allantoic sac route. Amino-allantoic fluid was harvested from all embryos dying later than 24 h post inoculation or from surviving embryos after 6 days and were tested for haemagglutinating activity. Samples that showed no haemagglutination were passaged a second time by inoculation of 0.2 ml amino-allantoic fluid into embryonated eggs. Embryo deaths within 24 h post inoculation were regarded as non-specific and the test was repeated.
Haemagglutination
The microplate haemagglutination test was performed as described by the OIE (Citation2009) with the modification that sterile NaCl solution was used instead of phosphate-buffered saline. Results are expressed as reciprocal values (log2 X) of the highest dilution, which showed complete haemagglutination.
Confirmation and characterization of APMV-1 samples
All APMV-1 organ samples of the clinical outbreaks were sent to the national reference laboratory for APMVs (Friedrich Loeffler-Institute, Insel Riems, Germany) for further characterization. All samples were confirmed as APMV-1 by M-gene and F-gene specific rRT-PCR as described by Wise et al. (Citation2004). Positive samples were sequenced directly on an amplified part of the F-gene as published by Aldous et al. (Citation2004) and used for subsequent determination of amino acids of the proteolytic cleavage site (OIE, Citation2009) and typing of subtypes by applying a BLAST search (NCBI; Rockville Pike, Maryland, USA).
Haemagglutination inhibition assay
The HI assay was also performed according to the OIE (Citation2009) with the same modifications as described above. The strain Montana was used as antigen (Knoll et al., Citation1986). The highest dilution, which showed complete HI, is shown as a reciprocal value (log2 X). Serum samples with HI titres ≥log2 4 are considered positive according to the OIE (Citation2009). A total of 3423 serum samples originating from 2271 adults and 1152 juvenile pigeons were tested for anti-APMV-1 antibodies.
Enzyme-linked immunosorbent assay
Serum samples from adult and juvenile pigeons of lofts that showed a positive or suspect AIV rRT-PCR result (n = 268) as well as 180 randomly chosen sera were tested by a commercial competitive multispecies influenza A ELISA (Type A Influenza Multi species Antibody Test Kit (AI MSp); BioCheck, London, UK) according to the manufacturer's instructions. Since the ELISA was not licensed for use on pigeon sera, further controls were included. Known AIV antibody-positive pigeon sera were included in the test, which were generously provided by Christian Grund, Friedrich Loeffler-Institute, Isle of Riems, Germany and Thierry van den Berg, Veterinary and Agrochemical Research Center in Ukkel, Belgium. These sera had been collected from pigeons that were experimentally infected either with H7N7 (A/tky/Egl/647/77) by the Friedrich Loeffler-Institute or with H5N1 (A/crested_eagle/Belgium/01/2004) by the Veterinary and Agrochemical Research Center.
Questionnaires
A total of 466 questionnaires were collected from the pigeon breeders to assess information about dates of vaccination against various pathogens, age, previous infections with APMV-1 and possibilities of contact with other animals including wild birds penetrating loft barriers.
Analysis of serum samples for APMV-1 antibodies
Only pigeons with known vaccination status were included in the analysis of APMV-1 antibody titres. According to the questionnaires, 876 juvenile pigeons had already been vaccinated before the time of sampling. Moreover, only sera of adult pigeons that had been vaccinated less than 12 months before the sampling were analysed since all manufacturers recommend an annual boost vaccination. In addition, for analysis of the results the sampled pigeons were allocated to subgroups: pigeons solely used for rearing (breeding pigeons), partners of racing pigeons (partner pigeons) or pigeons that participated in races. The allocation of 2057, the age of 2044 and the specific vaccine used for 1997 adult vaccinated pigeons was specified in the questionnaires and included in the comparative analysis of titres.
Statistical methods
Statistical analyses were carried out with the analysis software Statistix 9.0 (Analytical Software Tallahassee, Florida, USA) and with SAS, version 9.2 (2006; SAS Institute Inc., Cary, North Carolina, USA). Factors with P values < 0.05 in Kruskal–Wallis one-way analysis of variance with all-pairwise comparisons, in Wilcoxon rank-sum test or Wilcoxon two-sample test were considered statistically significant.
Results
Description of the sampled pigeons and participating lofts
Overall, the sampled pigeons consisted of 71% actively racing pigeons, 21% breeding pigeons and 8% partner pigeons. At the time of sampling, 76% of juvenile pigeons had already been vaccinated. Ninety per cent of adult pigeons had been APMV-1 vaccinated within the last 12 months. Five different inactivated vaccines had been used for APMV-1 vaccination during our study according to the questionnaires. Four vaccines were based on the vaccine strain LaSota. One of these vaccines also contained a live pigeon pox strain for simultaneous vaccination against both diseases. The fifth vaccine included a pigeon variant of APMV-1 (PPMV-1 strain P201). From the participating 172 different pigeon breeders, nine (5.2%) reported cases of paramyxovirosis within the last 5 years. During this time span, two of these lofts suffered from more than one outbreak.
The average number of pigeons housed in the participating lofts varied between 132 (standard deviation ± 53) in summer and 111 (standard deviation ± 54) in winter. Factors that may influence the spread of pathogens by racing pigeons are shown in . A trend in seasonal difference was observed between winter and summer samplings concerning the contact with wild animals in general (25 to 28% vs. 33 to 38%) and with wild birds in particular (45 to 52% vs. 73 to 74%). Although not statistically significant, the probability of contact was higher in trend during summer compared with winter samplings.
Table 1. Descriptive analysis of questionnaires collected from racing pigeon lofts (2008 to 2010).
Detection of APMV-1 antibodies in clinically healthy and vaccinated racing pigeons by haemagglutination inhibition
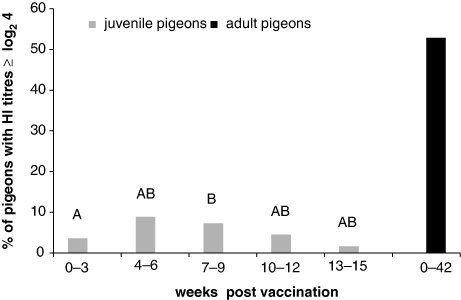
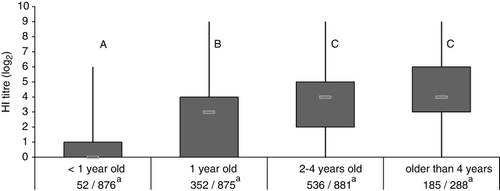
APMV-1-positive sera were already detected in juvenile pigeons within the first 3 weeks after immunization. During this 3-week time span, three of 84 analysed samples were positive. An increase in the percentage of HI titre-positive juvenile pigeons was observed up to 4 to 6 weeks post vaccination and declined over the next months (). In contrast, between 40.0 and 68.8% of vaccinated adult pigeons had HI titres ≥ log2 4 for over 10 months. Overall, only 5.9% of 876 investigated samples from vaccinated pigeons younger than 1 year old were positive according to the definition of the OIE (HI titre ≥ log2 4) whereas more than one-half (52.6%) of the 2057 vaccinated adult pigeons were positive for APMV-1 antibody. Age-related differences were also seen in HI titres, which significantly increased up to 2 years (). Comparing antibody titres, pigeons that participated in races had significantly higher APMV-1 titres with a mean of log2 3.3 (± 2.3) compared with partner pigeons with a mean of log2 2.8 (± 2.4) (Wilcoxon two-sample test, P < 0.05) and also higher levels compared with breeding pigeons (mean: log2 3.0 [± 2.4]). The vaccine based on the pigeon variant induced in adult pigeons significantly higher HI titres (mean: 4.0 ± 2.4) in comparison with each of three (mean: 3.1 ± 2.3, mean: 3.2 ± 2.2, and mean: 3.2 ± 2.4) out of four (mean: 3.4 ± 2.2) LaSota-based preparations (Wilcoxon rank-sum test, P < 0.05).
Avian paramyxovirus-1 detection in clinically healthy racing pigeons
Two of 439 pooled samples from racing pigeon lofts were APMV-1-positive by rRT-PCR (). The CT values were 33 and 34, respectively, but no virus could be isolated.
Table 2. Detection of APMV-1 and AIV by rRT-PCR in clinically healthy racing pigeon lofts.
Avian paramyxovirus-1 detection in clinically sick racing pigeons
Clinical signs indicative for PPMV-1 were observed in two participating lofts and samples were included in this study. All examined samples originated from clinically sick juvenile birds, which were infected in the second half of the year (July, October). The CT values for APMV-1 in brains and kidneys ranged from 12 to 25 whereas CT values from pharyngeal swabs taken from the same pigeons (n = 2) displayed CT values ranging between 24 and 28. The rRT-PCR results were confirmed using rRT-PCRs targeting the M-gene and virulent pathotype specific F-gene sequences (Wise et al., Citation2004). Subsequent direct sequencing of PCR products of organ samples revealed multiple basic amino acids and phenylalanine at the cleavage site (GGRRQKR*FIG), characteristic for virulent APMV-1.
Phylogenetic analyses showed that the obtained isolates clustered within class 2 APMV-1 in genotype VIb, and belonged to subgroup f (Aldous et al., Citation2004) (data not shown). Furthermore, the outbreaks were caused by two independent PPMV-1 strains.
Three of six serum samples from diseased pigeons showed no detectable antibodies whereas three sera yielded HI titres (log2) of 4, 6, and 8 respectively.
Detection of AIV antibodies in clinically healthy racing pigeons by ELISA
No specific AIV antibodies were detected by ELISA in the selected 448 serum samples.
Avian influenza virus detection in clinically healthy racing pigeons
Overall, 13 lofts were tested positive by generic AIV-M rRT-PCR (). The detected CT values ranged between 31 and 35. In six lofts, AIV was detected in pharyngeal swabs of adult pigeons and in seven lofts in samples of juvenile pigeons but no replicating virus could be isolated.
Discussion
This study focused on the APMV-1 and AIV infection status of apparently healthy racing pigeons considering that subclinically infected racing pigeons may serve as an unnoticed threat for commercial poultry and may spread notifiable pathogens during their racing flights. Furthermore, PPMV-1 is known to circulate in domesticated and wild pigeons (Alexander, Citation2011) and field virus may be present even in vaccinated racing pigeons without affecting their performance (Rupiper, Citation1998). Therefore it is of concern that APMV-1 may circulate unnoticed in vaccinated birds due to the lack of clinical signs (Capua et al., Citation2002). This may be a threat for unvaccinated pigeons or for birds that did not achieve sufficiently protective immunity.
According to the questionnaires, racing pigeons had manifold possibilities of contact with other animal species. Despite this, the detection rates for AIV and APMV-1 specific RNA were low with 0 to 6.7% and 0 to 1.4% of lofts positive, respectively.
In this study, we used oropharyngeal swabs for virus screening by rRT-PCR to avoid interference with possible PCR inhibitors that may have occurred within cloacal swabs (Das et al., Citation2006; Spackman & Suarez, Citation2008). Cloacal swabs were only investigated for virus isolation of samples from suspicious lofts. However, no sample was positive by virus isolation. This discrepancy between the results of rRT-PCR and virus isolation has been described in previous surveillance studies (Munster et al., Citation2007; Terregino et al., Citation2007; Ip et al., Citation2008; Haynes et al., Citation2009; Jindal et al., Citation2010). Probably transport, storage as well as repeated thawing affected the quality of the sample, avoiding the isolation of replicating virus or further characterization including the identification of subtypes or pathogenicity markers (Hoffmann et al., Citation2009). Furthermore, the high CT values detected in our study relate to low amounts of virus or virus particles in the samples (Munster et al., Citation2007) presumably not sufficient for detection of replicating virus in embryonated eggs (Jindal et al., Citation2010). Possibly, cell culture systems would have supported viral replication in virus-positive swabs, and should be considered in future studies.
The detection rate of APMV-1 found in this study is in accordance with the findings of Knoll et al. (Citation1986), who isolated the virus in 0.8% of examined racing pigeons. Overall, the low detection rate of APMV-1 may be due to routine vaccination of racing pigeons. APMV-1 vaccination may lead to a reduction in the number of pigeons that shed virus after infection (Duchatel et al., Citation1992; Carrasco et al., Citation2009) as well as the amount of shed virus (Kapczynski et al., Citation2006). The importance of an appropriate vaccination programme is supported by the observation of APMV-1 outbreaks in improperly vaccinated lofts during our study. The outbreak in July was probably associated with the racing season (Alexander et al., Citation1984b). Older vaccinated pigeons might have served as subclinical carriers of field virus, and transmitted APMV-1 to juvenile birds. We may speculate that despite vaccination 10 days before the outbreak, vaccine-induced anti-APMV-1 immunity may still have been insufficient to protect juvenile birds of the loft. In the second outbreak in October 2009, the virus might have been introduced by newly purchased birds as reported by the pigeon breeder. Only unvaccinated pigeons showed signs, which highlights the importance of vaccination of all pigeons in a loft (Alexander et al., Citation1986).
The strains from the outbreaks were identified as mesogenic to velogenic pathotypes, which is typical for PPMV-1 isolates (Hlinak et al., Citation1998). Moreover, the isolates were further characterized and identified as PPMV-1 with the amino acid sequence 112RRQKRF117 at the cleavage site. This amino acid motif is found in the majority of PPMV-1 isolates (Meulemans et al., Citation2002; Alexander, Citation2011).
Our findings are in accordance with recent phylogenetic analyses of APMV-1 isolates collected from pigeons in Germany in 2008 to 2012. All of the detected APMV-1 isolates clustered within genotype VIb, lineage d or f, and confirmed the co-circulation of different strains in the German pigeon population (Grund et al., Citation2013). Detection of APMV vaccine strains by rRT-PCR can be excluded since all licensed vaccines for pigeons are inactivated (Bönner et al., Citation2003) and have to be applied subcutaneously. Unfortunately, the HI test does not allow the differentiation between antibodies due to a natural APMV-1 infection or vaccination. Since only two samples from clinically healthy pigeons were positive for APMV-1 by rRT-PCR, no correlation between antibody level and infection status could be calculated. Interestingly, only 25.2 and 74.9% of sera from juvenile and adult pigeons, respectively, showed detectable and overall relatively low antibody levels after vaccination. This could be due to the overall poor ability of pigeons to mount an antibody response compared with other species (Guttman et al., Citation1971; Higgins, Citation1996). Other authors confirmed the low rate of seroconversion and low HI titres after APMV-1 vaccination of pigeons (Duchatel et al., Citation1992; Bönner et al., Citation2003). Nevertheless, circulating HI titres should not be over-interpreted as they cannot be reliably used to estimate protection against a challenge (Duchatel et al., Citation1992; Seal et al., Citation2000). The local innate immunity and also acquired immunity at the site of virus entry is considered the first line of defence against APMV-1 (Al-Garib et al., Citation2003). Besides the importance of the local humoral immunity, studies in poultry have clearly demonstrated the role of cell-mediated mechanisms in protection against APMV-1 (Kapczynski et al., Citation2013), which is difficult to determine in pigeons due to a lack of specific reagents.
The APMV-1 antibody response of juvenile racing pigeons was lower than that of adult pigeons (Tsai & Lee, Citation2006), possibly due to the still developing immune system and the fact that only a single vaccination with an inactivated vaccine was applied. In contrast, adult pigeons had been boosted annually by vaccination and perhaps even by PPMV-1 field exposure, with racing pigeons showing the highest mean APMV-1 antibody titres. We may speculate that pigeons taking part in free flights for training or racing are more prone to APMV-1 field exposure, which may even lead to a stronger humoral response than the application of an inactivated vaccine.
Overall, from 678 investigated samples, 1.9% were AIV-positive by rRT-PCR. Other authors reported similar AIV prevalence in domesticated pigeons in China ranging from 0.5% (Liu et al., Citation2003a) to 2.9% (Guan et al., Citation2000). In contrast, the majority of studies of feral or wild pigeons detected no AIV by virus isolation, RT-PCR or real-time RT-PCR (Motha et al., Citation1997; Lillehaug et al., Citation2005; Kohls et al., Citation2011). The detection of AIV by rRT-PCR in this study suggests that racing pigeons had contact with the virus but presumably no or very low replication and shedding occurred. Hence no isolation of AIV was possible in embryonated eggs, and no humoral response could be detected. Owing to the low virus copy numbers in the samples, the determination of AIV subtypes was impaired. Low AIV replication and shedding in pigeons has been demonstrated previously in AIV infection studies in pigeons by the lack of AIV-infected sentinel animals (Werner et al., Citation2007; Smietanka et al., Citation2011; Yamamoto et al., Citation2012). Moreover, an experimental infection of pigeons with a high dose of HPAIV H5N1 led only to minute excretion of the virus in contrast to the marked shedding of inoculated chickens (Werner et al., Citation2007). Infection of pigeon tracheal organ cultures with different AIV subtypes resulted in the lowest viral titres and failed to induce ciliostasis in comparison with AIV-infected tracheal organ cultures of Pekin duck, chicken or turkey (Petersen et al., Citation2012).
The respiratory tract of pigeons shows mainly α-2,6-linked and no α-2,3-linked sialic acids like in chickens (Liu et al., Citation2009). As AIV preferentially binds to α-2,3-linked sialic acids (Rogers et al., Citation1983), we may expect reduced AIV susceptibility of the pigeon. Furthermore, the innate immune response of the host may affect its susceptibility for the establishment of AIV infection (Cornelissen et al., Citation2012). Different to chickens, pigeons—like ducks, which exhibit a certain natural resistance to AIV—possess the retinoic acid-inducible gene I, which triggers a strong antiviral interferon response (Barber et al., Citation2010) possibly controlling AIV replication in a sufficient way. The source of AIV infection in the examined pigeons remains unclear. Transmissions due to contact with wild birds seems the most likely. Nevertheless, a transmission of human influenza virus by breeders cannot be fully excluded. Previous studies have documented the possible transmission of H1N1 from humans to poultry (Liu et al., Citation2003b; Reid et al., Citation2012) and companion animals (Trujillo et al., Citation2012). The applied AIV rRT-PCR can also detect human influenza viruses (Spackman et al., Citation2002), and transmissions from humans to pigeons seem possible (Romvary & Tanyi, Citation1975; Romvary et al., Citation1976). Moreover, antibodies against human influenza virus have been detected in pigeons (Zupančić et al., Citation1986; Abubakar et al., Citation2008) and the AIV receptor profile in the respiratory tract of pigeons mimics those of humans (Liu et al., Citation2009). Thus, further studies are needed to better understand the epidemiology of AIV in pigeons. Data should be collected about the infectious status of pigeon breeders, with a specific focus on influenza viruses. But also, more information should be made available regarding the proximity of migratory birds, lakes, rivers and swamp areas close to pigeon lofts.
Many seroepidemiological studies found no (Motha et al., Citation1997) or low percentages (< 2%) of wild pigeons with AIV antibodies (Kohls et al., Citation2011; Siengsanan-Lamont et al., Citation2011), which is in accordance with our observations. In the case of low viral replication, possibly only in epithelial cells at the virus entry side, the virus load may not have been sufficient to induce circulating antibodies explaining the lack of anti-AIV antibodies in this study (Thomas et al., Citation2008). Even after experimental infection of pigeons with a high dose of a highly pathogenic AIV (108 median embryo infective dose), pigeons developed only low HI antibody levels with a maximum titre of log2 6 (Klopfleisch et al., Citation2006).
This study suggests that AIV and PPMV-1 may circulate unnoticed in subclinically infected racing pigeons, but overall the low detection rate suggests that healthy and APMV-1 vaccinated racing pigeons may play only a minor epidemiological role in the distribution of these pathogens.
Acknowledgements
The authors would like to thank the Prof. Dr Kohaus-Förderverein e.V. for the support of this study. They greatly appreciate the cooperation of the national reference laboratory for APMVs (Friedrich Loeffler-Institute, Insel Riems, Germany) and in particular the valuable support by PD Dr C. Grund. Moreover, we thank L. Kreienbrock, M. Hartmann, G. Rechter and B. Bosse from the Department of Biometry, Epidemiology and Information Processing, WHO-Collaborating Centre for Research and Training in Veterinary Public Health at the University of Veterinary Medicine in Hannover, Germany for their great help in designing the questionnaires and data management. The authors also thank T. van den Berg and M. Steensels from the Veterinary and Agrochemical Research Center in Ukkel, Belgium for generously providing AIV antibody-positive pigeon sera. Furthermore we appreciate the assistance of V. Lebed concerning serological investigations.
References
- Abubakar, M.B., Ei-Yuguda, A.D. & Baba, S.S. (2008). Serological evidence of influenza virus infections in domestic animals and birds in North-Eastern Nigeria. Journal of Food Agriculture & Environment, 6, 67–70.
- Al-Garib, S.O., Gielkens, A.L.J., Gruys, E. & Koch, G. (2003). Review of Newcastle disease virus with particular references to immunity and vaccination. World's Poultry Science Journal, 59, 185–200. 10.1079/WPS20030011
- Aldous, E.W., Fuller, C.M., Mynn, J.K. & Alexander, D.J. (2004). A molecular epidemiological investigation of isolates of the variant avian paramyxovirus type 1 virus (PPMV-1) responsible for the 1978 to present panzootic in pigeons. Avian Pathology, 33, 258–269. 10.1080/0307945042000195768
- Aldous, E.W., Mynn, J.K., Banks, J. & Alexander, D.J. (2003). A molecular epidemiological study of avian paramyxovirus type 1 (Newcastle disease virus) isolates by phylogenetic analysis of a partial nucleotide sequence of the fusion protein gene. Avian Pathology, 32, 239–256. 10.1080/030794503100009783
- Alexander, D.J. (2011). Newcastle disease in the European Union 2000 to 2009. Avian Pathology, 40, 547–558. 10.1080/03079457.2011.618823
- Alexander, D.J., Lister, S.A. & Wilson, G.W.C. (1986). Avian paramyxovirus type-1 infection of racing pigeons: 5 continued spread in 1984. The Veterinary Record, 118, 424–427. 10.1136/vr.118.15.424
- Alexander, D.J. & Parsons, G. (1984). Avian paramyxovirus type 1 infections of racing pigeons: 2 pathogenicity experiments in pigeons and chickens. The Veterinary Record, 114, 466–469. 10.1136/vr.114.19.466
- Alexander, D.J., Russell, P.H. & Collins, M.S. (1984a). Paramyxovirus type 1 infections of racing pigeons: 1 characterisation of isolated viruses. The Veterinary Record, 114, 444–446. 10.1136/vr.114.18.444
- Alexander, D.J., Wilson, G.W., Thain, J.A. & Lister, S.A. (1984b). Avian paramyxovirus type 1 infection of racing pigeons: 3 epizootiological considerations. The Veterinary Record, 115, 213–216. 10.1136/vr.115.9.213
- Ballagi-Pordany, A., Wehmann, E., Herczeg, J., Belak, S. & Lomniczi, B. (1996). Identification and grouping of Newcastle disease virus strains by restriction site analysis of a region from the F gene. Archives of Virology, 141, 243–261. 10.1007/BF01718397
- Barber, M.R.W., Aldridge, J.R., Webster, R.G. & Magor, K.E. (2010). Association of RIG-I with innate immunity of ducks to influenza. Proceedings of the National Academy of Sciences of the United States of America, 107, 5913–5918. 10.1073/pnas.1001755107
- BMELV. (2013). Statistischer Monatsbericht—Anzeigepflichtige Tierseuchen der Bundesrepublik Deutschland. Bonn: Federal Ministry of Food, Agriculture and Consumer Protection. Retrieved from http://www.bmelv-statistik.de/de/statistischer-monatsbericht/einzelne-monatsberichte/.
- Bönner, B.M., Koehler, K., Reichel, U., Beck, I., Jaeger, S., Barbeito, J., Redmann, T. & Kaleta, E.F. (2003). Undesirable reactions of domestic pigeons to vaccination against paramyxovirus type 1. Deutsche Tierärztliche Wochenschrift, 110, 403–406.
- Camenisch, G., Bandli, R. & Hoop, R. (2008). Monitoring of wild birds for Newcastle disease virus in Switzerland using real time RT-PCR. Journal of Wildlife Diseases, 44, 772–776. 10.7589/0090-3558-44.3.772
- Capua, I., Dalla Pozza, M., Mutinelli, F., Marangon, S. & Terregino, C. (2002). Newcastle disease outbreaks in Italy during 2000. The Veterinary Record, 150, 565–568. 10.1136/vr.150.18.565
- Carrasco, A.O., Seki, M.C., de Sousa, R.L., Raso, T.F. & Pinto, A.A. (2009). Protection levels of vaccinated pigeons (Columba livia) against a highly pathogenic Newcastle disease virus strain. Tropical Animal Health and Production, 41, 1325–1333. 10.1007/s11250-009-9318-7
- Collins, M.S., Alexander, D.J., Brockman, S., Kemp, P.A. & Manvell, R.J. (1989). Evaluation of mouse monoclonal antibodies raised against an isolate of the variant avian paramyxovirus type 1 responsible for the current panzootic in pigeons. Archives of Virology, 104, 53–61. 10.1007/BF01313807
- Cornelissen, J.B.W.J., Post, J., Peeters, B., Vervelde, L. & Rebel, J.M.J. (2012). Differential innate responses of chickens and ducks to low-pathogenic avian influenza. Avian Pathology, 41, 519–529. 10.1080/03079457.2012.732691
- Das, A., Spackman, E., Senne, D., Pedersen, J. & Suarez, D.L. (2006). Development of an internal positive control for rapid diagnosis of avian influenza virus infections by real-time reverse transcription-PCR with lyophilized reagents. Journal of Clinical Microbiology, 44, 3065–3073. 10.1128/JCM.00639-06
- de Oliveira Torres Carrasco, A., Seki, M.C., de Freitas Raso, T., Paulillo, A.C. & Pinto, A.A. (2008). Experimental infection of Newcastle disease virus in pigeons (Columba livia): humoral antibody response, contact transmission and viral genome shedding. Veterinary Microbiology, 129, 89–96. 10.1016/j.vetmic.2007.11.012
- Dortmans, J.C.F.M., Fuller, C.M., Aldous, E.W., Rottier, P.J.M. & Peeters, B.P.H. (2010). Two genetically closely related pigeon paramyxovirus type 1 (PPMV-1) variants with identical velogenic fusion protein cleavage sites but with strongly contrasting virulence. Veterinary Microbiology, 143, 139–144. 10.1016/j.vetmic.2009.11.021
- Duchatel, J.P., Flore, P.H., Hermann, W. & Vindevogel, H. (1992). Efficacy of an inactivated aqueous-suspension Newcastle-disease virus-vaccine against paramyxovirus type-1 infection in young pigeons with varying amounts of maternal antibody. Avian Pathology, 21, 321–325. 10.1080/03079459208418847
- Duchatel, J.P. & Vindevogel, H. (1986). Assessment of vaccination of pigeons against paramyxovirus type 1 infection with inactivated aqueous-suspension or oil-emulsion vaccines. Avian Pathology, 15, 455–465. 10.1080/03079458608436307
- Ellis, T.M., Bousfield, R.B., Bissett, L.A., Dyrting, K.C., Luk, G.S., Tsim, S.T., Sturm-Ramirez, K., Webster, R.G., Guan, Y. & Malik Peiris, J.S. (2004). Investigation of outbreaks of highly pathogenic H5N1 avian influenza in waterfowl and wild birds in Hong Kong in late 2002. Avian Pathology, 33, 492–505. 10.1080/03079450400003601
- Fuller, C.M., Brodd, L., Irvine, R.M., Alexander, D.J. & Aldous, E.W. (2010). Development of an L gene real-time reverse-transcription PCR assay for the detection of avian paramyxovirus type 1 RNA in clinical samples. Archives of Virology, 155, 817–823. 10.1007/s00705-010-0632-1
- Gall, A., Hoffmann, B., Harder, T., Grund, C. & Beer, M. (2008). Universal primer set for amplification and sequencing of HA0 cleavage sites of all influenza A viruses. Journal of Clinical Microbiology, 46, 2561–2567. 10.1128/JCM.00466-08
- Gao, R., Cao, B., Hu, Y., Feng, Z., Wang, D., Hu, W., Chen, J., Jie, Z., Qiu, H., Xu, K., Xu, X., Lu, H., Zhu, W., Gao, Z., Xiang, N., Shen, Y., He, Z., Gu, Y., Zhang, Z., Yang, Y., Zhao, X., Zhou, L., Li, X., Zou, S., Zhang, Y., Yang, L., Guo, J., Dong, J., Li, Q., Dong, L., Zhu, Y., Bai, T., Wang, S., Hao, P., Yang, W., Han, J., Yu, H., Li, D., Gao, G. F., Wu, G., Wang, Y., Yuan, Z. & Shu, Y. (2013). Human infection with a novel avian-origin influenza A (H7N9) virus. The New England Journal of Medicine, 368, 1888–1897. 10.1056/NEJMoa1304459
- Gronesova, P., Mizakova, A. & Betakova, T. (2009). Determination of hemagglutinin and neuraminidase subtypes of avian influenza A viruses in urban pigeons by a new nested RT-PCR. Acta Virologica, 53, 213–216. 10.4149/av_2009_03_213
- Grund, C., Harder, T. & Beer, M. (2013). Phylogenetic analysis of pigeontype paramyxovirus 1 in Germany. Proceedings of the 23rd Annual Meeting of the Society for Virology (p. 71). Kiel, Germany. Retrieved from http://www.virology-meeting.de/fileadmin/media/2013/gfv/Druckelemente/GfV2013_Programme.pdf
- Guan, Y., Shortridge, K.F., Krauss, S., Chin, P.S., Dyrting, K.C., Ellis, T.M., Webster, R.G. & Peiris, M. (2000). H9N2 influenza viruses possessing H5N1-like internal genomes continue to circulate in poultry in southeastern China. Journal of Virology, 74, 9372–9380. 10.1128/JVI.74.20.9372-9380.2000
- Guttman, R.M., Tebo, T., Edwards, J., Barboriak, J.J. & Fink, J.N. (1971). The immune response of the pigeon (Columba livia). Journal of Immunology, 106, 392–396.
- Han, J., Jin, M., Zhang, P., Liu, J., Wang, L., Wen, D., Wu, X., Liu, G., Zou, Y., Lv, X., Dong, X., Shao, B., Gu, S., Zhou, D., Leng, Q., Zhang, C. & Lan, K. (2013). Epidemiological link between exposure to poultry and all influenza A (H7N9) confirmed cases in Huzhou city, China, March to May 2013. Eurosurveillance, 18, 6–11.
- Haynes, L., Arzey, E., Bell, C., Buchanan, N., Burgess, G., Cronan, V., Dickason, C., Field, H., Gibbs, S., Hansbro, P.M., Hollingsworth, T., Hurt, A.C., Kirkland, P., McCracken, H., O'Connor, J., Tracey, J., Wallner, J., Warner, S., Woods, R. & Bunn, C. (2009). Australian surveillance for avian influenza viruses in wild birds between July 2005 and June 2007. Australian Veterinary Journal, 87, 266–272. 10.1111/j.1751-0813.2009.00446.x
- Higgins, D.A. (1996). Comparative immunology of avian species. In T.F. Davison, T.R. Morris & L.N. Payne (Eds.), Poultry Immunology Vol. 24 (pp.149–205). Abingdon: Carfax Publishing.
- Hlinak, A., Werner, O. & Ziedler, K. (1998). Occurrence of paramyxovirus-1 (PMV 1) infections in pigeons. Berliner und Münchener Tierärztliche Wochenschrift, 111, 332–336.
- Hoffmann, B., Beer, M., Reid, S.M., Mertens, P., Oura, C.A., van Rijn, P.A., Slomka, M.J., Banks, J., Brown, I.H., Alexander, D.J. & King, D.P. (2009). A review of RT-PCR technologies used in veterinary virology and disease control: sensitive and specific diagnosis of five livestock diseases notifiable to the World Organisation for Animal Health. Veterinary Microbiology, 139, 1–23. 10.1016/j.vetmic.2009.04.034
- Hoffmann, B., Depner, K., Schirrmeier, H. & Beer, M. (2006). A universal heterologous internal control system for duplex real-time RT-PCR assays used in a detection system for pestiviruses. Journal of Virological Methods, 136, 200–209. 10.1016/j.jviromet.2006.05.020
- Hoque, M.A., Burgess, G.W., Karo-Karo, D., Cheam, A.L. & Skerratt, L.F. (2012). Monitoring of wild birds for Newcastle disease virus in north Queensland, Australia. Preventive Veterinary Medicine, 103, 49–62. 10.1016/j.prevetmed.2011.08.013
- Ip, H.S., Flint, P.L., Franson, J.C., Dusek, R.J., Derksen, D.V., Gill, R. E., Jr., Ely, C.R., Pearce, J.M., Lanctot, R.B., Matsuoka, S.M., Irons, D.B., Fischer, J.B., Oates, R.M., Petersen, M.R., Fondell, T.F., Rocque, D.A., Pedersen, J.C. & Rothe, T.C. (2008). Prevalence of influenza A viruses in wild migratory birds in Alaska: patterns of variation in detection at a crossroads of intercontinental flyways. Virology journal, 5, 71. 10.1186/1743-422X-5-71
- Jindal, N., de Abin, M., Primus, A.E., Raju, S., Chander, Y., Redig, P.T. & Goyal, S.M. (2010). Comparison of cloacal and oropharyngeal samples for the detection of avian influenza virus in wild birds. Avian Diseases, 54, 115–119.
- Kaleta, E.F. & Honicke, A. (2004). Review of the literature on avian influenza A viruses in pigeons and experimental studies on the susceptibility of domestic pigeons to influenza A viruses of the haemagglutinin subtype H7. Deutsche Tierärztliche Wochenschrift, 111, 467–472.
- Kalhoro, N.H., Veits, J., Rautenschlein, S. & Zimmer, G. (2009). A recombinant vesicular stomatitis virus replicon vaccine protects chickens from highly pathogenic avian influenza virus (H7N1). Vaccine, 27, 1174–1183. 10.1016/j.vaccine.2008.12.019
- Kapczynski, D.R., Afonso, C.L. & Miller, P.J. (2013). Immune responses of poultry to Newcastle disease virus. Developmental and Comparative Immunology, 41, 447–453. 10.1016/j.dci.2013.04.012
- Kapczynski, D.R., Wise, M.G. & King, D.J. (2006). Susceptibility and protection of naive and vaccinated racing pigeons (Columbia livia) against exotic Newcastle disease virus from the California 2002-2003 outbreak. Avian Diseases, 50, 336–341. 10.1637/7479-112905R.1
- Klopfleisch, R., Werner, O., Mundt, E., Harder, T. & Teifke, J. P. (2006). Neurotropism of highly pathogenic avian influenza virus A/chicken/Indonesia/2003 (H5N1) in experimentally infected pigeons (Columbia livia f. domestica). Veterinary Pathology, 43, 463–470. 10.1354/vp.43-4-463
- Knoll, M., Kosters, J. & Lutticken, D. (1986). Duration of immunity after vaccination against paramyxovirus infection in pigeons with an oil-emusified homologous vaccine—results of a long-term trial under laboratory conditions. Der Praktische Tierarzt, 67, 975–980.
- Kohls, A., Luschow, D., Lierz, M. & Hafez, H.M. (2011). Influenza A virus monitoring in urban and free-ranging pigeon populations in Germany, 2006–2008. Avian Diseases, 55, 447–450. 10.1637/9567-100710-ResNote.1
- Lana, D.P., Snyder, D.B., King, D.J. & Marquardt, W.W. (1988). Characterization of a battery of monoclonal antibodies for differentiation of Newcastle disease virus and pigeon paramyxovirus-1 strains. Avian Diseases, 32, 273–281. 10.2307/1590814
- Li, K.S., Guan, Y., Wang, J., Smith, G.J.D., Xu, K.M., Duan, L., Rahardjo, A.P., Puthavathana, P., Buranathai, C., Nguyen, T.D., Estoepangestie, A.T.S., Chaisingh, A., Auewarakul, P., Long, H. T., Hanh, N.T.H., Webby, R.J., Poon, L.L.M., Chen, H., Shortridge, K.F., Yuen, K.Y., Webster, R.G. & Peiris, J.S.M. (2004). Genesis of a highly pathogenic and potentially pandemic H5N1 influenza virus in eastern Asia. Nature, 430, 209–213. 10.1038/nature02746
- Li, Q., Zhou, L., Zhou, M., Chen, Z., Li, F., Wu, H., Xiang, N., Chen, E., Tang, F., Wang, D., Meng, L., Hong, Z., Tu, W., Cao, Y., Li, L., Ding, F., Liu, B., Wang, M., Xie, R., Gao, R., Li, X., Bai, T., Zou, S., He, J., Hu, J., Xu, Y., Chai, C., Wang, S., Gao, Y., Jin, L., Zhang, Y., Luo, H., Yu, H., Gao, L., Pang, X., Liu, G., Shu, Y., Yang, W., Uyeki, T.M., Wang, Y., Wu, F. & Feng, Z. (2013). Preliminary report: epidemiology of the avian influenza A (H7N9) outbreak in China. The New England journal of medicine.
- Lillehaug, A., Monceyron Jonassen, C., Bergsjo, B., Hofshagen, M., Tharaldsen, J., Nesse, L.L. & Handeland, K. (2005). Screening of feral pigeon (Colomba livia), mallard (Anas platyrhynchos) and graylag goose (Anser anser) populations for Campylobacter spp., Salmonella spp., avian influenza virus and avian paramyxovirus. Acta Veterinaria Scandinavica, 46, 193–202. 10.1186/1751-0147-46-193
- Liu, M., Guan, Y., Peiris, M., He, S., Webby, R.J., Perez, D. & Webster, R.G. (2003a). The quest of influenza A viruses for new hosts. Avian Diseases, 47, 849–856. 10.1637/0005-2086-47.s3.849
- Liu, M., He, S.Q., Walker, D., Zhou, N.N., Perez, D.R., Mo, B., Li, F., Huang, X.T., Webster, R.G. & Webby, R.J. (2003b). The influenza virus gene pool in a poultry market in South Central China. Virology, 305, 267–275. 10.1006/viro.2002.1762
- Liu, Y.H., Han, C.H., Wang, X.Q., Lin, J., Ma, M., Shu, Y.L., Zhou, J., Yang, H.C., Liang, Q., Guo, C.T., Zhu, J.J., Wei, H.T., Zhao, J.Y., Ma, Z.J. & Pan, J. (2009). influenza A virus receptors in the respiratory and intestinal tracts of pigeons. Avian Pathology, 38, 263–266. 10.1080/03079450903055363
- Meulemans, G., van den Berg, T.P., Decaesstecker, M. & Boschmans, M. (2002). Evolution of pigeon Newcastle disease virus strains. Avian Pathology, 31, 515–519. 10.1080/0307945021000005897
- Motha, J., Gibbons, A.M. & Reed, C.E.M. (1997). A survey for avian paramyxoviruses and influenza viruses in feral pigeons and native birds in New Zealand. New Zealand Veterinary Journal, 45, 215–216. 10.1080/00480169.1997.36032
- Munster, V.J., Baas, C., Lexmond, P., Waldenstrom, J., Wallensten, A., Fransson, T., Rimmelzwaan, G.F., Beyer, W.E., Schutten, M., Olsen, B., Osterhaus, A.D. & Fouchier, R.A. (2007). Spatial, temporal, and species variation in prevalence of influenza A viruses in wild migratory birds. PLoS Pathogens, 3, 630–638. 10.1371/journal.ppat.0030061
- OIE. (2009). Chapter 2.3.14, Newcastle disease. In O.I.d. Epizooties (Ed.), Manual of Diagnostic Tests and Vaccines for Terrestrial Animals (pp.576–589). Paris: World Organisation for Animal Health.
- OIE. (2013). OIE expert mission finds live bird markets play a key role in poultry and human infections with influenza A(H7N9). Paris: World Organisation for Animal Health. Retrieved from http://www.oie.int/en/for-the-media/press-releases/detail/article/oie-expert-mission-finds-live-bird-markets-play-a-key-role-in-poultry-and-human-infections-with-infl/.
- Panigrahy, B., Senne, D.A., Pedersen, J.C., Shafer, A.L. & Pearson, J.E. (1996). Susceptibility of pigeons to avian influenza. Avian Diseases, 40, 600–604. 10.2307/1592270
- Perkins, L.E. & Swayne, D.E. (2002). Pathogenicity of a Hong Kong-origin H5N1 highly pathogenic avian influenza virus for emus, geese, ducks, and pigeons. Avian Diseases, 46, 53–63. 10.1637/0005-2086(2002)046[0053:POAHKO]2.0.CO;2
- Petersen, H., Matrosovich, M., Pleschka, S. & Rautenschlein, S. (2012). Replication and adaptive mutations of low pathogenic avian influenza viruses in tracheal organ cultures of different avian species. Plos One, 7, e42260. 10.1371/journal.pone.0042260
- Rabl, S., Rinder, M., Neubauer-Juric, A., Bogner, K.H., Korbel, R. & Buttner, M. (2009). [Surveillance of wild birds for avian influenza A virus (AIV) in Bavaria in the years 2007 and 2008]. Berliner und Münchener Tierärztliche Wochenschrift, 122, 486–493.
- Reid, S.M., Cox, W.J., Ceeraz, V., Sutton, D., Essen, S.C., Howard, W.A., Slomka, M.J., Irvine, R.M. & Brown, I.H. (2012). First reported detection of influenza A (H1N1)pdm09 in Turkeys in the United Kingdom. Avian Diseases, 56, 1062–1067. 10.1637/10178-041012-Reg.1
- Rogers, G.N., Pritchett, T.J., Lane, J.L. & Paulson, J.C. (1983). Differential sensitivity of human, avian, and equine influenza A viruses to a glycoprotein inhibitor of infection: selection of receptor specific variants. Virology, 131, 394–408. 10.1016/0042-6822(83)90507-X
- Romvary, J., Meszaros, J., Tanyi, J., Rozsa, J. & Fabian, L. (1976). Spreading of virus-infection among wild birds and monkeys during influenza epidemic caused by Victoria(3)75 variant of A(H3N2) virus. Acta Veterinaria Academiae Scientiarum Hungaricae, 26, 369–376.
- Romvary, J. & Tanyi, J. (1975). Occurrence of Hong-Kong influenza A (H3N2) virus-infection in Budapest zoo. Acta Veterinaria Academiae Scientiarum Hungaricae, 25, 251–254.
- Rupiper, D.J. (1998). Diseases that affect race performance of homing pigeons. Part I: husbandry, diagnostic strategies, and viral diseases. Journal of Avian Medicine and Surgery, 12, 70–77.
- Seal, B.S., King, D.J. & Sellers, H.S. (2000). The avian response to Newcastle disease virus. Developmental and Comparative Immunology, 24, 257–268. 10.1016/S0145-305X(99)00077-4
- Siengsanan-Lamont, J., Robertson, I., Blacksell, S.D., Ellis, T., Fenwick,S., Saengchoowong, S., Suwanpukdee, S., Yongyuttawichai, P., Sariya, L., Prompiram, P., Chaichoun, K., Wiriyarat, W., Pothieng, D. & Ratanakorn, P. (2011). Virological and molecular epidemiological investigations into the role of wild birds in the epidemiology of influenza A/H5N1 in central Thailand. Veterinary Microbiology, 148, 213–218. 10.1016/j.vetmic.2010.09.028
- Smietanka, K., Minta, Z., Wyrostek, K., Jozwiak, M., Olszewska, M., Domanska-Blicharz, A.K., Reichert, A.M., Pikula, A., Habyarimana, A. & van den Berg, T. (2011). Susceptibility of pigeons to clade 1 and 2.2 high pathogenicity avian influenza H5N1 virus. Avian Diseases, 55, 106–112. 10.1637/9514-090110-ResNote.1
- Songserm, T., Amonsin, A., Jam-On, R., Sae-Heng, N., Meemak, N., Pariyothorn, N., Payungporn, S., Theamboonlers, A. & Poovorawan, Y. (2006). Avian influenza H5N1 in naturally infected domestic cat. Emerging Infectious Diseases, 12, 681–683. 10.3201/eid1204.051396
- Spackman, E., Senne, D.A., Myers, T.J., Bulaga, L.L., Garber, L.P., Perdue, M.L., Lohman, K., Daum, L.T. & Suarez, D.L. (2002). Development of a real-time reverse transcriptase PCR assay for type A influenza virus and the avian H5 and H7 hemagglutinin subtypes. Journal of Clinical Microbiology, 40, 3256–3260 10.1128/JCM.40.9.3256-3260.2002
- Spackman, E. & Suarez, D.L. (2008). Avian influenza virus RNA extraction from tissue and swab material. In E. Spackman (Ed.), Avian Influenza Virus (pp.13–18). Totowa, New Jersey, USA: Humana Press.
- Terregino, C., De Nardi, R., Guberti, V., Scremin, M., Raffini, E., Martin, A.M., Cattoli, G., Bonfanti, L. & Capua, I. (2007). Active surveillance for avian influenza viruses in wild birds and backyard flocks in Northern Italy during 2004 to 2006. Avian Pathology, 36, 337–344. 10.1080/03079450701488345
- Thomas, C., Manin, T.B., Andriyasov, A.V. & Swayne, D.E. (2008). Limited susceptibility and lack of systemic infection by an H3N2 swine influenza virus in intranasally inoculated chickens. Avian Diseases, 52, 498–501. 10.1637/8210-011408-RESNOTE.1
- Trujillo, J., Sponseller, B., Lohr, C. & Nara, P. (2012). Reverse zoonosis of influenza A virus in companion animals; what it means to veterinary and human medicine. International Journal of Infectious Diseases, 16, E274–E274. 10.1016/j.ijid.2012.05.931
- Tsai, H.J. & Lee, C.Y. (2006). Serological survey of racing pigeons for selected pathogens in Taiwan. Acta Veterinaria Hungarica, 54, 179–189. 10.1556/AVet.54.2006.2.5
- Tudor, D.C. (1991). Avian influenza. In D.C. Tudor (Ed.), Pigeon Health and Disease 1st edn (pp.41–44). Ames, Iowa, USA: Iowa State University Press.
- Wakamatsu, N., King, D.J., Kapczynski, D.R., Seal, B.S. & Brown, C.C. (2006). Experimental pathogenesis for chickens, turkeys, and pigeons of exotic Newcastle disease virus from an outbreak in California during 2002-2003. Veterinary Pathology, 43, 925–933. 10.1354/vp.43-6-925
- Werner, O., Starick, E., Teifke, J., Klopfleisch, R., Prajitno, T.Y., Beer, M., Hoffmann, B. & Harder, T.C. (2007). Minute excretion of highly pathogenic avian influenza virus A/chicken/Indonesia/2003 (H5N1) from experimentally infected domestic pigeons (Columbia livia) and lack of transmission to sentinel chickens. Journal of General Virology, 88, 3089–3093. 10.1099/vir.0.83105-0
- Wise, M.G., Suarez, D.L., Seal, B.S., Pedersen, J.C., Senne, D.A., King, D.J., Kapczynski, D.R. & Spackman, E. (2004). Development of a real-time reverse-transcription PCR for detection of Newcastle disease virus RNA in clinical samples. Journal of Clinical Microbiology, 42, 329–338. 10.1128/JCM.42.1.329-338.2004
- Yamamoto, Y., Nakamura, K., Yamada, M. & Mase, M. (2012). Limited susceptibility of pigeons experimentally inoculated with H5N1 highly pathogenic avian influenza viruses. Journal of Veterinary Medical Science, 74, 205–208. 10.1292/jvms.11-0312
- Zupančić, Z., Ugrčić, I., Jelavić, V.S.I.-P., Gregurić, J., Jukić, B. & Smerdel, S. (1986). Antibodies for human influenza type A virus in bird sera. Veterinarski Arhiv, 56, 217–225.