Abstract
Passerines are frequently parasitized by coccidia, especially species of the genus Isospora, with extra-intestinal stages that can be highly pathogenic causing serious clinical damage in young birds. Whilst there is still no effective treatment to completely clear isosporoid coccidia with extra-intestinal stages from a host species, our results showed that prolonged treatment with toltrazuril (BAYER AG, Leverkusen, Germany) can decrease the oocysts in faeces and thus reduce the extra-intestinal phase of the infection. The toltrazuril treatment is therefore probably indirectly effective against the systemic form of atoxoplasmosis.
Introduction
Parasitic diseases in birds in captivity are quite common and the most frequent parasites found in these captive birds are coccidia, especially the genus Isospora spp. The genus Isospora combines coccidia with two different lifecycles (Atkinson et al., Citation2008). Isospora spp. with extra-intestinal proliferative stages cause atoxoplasmosis, primarily of passerine birds (Box, Citation1966; Partington et al., Citation1989). The organism is transmitted via a faecal–oral route and has an asexual lifecycle in the leucocytes and a sexual lifecycle in the intestinal mucosa. Oocysts are then passed in the faeces. Clinical signs are non-specific. Atoxoplasmosis is therefore mostly indicated by the presence of endogenous stages in mononuclear blood cells or oocysts in faeces (Box, Citation1970; Levine, Citation1982; Tully et al., Citation2000).
Coccidia with extra-intestinal stages are pathogenic, causing serious clinical changes in young birds. Mortalities reaching up to 80% have been observed (Partington et al., Citation1989; Upton et al., Citation2001; Adkesson et al., Citation2005). In captive populations of Blue-crowned Laughing Thrushes (Dryonastes courtoisi; Ménégaux, 1923) at Durrell Wildlife Conservation Trust, the mortality caused by Isospora spp. causing atoxoplasmosis was almost 44% (Barbon et al., Citation2013). There is still no effective treatment completely clearing isosporoid coccidian with extra-intestinal stages from a host species (Norton et al., Citation2003).
The Blue-crowned Laughing Thrush is an endemic species and occupies an extremely small fragmented breeding range in Jiangxi Province in China. The bird is classified as Critically Endangered (Wilkinson & Gardner, Citation2011; IUCN, Citation2012). In this study we focused on using toltrazuril treatment against Isospora spp. causing atoxoplasmosis in the Blue-crowned Laughing Thrush.
Materials and Methods
A group of six birds of D. curtoisi were kept in separate cages in the Veterinary Department at Durrell Wildlife Conservation Trust in Jersey for a period of 3 months (the trial period) from 8 September 2010 to 22 December 2010. These birds were considered positive for atoxoplasmosis based on clinical signs and previous examination (blood smears).
The treatment protocol consisted of the administration of toltrazuril (Baycox® 2.5%) at 12.5 mg/kg orally for 2 days, cessation for 5 days and then this 7-day cycle repeated throughout the trial period. The investigation was part of a prolonged treatment for sick birds with routine follow ups in order to establish efficacy of the treatment.
Faecal samples were collected at the end of each day and placed into vials containing 2.5% aqueous (w/v) potassium dichromate (K2Cr2O7) solution. The vials were maintained at room temperature (∼23°C) for between 5 and 7 days to allow the oocysts to sporulate (Long et al., Citation1976). The oocysts were concentrated by modified Sheather's sugar solution flotation (Sheather, Citation1923) and were concentrated and quantified according to Roepstorff & Nansen (Citation1998) using McMasters' chamber. Blood samples were taken once a week from the right ulnar vein using a 0.3 ml insulin syringe. Buffy coat smears were prepared according to Franklin (Citation1983) by the centrifugation of peripheral blood in a capillary tube for 3 min using a microhaematocrit centrifuge. The slides were stained with Diff-Quick stain kit, Fisher Scientific, United Kindom and examined with a Leica Amersham, Bucks, United Kindom DM 1000 LED microscope 100 × lens using oil immersion. One thousand leucocytes were counted in each smear in order to determine a percentage of infected cells in the blood.
Results
Birds were observed looking lethargic, with fluffy feathers, not flying well and with laboured breathing. On handling, swollen mottled pectoral muscles, hepatomegaly (“black spot”) and visible distended intestines were also observed.
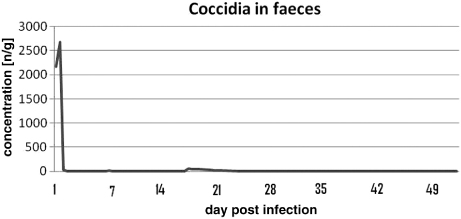
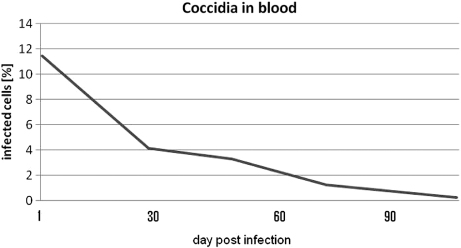
The only parasites to be found in faeces or blood were Isospora spp. The toltrazuril treatment reduced all intestinal stages of Isospora spp. within 7 days () and the clinical signs had disappeared, while extra-intestinal stages in white blood cells were reduced to a minimum within 3 months (). Ten days after the trial terminated, the levels of coccidia found in faeces were almost at the initial levels and extra-intestinal stages in white blood cells had reappeared (data not published).
No bacterial or viral infections were diagnosed in these birds.
Discussion
It is well known that toltrazuril is effective against all species of Eimeria infecting chickens (Mehlhorn et al., Citation1988; Greif, Citation2000) and several treatment protocols for atoxoplasmosis have been published by Norton et al. (Citation2003). These recorded that drugs such as sulfachlorpyrazine (ESB3) and toltrazuril affect only the intestinal stages of the parasite and are effective in drinking water. Sulfachlorpyridazine (Vetisulid®, Vetmedica INC, Boehringer Ingelheim, St. Joseph, MO, USA) may be substituted for sulfachlorpyrazine, but, in this case, a vitamin B12 supplement should be used during treatment. There are also studies comparing the efficacy of sulphaquinoxaline, sulfamethazine and diclazuril in green-winged saltators (Coelho et al., Citation2012a), lesser seed-finches and great-billed seed-finches infected with Isospora spp. (Coelho et al., Citation2012b). As far as we know, toltrazuril is eliminating all intestinal stages and therefore, according to our findings, we conclude that the toltrazuril treatment is preventing re-infection from the intestine that, eventually, could result in a reduction of systematic infection, as the Isospora spp. can only multiply a certain number of times asexually (Box, Citation1977).
The oral administration treatment regime described above provided certainty of dosage for each individual bird, which would not have been possible if the birds had been allowed unlimited access to water treated with the drug. There was no control group and therefore it seems difficult to distinguish between treatment-related decrease of parasite burden and spontaneous recovery. Furthermore, there is no proof that extra-intestinal stages were affected by toltrazuril treatment, but there are some indications. According to our findings, extra-intestinal forms of isosporoid parasite can cause re-infection even after the treatment, resulting in the reappearance of intestinal forms.
Infectious diseases, including parasitic infections, pose serious risks for endangered populations of wild birds. It is necessary to monitor infection in populations that are managed in the wild and populations of captive bred specimens which are to be re-introduced as a part of species conservation programme. In addition, reducing intestinal and extra-intestinal stages of parasites in young birds will help them to overcome the critical period, until they are immune to coccidia infections. Further investigations are necessary to compare different protocols and confirm the findings of this study.
Acknowledgements
The authors wish to thank all those who have provided input and comments in the drafting of this paper. Our particular thanks to Melissa Frost, veterinary technician at Durrell Wildlife Conservation Trust, and to Cristian Segura Cortijos for their assistance with sampling. This work was funded by grant IGA VFU Brno 232/2010.
References
- Adkesson, M.J., Zdziarski, M.J. & Little, S.E. (2005). Atoxoplasmosis in tanagers. Journal of Zoo and Wildlife Medicine, 36, 265–272. 10.1638/03-091.1
- Atkinson, C.T., Thomas, N.J. & Hunter, D.B. (2008). Parasitic Diseases of Wild Birds. Iowa: Willey-Blackwell.
- Barbon, A.R., Jamriška, J. & Lopéz, J.F. (2013). Atoxoplasmosis in captive blue crowned laughing thrush (Dryonastes courtoisi) at Durrell Wildlife Conservation Trust. ICARE, Wiesbaden, Germany, 119.
- Box, E.D. (1966). Blood and tissue protozoa of the English sparrow (Passer domesticus domesticus) in Galveston, Texas. Journal of Protozoology, 13, 204–208. 10.1111/j.1550-7408.1966.tb01895.x
- Box, E.D. (1970). Atoxoplasma associated with an isosporan oocyst in canaries. The Journal of Protozoology, 17, 391–396. 10.1111/j.1550-7408.1970.tb04700.x
- Box, E.D. (1977). Life cycle of two Isospora species in the canary, Serinus canarius Linnaeus. The Journal of Protozoology, 24, 57–67. 10.1111/j.1550-7408.1977.tb05281.x
- Coelho, C.D., Berto, B.P., Neves, D.M., de Oliveira, V.M., Flausino, W. & Lopes, C.W.G. (2012a). Diagnosis and treatment of coccidiosis in Green-winged Saltator Saltator similis D' Orbigny Lafresnaye, 1837 kept under quarantine conditions. Revista Brasileira de Medicina Veterinária, 34, 46–54.
- Coelho, C.D., Berto, B.P., de Oliveira, V.M., Neves, D.M., Flausino, W. & Lopes, C.W.G. (2012b). Treatment of coccidiosis from the genus Isospora Schneider, 1881 in Sporophila angolensis Linnaeus, 1766 and Sporophila maximiliani Cabanis, 1851 under quarantine conditions. Revista Brasileira de Medicina Veterinária, 34, 102–108.
- Franklin, I.M. (1983). A comparison of peripheral blood and Buffy coat smear examination for the prediction of bone marrow relapse of acute lymphoblastic leukaemia in childhood. Journal of Clinical Pathology, 36, 192–194. 10.1136/jcp.36.2.192
- Greif, G. (2000). Immunity to coccidiosis after treatment with toltrazuril. Parasitology Research, 86, 787–790. 10.1007/s004360000218
- IUCN (2012). IUCN red list of threatened species. Garrulax courtoisi. Version 2012.2. < www.iucnredlist.org>
- Levine, N.D. (1982). The genus Atoxoplasma(Protozoa, Apicomplexa). The Journal of Parasitology, 68, 719–723. 10.2307/3280933
- Long, P.L., Millard, B.J., Joyner, L.P. & Norton, C.C. (1976). A guide to laboratory techniques used in the study and diagnosis of avian coccidiosis. Folia Veterinaria Latina, 6, 210–217. 10.1007/BF00931192
- Mehlhorn, H., Schmahl, G. & Haberkorn, A. (1988). Toltrazuril effective against a broad spectrum of protozoan parasites. Parasitology Research, 75, 64–66. 10.1007/BF00931192
- Norton, T.L., Neiffer, D.L., Seibels, B., Benson, K., McAloose, D., Travis, D., Greiner, E., Latimer, K., Little, S.E., Zdziarski, J.M., Schrenzel, M., Rideout, B., Vince, M. & Gentz, N. (2003). Atoxoplasma medical protocols recommended by the passerine atoxoplasma working group. Eastern Regional AZA conference in Columbia. http://www.riverbanks.org/subsite/aig/Atoxo-recommendations.htm
- Partington, C.C.J., Gardiner, Ch.H., Fitz, D., Phillips, L.G. & Montali, R.J. (1989). Atoxoplasmosis in Bali Mynahs (Leucopsar rothschildi). Journal of Zoo Wildlife Medicine, 20, 328–335.
- Roepstorff, A. & Nansen, P. (1998). The Epidemiology, Diagnosis and Control of Helminth Parasites of Swine. A FAO Animal Health Manual, vol. 3. Rome: FAO.
- Sheather, A.L. (1923). The detection of intestinal protozoa and mange parasites by a flotation technique. Journal of Comparative Pathology, 36, 266–275.
- Tully, T.N., Jr., Dorrestein, G.M. & Jones, A.K. (2000). Handbook of Avian Medicine 2nd edn, Oxford: Elsevier.
- Upton, S.J., Wilson, S.C., Norton, T.M. & Greiner, E.C. (2001). A new species of Isospora Schneider, 1881 (Apicomplexa: Eimeriidae) from the Bali (Rothschild's) mynah Leucopsar rothschildi (Passeriformes: Sturnidae), and comments concerning the genera Atoxoplasma Garham, 1950 and Isospora. Systematic Parasitology, 48, 47–53. 10.1023/A:1026520206525
- Wilkinson, R. & Gardner, L. (2011). No laughing matter. Zooquaria, 74, 12–13.