Abstract
Avian pathogenic Escherichia coli (APEC) are a substantial burden to the global poultry industry. APEC cause a syndromic poultry infection known as colibacillosis, which has been previously associated with broiler chickens over 2 weeks old. We recently reported that the intestinal tract of 1-day-old broilers harbours a rich reservoir of potentially pathogenic E. coli. Prior infections of the reproductive tract of breeders, egg hygiene and transportation all contribute to early colonization of the neonatal gut. Up to one-half of all flock deaths occur in the first week of production, but few data are available describing the contribution of E. coli. In the present study, all dead birds collected on the first daily welfare walk 48 and 72 h after chick placement underwent post-mortem examination. Diseased tissues were selectively cultured for E. coli and isolates subsequently virulotyped using 10 APEC virulence-associated genes (VAGs): astA, iss, irp2, iucD, papC, tsh, vat, cvi, sitA and ibeA. Approximately 70% of birds displayed signs of colibacillosis. Thirty distinct virulence profiles were identified among 157 E. coli. Isolates carried between zero and seven VAGs; ∼30% of E. coli isolates carried five to seven VAGs, with 12.7% sharing the same VAG profile (astA, iss, irp2, iucD, tsh, cvi and sitA). Overall, this study demonstrates the significant contribution of E. coli infections to early broiler mortalities. The identification of a diverse E. coli population is unsurprising based on our previous findings. This work emphasizes the need for an effective vaccination programme and provides preliminary data for vaccine production.
Introduction
Avian pathogenic Escherichia coli (APEC) are an ill-defined pathotype of the extra-intestinal pathogenic E. coli group. APEC are the aetiological agent of an avian syndromic disease characterized by fibrinous lesions around visceral organs collectively termed colibacillosis. Airsacculitis, cellulitis, pericarditis, perihepatitis and respiratory distress are among the most commonly associated signs of colibacillosis (Barnes, Citation1997). The broiler (meat chicken) industry is substantial, with over 900 million broiler chickens being reared for consumption in the UK alone (DEFRA, Citation2013). Colibacillosis is an endemic disease in commercial flocks and is responsible for substantial economic losses globally.
The APEC pathotype shows high diversity. Recently, the evolution of APEC strains from multiple E. coli lineages following the acquisition of virulence-associated genes (VAGs) highlighted the high genetic diversity within this pathogenic group (Dziva et al., Citation2013). Genes involved in adhesion, invasion, toxin production, serum survival and iron acquisition have been shown to contribute to APEC pathogenesis (Skyberg et al., Citation2003; Ewers et al., Citation2005; Germon et al., Citation2005; McPeake et al., Citation2005; Rodriguez-Siek et al., Citation2005b; Kariyawasam et al., Citation2006). Such pathogen diversity has hindered the production of an effective vaccination programme suitable against heterologous challenge.
The intestinal E. coli population has been identified as a potential APEC reservoir (Ewers et al., Citation2009). A recent study undertaken by our research group identified the intestinal tract of 1-day-old commercial broiler chicks to be rich in potentially pathogenic E. coli (termed potential APEC) (Kemmett et al., Citation2013). These potential APEC were identified by their possession of at least five VAGs previously identified as contributors to APEC pathogenesis (Skyberg et al., Citation2003; Ewers et al., Citation2005; Rodriguez-Siek et al., Citation2005b). In 1-day-old broiler chicks, almost 25% of the faecal E. coli population sampled carried at least five of the 10 VAGs used in virulotyping. Given the identification of such a potentially pathogenic burden it was of interest to determine the prevalence of E. coli-related systemic disease in these broiler chicks, particularly as many deaths occur within the first few days of production.
First week mortalities can account for up to 50% of total flock loses (Yassin et al., Citation2009; Olsen et al., Citation2012). Early mortalities reportedly reflect overall flock performance, which has led to contracts between hatcheries and broiler farmers often stating an adjusted cost per chick based on the performance of chicks during the first week (Goodhope, Citation1991; Yassin et al., Citation2009). Chick survival during this initial period has been reported to be associated with the broiler breeder farm and hatcheries with emphasis on breeder flock management (nutrition, lighting and age) (Renema et al., Citation2008). Early mortalities also correlate with the extreme breeder ages, egg storage length, breeder feed and the hatchery used (Suarez et al., Citation1997; Vieira & Moran, Citation1998, Citation1999; Pedroso et al., Citation2005).
Olsen et al. (Citation2012) reported that bacterial infections, primarily E. coli, accounted for ∼50% of layer flock mortalities during the first week (Olsen et al., Citation2012). Omphalitis and/or yolk sac infections, with or without septicaemia, were reported. Such infections may originate from infected breeder hens with salpingitis where the yolk sac becomes infected in ovo, or through the hatchery environment (Vandekerchove et al., Citation2004; Mokady et al., Citation2005). Additionally, Petersen et al. (Citation2006) demonstrated the potential vertical transmission of fluoroquinolone-resistant E. coli (Petersen et al., Citation2006).
Investigations into broiler flock infection mortalities are rarely conducted. This present study reports on the contribution of E. coli to broiler chick mortalities in the first 72 h of production.
Materials and Methods
Ethics statement
The following protocol did not involve any invasive procedures; no approval under the Animals (Scientific Procedures) Act (1986) was needed. No birds were culled for the purpose of this study and all dead birds intended for post-mortem examination were collected on the first daily welfare walk conducted by farmers. The study was conducted in strict accordance with the University of Liverpool Research Governance policies and permission for sampling on the broiler farms was granted by the farms.
Standard commercial broiler farm
In commercial production, day-of-hatch and 1-day-old broiler chicks are transported to the broiler farm for rearing. The day they arrive on the broiler farm may be referred to as “Day 1 of placement/production”.
Dead standard commercial chicks were collected during the first daily welfare walk on a standard commercial broiler chicken farm in the UK 48 and 72 h after placement. The flock used in this study was from a commercial farm, which routinely vaccinated as standard within the industry in the UK for avian pneumovirus (7 days old), infectious bronchitis virus (14 days old) and infectious bursal disease (16 days old). Prior to this, at the hatchery, birds were vaccinated against the infectious bronchitis virus. No prior veterinary intervention was undertaken.
Post-mortem examination of dead broiler chicks
To minimize the detection of systemic E. coli resulting from a loss of intestinal integrity following death, only birds identified as recently dead were included. Birds were only selected for post-mortem examination if they did not show signs of physical injury, including extensive pecking injuries, had not been trodden on (flattened appearance) and/or did not have broken legs. All birds were examined for classical signs of colibacillosis, including ascites, airsacculitis, cellulitis, enlarged spleen, pericarditis and perihepatitis and yolk sac infection (Barnes, Citation1997). For each bird, up to 1 g each of heart, kidney, liver, lung and spleen tissues were collected using sterile forceps and scalpels. Enough sterile phosphate-buffered saline was added to each sample for homogenization using a Biomaster Micro-stomacher 80 (Seward, Worthing, West Sussex, UK) for 60 sec at high speed. Then 50 µl each homogenate was streaked onto eosin–methylene blue agar and incubated overnight at 37°C. All media used were obtained from LabM Ltd (Bury, UK). Two to three E. coli colonies per positive tissue sample were picked, re-plated onto nutrient agar and incubated overnight at 37°C. E. coli identification was confirmed using a polymerase chain reaction (PCR) targeting the uidA gene (McDaniels et al., Citation1996).
Virulotyping of extra-intestinal E. coli
One colony from above was added to 300 µl of 20% (w/v) Chelex-100 in 10 mM Tris–HCl, 1 mM ethylenediamine tetraacetic acid, pH 8.0 (Bio-Rad, Hemel Hempstead, Hertfordshire, UK). Bacterial DNA was extracted using a protocol described previously (Walsh et al., Citation1991). Briefly, 300 µl Chelex-100 containing pooled colonies were incubated at 95°C for 10 min. Samples were centrifuged at 6700 × g for 2 min and 50 µl supernatant was added to 250 µl sterile double-distilled water. E. coli identification was confirmed using a PCR targeting the uidA gene (McDaniels et al., Citation1996).
All isolates were subjected to a full screen of 10 virulence genes and each was given a corresponding VAG profile. The individual isolate DNA templates were screened for 10 VAGs; astA, iss, irp2, iucD, papC, tsh, vat, cvi, sitA and ibeA. Three separate PCR assays were performed; one multiplex PCR described previously by Ewers et al. (Citation2005), and two single PCR assays for ibeA and sitA outlined by Timothy et al. (2008). Primer sequences are presented in . All primers were obtained from Eurofins MWG operon (Ebersberg, Germany) and all molecular reagents from Thermo Scientific (Epsom, Surrey, UK).
Table 1. Gene target, primer sequences, accession numbers and product length for virulence-associated genes used for virulotyping extra-intestinal E. coli.
Briefly, for a 25 µl multiplex PCR, 4 µl of 25 mM MgCl2, 13.9 µl sterile water, 2.5 µl of 10× PCR buffer, 0.5 µl 20 mM dNTPs, 0.1 µl each 100 pmol forward and reverse primer, 0.5 µl of 5 U/µl Taq polymerase and 2 µl DNA template were used. Multiplex PCR thermocycler conditions were as follows: initial denaturation 94°C for 3 min; 25 cycles of 94°C for 30 sec, 58°C for 30 sec, 68°C for 3 min; final extension at 72°C for 10 min; and hold at 4°C. Each individual PCR contained 1 µl DNA template, 1 µl each primer (100 pmol) and 22 µl of 1.1× Reddymix PCR mastermix with 1.5 mM MgCl2. Thermocycler conditions for sitA and ibeA were identical: 95°C for 12 min; 25 cycles of 94°C for 30 sec, 63°C for 30 sec, 68°C for 3 min; with a final 72°C for 10 min. The mixture was held at 4°C. PCR products were subject to electrophoresis as above. The presence or absence of the 10 VAGs produced a series of 10 numbers, which denoted the VAG profile for each isolate (presence “1” or absence “0”).
Phylogenetic analysis
E. coli were assigned to one of four phylogenetic groups (A, B1, B2 or D) using a triplex PCR targeting chuA, yjaA and the DNA fragment TSPE4.C2 (Clermont et al., Citation2000). All reagents were obtained from Thermo Scientific. Each 25 µl PCR reaction contained 3 µl template DNA extract, 0.2 µl each forward and reverse 100 pmol primer (Eurofins MWG operon), 2.5 µl dNTPs, 4 µl MgCl2, 2 µl of 10× PCR buffer and 0.25 µl of 5 U/µl Taq polymerase. Thermocycler conditions were as follows: initial denaturation at 94°C for 5 min; 30 cycles of 30 sec at 94°C, 30 sec at 59°C and 30 sec at 72°C; with a final extension at 72°C for 7 min. PCR products were separated by electrophoresis. Phylogenetic group classification was based on the combination of chuA, yjaA and TSPE4.C2: group A (chuA−, TSPE4.C2−, yjaA+), group B1 (chuA−, TSPE4.C2+, yjaA−), group B2 (chuA+, TSPE4.C2−/ + , yjaA+) and group D (chuA+, TSPE4.C2−/ + , yjaA−). E. coli negative for all products were assigned to group A0.
Results
Post-mortem examinations
At placement, 25,700 chicks were placed in the rearing shed. The overall flock mortality rate for the sampled flock at the point of slaughter was 4.36%, while flock mortality in the first week was recorded as 1.03% and 0.44% for the first 72 h.
Overall, 37 birds (n = 14 at 48 h and n = 23 at 72 h after placement) were collected on the first daily welfare walk and were subject to post-mortem. Twenty-six out of 37 birds (70.27%) showed clinical signs associated with colibacillosis (n = 10 at 48 h and n = 16 at 72 h after placement) (). E. coli was isolated by pure culture from all pathological lesions tested, although quantification was not undertaken. summarizes the pathology observed at post-mortem.
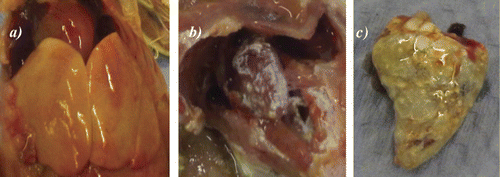
Table 2. Prevalence of pathological lesions associated with colibacillosis identified during post-mortem examination of broiler chicks.
E. coli virulotyping
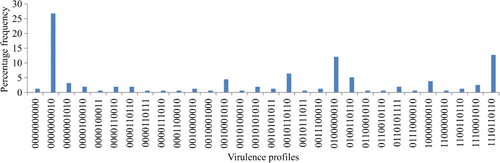
One hundred and fifty-seven extra-intestinal E. coli were screened for carriage of up to 10 VAGs. The overall presence of each VAG is represented in and a summary of the distribution of VAG profiles is presented in .
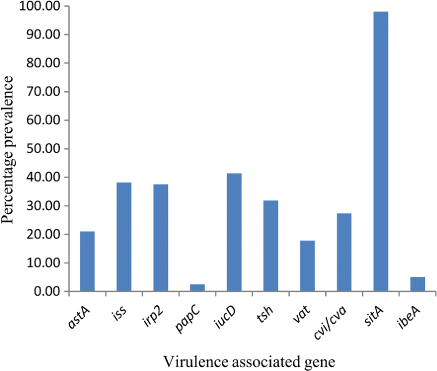
Table 3. Frequency of VAG profiles in E. coli isolates.
Three of the four most prevalent genes identified among the extra-intestinal E. coli are ones involved in iron acquisition (sitA, iucD and irp2, positive in 98.09%, 41.40% and 37.58% of the population respectively). The iss gene is involved in serum survival and was detected in 38.22% of the isolates tested. In the present study only 2.55% of isolates carried the pyelonephritis-associated pili gene (papC). Toxin-producing genes astA and vat were identified in 21.02% and 17.83% of isolates respectively.
Thirty distinct virulence profiles were identified. Isolates with 0/10 VAGs accounted for 1.27% of the total, while the maximum number of VAGs identified in an individual isolate was 7/10 (14.65%). Approximately 30% of E. coli carried five to seven VAGs. In total, 12.7% of E. coli harboured the same VAG profile, positive for astA, iss irp2, iucD, tsh, cvi and sitA, and negative for papC, vat and ibeA. These E. coli were isolated from multiple birds and organs (heart, liver and cellulitis swab). The most abundant virulence profile, representing 42/157 isolates (26.8%), was positive for sitA whilst negative for the remaining nine genes. Multiple VAG profiles were carried by E. coli isolated from the same bird, supporting our previous findings (Kemmett et al., Citation2013). These data are summarized in and . Simpson's diversity index calculations suggest a large degree of diversity among the E. coli sampled (D = 0.915).
E. coli phylogenetic analysis
summarizes the distribution of E. coli into the five phylogenetic groups (A, A0, B1, B2 and D). The most common phylogenetic group was group A (29.94%), while phylogenetic groups largely expected to represent pathogenic E. coli (groups B2 and D) represented 12.74% and 15.29% of E. coli respectively. E. coli with the VAG profile astA, iss, irp2, iucD, tsh, cvi and sitA mentioned above, were assigned to group A or A0 (untypable). Other E. coli with seven VAGs (distinct to the described profile) were classified as group A, B2 or D E. coli, suggesting these isolates were not all clonal. There appeared to be no association between the number of VAGs carried and the phylogentic group: only 75% of group B2 E. coli carried less than five VAGs; and phylogroup A represented the majority of E. coli, carrying seven VAGs.
Table 4. Phylogenetic analysis of extra-intestinal E. coli.
Discussion
In a recent study, we investigated the carriage of a panel of VAGs associated with APEC pathogenicity in commercial broiler intestinal populations of E. coli. This identified the intestines of 1-day-old chicks to be a reservoir rich in potentially pathogenic E. coli. In the present study, we report on the contribution of extra-intestinal E. coli infections to mortalities in the first few days of commercial broiler flock production. Approximately 70% of birds in the present study showed signs of colibacillosis. Olsen et al. (Citation2012) previously reported ∼50% of mortalities in commercial Danish layer flocks were due to E. coli or Enterococcus faecalis infection (Olsen et al., Citation2012).
Poor flock performance has been correlated with increased early mortality rates (Goodhope, Citation1991; Yassin et al., Citation2009). Increased early mortalities have previously been related to hatchery practices (including collection and egg storage length) and broiler breeders (Heier et al., Citation2002; Yassin et al., Citation2009). Young broiler breeders often produce smaller eggs containing increased levels of albumen and lead to lower live chick weights (Vieira & Moran, Citation1998, Citation1999). Older breeders suffer increased navel to yolk sac infections and eggs frequently hatch sooner leading to increased chick dehydration at the hatchery (Suarez et al., Citation1997). Acting as a key indicator, reasons behind early broiler flock mortalities could be central to managing the flock and decreasing overall mortality rates.
Colibacillosis is an economically important poultry infection resulting in increased mortality rates and increased rejections of carcasses at slaughter. Colibacillosis has often been associated with a disease of older broiler chickens (>2 weeks old), but several studies have taken an “integrated poultry production” approach and suggested broiler breeders and hatcheries may be significant reservoirs of early APEC infections either via environmental contamination or vertical transmission (Giovanardi et al., Citation2005; Petersen et al., Citation2006).
The present study supports similar findings to those observed in layer flocks, with E. coli infections being due to multiple strains of E. coli (Olsen et al., Citation2012). Thirty different virulence profiles were identified from 157 E. coli strains. The most prevalent virulence profile was positive for sitA and negative for the other nine genes (0000000010), and sitA was carried by ∼98% of all the isolates. The sitABCD operon encodes an iron and manganese transport system and was shown to contribute to the virulence of APEC chi7122 in a chicken infection model, with a possible additional role in protection against oxidative stress (T. J. Johnson et al., Citation2006; Sabri et al., Citation2006). The sitABCD operon has been identified in over 85% of APEC populations and has been located to ColV plasmids, associated with APEC pathogenicity (Rodriguez-Siek et al., Citation2005a).
Around 30% of isolates carried five to seven VAGs. A conserved profile of astA, iss, irp2, iucD, tsh, cvi and sitA was observed in 12.7% of isolates. The identification of this profile in more than one bird and organ at the same time point (72 h) may suggest a common or related E. coli strain. Phylogenetic analysis using a widely accepted protocol suggests these E. coli fall into phylogroup A or were untypable (A0) (Clermont et al., Citation2000). Phylogroup A has previously largely been associated with non-pathogenic and environmental E. coli (Gordon, Citation2004; Touchon et al., Citation2009). This profile was not identified in the faeces of 1-day-old broilers in the previous study (Kemmett et al., Citation2013). From previous work we know that the intestine of 1-day-old broiler chicks is rich in potentially pathogenic E. coli, with ∼24% of the E. coli population carrying five or more VAGs (Kemmett et al., Citation2013). Phylogenetic analysis highlights the high genetic diversity seen among extra-intestinal E. coli in diseased broiler chicks, with all four major phylogenetic groups represented in the 157 isolates.
The identification of key VAGs involved in systemic infection could be used in developing recombinant vaccines. Lynne et al. (Citation2012) previously tested a recombinant Iss-based vaccine, which showed some promise leading to both serum and mucosal humoral immune responses (Lynne et al., Citation2012). In the present study, the iss gene was carried by almost 40% of E. coli; other protein targets would therefore need to be sought. The vaccination of broiler breeders and the subsequent transfer of maternal antibodies may be the favourable control measure due to the early detection of colibacillosis and the short lifespan of a broiler chicken. Past studies have shown some promise in the ability to transfer protective egg yolk immunoglobulin Y and other immunomodulatory peptides to chicks, although further work is required (Kariyawasam et al., Citation2004; Liou et al., Citation2011; Polanowski et al., Citation2012).
The panel of VAGs used in this investigation is not an exhaustive list of potential targets. Genes involved in bacterial adhesion, invasion, toxin production, serum survival and iron acquisition have all been associated with APEC pathogenesis. The 10 genes used in the current investigation reflect these traits.
To our knowledge, this is the first study to assess the contribution of colibacillosis to early broiler deaths. In summary, the present investigation highlights the significant proportion of early mortalities related to colibacillosis. Furthermore, the identification of a genotypic diverse population in respect to VAG carriage supports the work of others. These findings are unsurprising based on previous findings of a rich reservoir of potentially pathogenic E. coli in the intestinal tract of 1-day-old chicks. This work emphasizes the need for an effective vaccination programme and provides preliminary data, which may be used in deciphering an effective recombinant protein vaccination target. Additionally, improved egg and chick hygiene at the hatchery and during transportation is needed. Further work including the sampling of more farms, hatcheries and breeders along with an increased panel of VAGs will strengthen our knowledge on early mortalities related to colibacillosis in broiler flocks.
Acknowledgements
This work was funded by the Biotechnical and Biological Sciences Research Council and would not have been possible without the continued support of the UK Poultry Industry.
References
- Barnes, H.J. & Gross, W.B. (1997). In B.W. Calnek, H.J. Barnes, C.W. Beard, L.R. McDougald & Y.M. Saif (Eds.), Diseases of Poultry 10th edn (pp. 131–141). Ames: Iowa State University Press.
- Clermont, O., Bonacorsi, S. & Bingen, E. (2000). Rapid and simple determination of the Escherichia coli phylogenetic group. Applied and Environmental Microbiology, 66, 4555–4558. 10.1128/AEM.66.10.4555-4558.2000
- DEFRA. (2013). Poultry and Poultry Meat: Joint Announcement of the Agricultural Departments of the United Kingdom. London: DEFRA.
- Dozois, C.M., Fairbrother, J.M., Harel, J. & Bosse, M. (1992). pap- and pil-related DNA sequences and other virulence determinants associated with Escherichia coli isolated from septicemic chickens and turkeys. Infection and Immunity, 60, 2648–2656.
- Dziva, F., Hauser, H., Connor, T.R., van Diemen, P.M., Prescott, G., Langridge, G.C., Eckert, S., Chaudhuri, R.R., Ewers, C., Mellata, M., Mukhopadhyay, S., Curtiss, R., III, Dougan, G., Wieler, L.H., Thomson, N.R., Pickard, D.J. & Stevens, M.P. (2013). Sequencing and functional annotation of avian pathogenic Escherichia coli serogroup O78 strains reveal the evolution of E. coli lineages pathogenic for poultry via distinct mechanisms. Infection and Immunity, 81, 838–849. 10.1128/IAI.00585-12
- Ewers, C., Antao, E.M., Diehl, I., Philipp, H.C. & Wieler, L.H. (2009). Intestine and environment of the chicken as reservoirs for extraintestinal pathogenic Escherichia coli strains with zoonotic potential. Applied and Environmental Microbiology, 75, 184–192. 10.1128/AEM.01324-08
- Ewers, C., Janssen, T., Kiessling, S., Philipp, H.C. & Wieler, L.H. (2005). Rapid detection of virulence-associated genes in avian pathogenic Escherichia coli by multiplex polymerase chain reaction. Avian Diseases, 49, 269–273. 10.1637/7293-102604R
- Franck, S.M., Bosworth, B.T. & Moon, H.W. (1998). Multiplex PCR for enterotoxigenic, attaching and effacing, and Shiga toxin-producing Escherichia coli strains from calves. Journal of Clinical Microbiology, 36, 1795–1797.
- Germon, P., Chen, Y.H., He, L., Blanco, J.E., Bree, A., Schouler, C., Huang, S.H. & Moulin-Schouleur, M. (2005). ibeA, a virulence factor of avian pathogenic Escherichia coli. Microbiology-Sgm, 151, 1179–1186. 10.1099/mic.0.27809-0
- Giovanardi, D., Campagnari, E., Ruffoni, L.S., Pesente, P., Ortali, G. & Furlattini, V. (2005). Avian pathogenic Escherichia coli transmission from broiler breeders to their progeny in an integrated poultry production chain. Avian Pathology, 34, 313–318. 10.1080/03079450500179046
- Goodhope, R.G. (1991). First week mortality—Influence on production. Second Western Meeting of Poultry Clinicians and Pathologists. Retrieved from http://www.westvet.com/1st_week_mortality.htm.
- Gordon, D.M. (2004). The influence of ecological factors on the distribution and genetic structure of Escherichia coli. In F. Neidhardt, (Ed.), Escherichia coli and Salmonella typhimurium: Cellular and Molecular Biology. Wahington, DC: American Society for Microbiology.
- Heier, B.T., Hogasen, H.R. & Jarp, J. (2002). Factors associated with mortality in Norwegian broiler flocks. Preventive Veterinary Medicine, 53, 147–158. 10.1016/S0167-5877(01)00266-5
- Janssen, T., Schwarz, C., Preikschat, P., Voss, M., Philipp, H.C. & Wieler, L.H. (2001). Virulence-associated genes in avian pathogenic Escherichia coli (APEC) isolated from internal organs of poultry having died from colibacillosis. International Journal of Medical Microbiology, 291, 371–378. 10.1078/1438-4221-00143
- Johnson, J.R. & Stell, A.L. (2000). Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. Journal of Infectious Diseases, 181, 261–272. 10.1086/315531
- Johnson, T.J., Siek, K.E., Johnson, S.J. & Nolan, L.K. (2006). DNA sequence of a ColV plasmid and prevalence of selected plasmid-encoded virulence genes among avian Escherichia coli strains. Journal of Bacteriology, 188, 745–758. 10.1128/JB.188.2.745-758.2006
- Kariyawasam, S., Johnson, T.J. & Nolan, L.K. (2006). The pap operon of avian pathogenic Escherichia coli strain O1: K1 is located on a novel pathogenicity island. Infection and Immunity, 74, 744–749. 10.1128/IAI.74.1.744-749.2006
- Kariyawasam, S., Wilkie, B.N. & Gyles, C.L. (2004). Resistance of broiler chickens to Escherichia coli respiratory tract infection induced by passively transferred egg-yolk antibodies. Veterinary Microbiology, 98, 273–284. 10.1016/j.vetmic.2003.10.022
- Kemmett, K., Humphrey, T., Rushton, S., Close, A., Wigely, P. & Williams, N.J. (2013). A longitudinal study simultaneously exploring the carriage of APEC virulence associated genes and the molecular epidemiology of faecal and systemic E. coli in commercial broiler chickens. PLOS One, 8, e67749. 10.1371/journal.pone.0067749
- Liou, J.F., Shiau, J.W., Tai, C.I. & Chen, L.R. (2011). Production of egg yolk immunoglobulin against Escherichia coli White Leghorn and Lohmann chickens. Journal of Animal and Veterinary Advances, 10, 2349–2356.
- Lynne, A.M., Kariyawasam, S., Wannemuehler, Y., Johnson, T.J., Johnson, S.J., Sinha, A.S., Lynne, D.K., Moon, H.W., Jordan, D.M., Logue, C.M., Foley, S.L. & Nolan, L.K. (2012). Recombinant iss as a potential vaccine for avian colibacillosis. Avian Diseases, 56, 192–199. 10.1637/9861-072111-Reg.1
- McDaniels, A.E., Rice, E.W., Reyes, A.L., Johnson, C.H., Haugland, R.A. & Stelma, G.N. (1996). Confirmational identification of Escherichia coli, a comparison of genotypic and phenotypic assays for glutamate decarboxylase and beta-D-glucuronidase. Applied and Environmental Microbiology, 62, 3350–3354.
- McPeake, S.J.M., Smyth, J.A. & Ball, H.J. (2005). Characterisation of avian pathogenic Escherichia coli (APEC) associated with colisepticaemia compared to faecal isolates from healthy birds. Veterinary Microbiology, 110, 245–253. 10.1016/j.vetmic.2005.08.001
- Mokady, D., Gophna, U. & Ron, E.Z. (2005). Extensive gene diversity in septicemic Escherichia coli strains. Journal of Clinical Microbiology, 43, 66–73. 10.1128/JCM.43.1.66-73.2005
- Olsen, R.H., Frantzen, C., Christensen, H. & Bisgaard, M. (2012). An investigation on first-week mortality in layers. Avian Diseases, 56, 51–57. 10.1637/9777-051011-Reg.1
- Pedroso, A.A., Andrade, M.A., Cafe, M.B., Leandro, N.S.M., Menten, J.F.M. & Stringhini, J.H. (2005). Fertility and hatchability of eggs laid in the pullet-to-breeder transition period and in the initial production period. Animal Reproduction Science, 90, 355–364. 10.1016/j.anireprosci.2005.03.001
- Petersen, A., Christensen, J.P., Kuhnert, P., Bisgaard, M. & Olsen, J.E. (2006). Vertical transmission of a fluoroquinolone-resistant Escherichia coli within an integrated broiler operation. Veterinary Microbiology, 116, 120–128. 10.1016/j.vetmic.2006.03.015
- Polanowski, A., Zablocka, A., Sosnowska, A., Janusz, M. & Trziszka, T. (2012). Immunomodulatory activity accompanying chicken egg yolk immunoglobulin Y. Poultry Science, 91, 3091–3096. 10.3382/ps.2012-02546
- Renema, R.A., Sikur, V.R., Robinson, F.E., Korver, D.R. & Zuidhof, M.J. (2008). Effects of nutrient density and age at photostimulation on carcass traits and reproductive efficiency in fast- and slow-feathering turkey hens. Poultry Science, 87, 1897–1908. 10.3382/ps.2007-00431
- Rodriguez-Siek, K.E., Giddings, C.W., Doetkott, C., Johnson, T.J., Fakhr, M.K. & Nolan, L.K. (2005a). Comparison of Escherichia coli isolates implicated in human urinary tract infection and avian colibacillosis. Microbiology-Sgm, 151, 2097–2110. 10.1099/mic.0.27499-0
- Rodriguez-Siek, K.E., Giddings, C.W., Doetkott, C., Johnson, T.J. & Nolan, L.K. (2005b). Characterizing the APEC pathotype. Veterinary Research, 36, 241–256. 10.1051/vetres:2004057
- Sabri, M., Leveille, S. & Dozois, C.M. (2006). A SitABCD homologue from an avian pathogenic Escherichia coli strain mediates transport of iron and manganese and resistance to hydrogen peroxide. Microbiology-Sgm, 152, 745–758.
- Sanger, F., Nicklen, S. & Coulson, A.R. (1977). DNA sequencing with chain-terminating inhibitors. Proceedings of the National Academy of Sciences of the United States of America, 74, 5463–5467. 10.1073/pnas.74.12.5463
- Skyberg, J.A., Horne, S.M., Giddings, C.W., Wooley, R.E., Gibbs, P.S. & Nolan, L.K. (2003). Characterizing avian Escherichia coli isolates with multiplex polymerase chain reaction. Avian Diseases, 47, 1441–1447. 10.1637/7030
- Suarez, M.E., Wilson, H.R., Mather, F.B., Wilcox, C.J. & McPherson, B.N. (1997). Effect of strain and age of the broiler breeder female on incubation time and chick weight. Poultry Science, 76, 1029–1036.
- Touchon, M., Hoede, C., Tenaillon, O., Barbe, V., Baeriswyl, S., Bidet, P., Bingen, E., Bonacorsi, S., Bouchier, C., Bouvet, O., Calteau, A., Chiapello, H., Clermont, O., Cruveiller, S., Danchin, A., Diard, M., Dossat, C., El Karoui, M., Frapy, E., Garry, L., Ghigo, J.M., Gilles, A.M., Johnson, J., Le Bouguenec, C., Lescat, M., Mangenot, S., Martinez-Jehanne, V., Matic, I., Nassif, X., Oztas, S., Petit, M.A., Pichon, C., Rouy, Z., Saint Ruf, C., Schneider, D., Tourret, J., Vacherie, B., Vallenet, D., Medigue, C., Rocha, E.P.C. & Denamur, E. (2009). Organised genome dynamics in the Escherichia coli species results in highly diverse adaptive paths. Plos Genetics, 5, e1000344. 10.1371/journal.pgen.1000344
- Timothy, S., Shafi, K., Leatherbarrow, A.H., Jordan, F.T.W. & Wigley, P. (2008). Molecular epidemiology of a reproductive tract-associated colibacillosis outbreak in a layer breeder flock associated with atypical avian pathogenic Escherichia coli. Avian Pathology, 37, 375–378. 10.1637/7030
- Vandekerchove, D., De Herdt, P., Laevens, H. & Pasmans, F. (2004). Colibacillosis in caged layer hens: characteristics of the disease and the aetiological agent. Avian Pathology, 33, 117–125. 10.1080/03079450310001642149
- Vieira, S.L. & Moran, E.T. (1998). Eggs and chicks from broiler breeders of extremely different age. Journal of Applied Poultry Research, 7, 372–376.
- Vieira, S.L. & Moran, E.T. (1999). Effects of egg of origin and chick post-hatch nutrition on broiler live performance and meat yields. World's Poultry Science Journal, 55, 125–142. 10.1079/WPS19990009
- Walsh, P.S., Metzger, D.A. & Higuchi, R. (1991). Chelex-100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotechniques, 10, 506–513.
- Yassin, H., Velthuis, A.G.J., Boerjan, M. & van Riel, J. (2009). Field study on broilers’ first-week mortality. Poultry Science, 88, 798–804. 10.3382/ps.2008-00292