Abstract
Chicks possess maternally derived antibody (MDA) against pathogens and vaccines previously encountered by the dams. This passive immunity is important in early life, when the immune system is immature and unable to fight off infection. On the other hand, MDA can also affect the development of the immune system and interfere with vaccination against avian diseases such as Newcastle disease (ND) and avian influenza (AI). The effect of MDA is generally investigated by studying the progeny of vaccinated dams, which is time-consuming, poorly flexible and expensive. Moreover, the antibody titres obtained are not homogeneous. In this study, a model was developed to offer a faster, more reproducible and cheaper way to study passive immunity in specific pathogen free chickens by injection of a polyclonal serum into the egg yolk at embryonic day 14, combined with an intraperitoneal injection at day 1. A satisfactory model, with consistent, homogeneous antibody titres, as well as persistence close to natural passive immunity, could be obtained for ND virus. On the other hand, the application of this optimized protocol in an H5 AI context induced only a low artificial passive immunity compared with that described in the literature for the progeny of AI vaccinated dams. This artificial model should facilitate future studies regarding the effect of passive immunity on vaccine efficacy at a young age and its effect on immune system development.
Introduction
Maternally derived passive immunity is obtained by the vertical transmission of antibodies from a mother to her offspring (Baintner, Citation2007). In birds, maternally derived antibody (MDA) is mainly composed of IgY and to a lesser extent IgA and IgM (Baintner, Citation2007; Alexander & Senne, Citation2008). The amount of IgY MDA in progeny is directly correlated with the IgY concentration in the maternal serum (Al-Natour et al., Citation2004; Hamal et al., Citation2006). During embryogenesis, maternally derived IgY antibodies, packaged into the egg yolk (EY) (Loeken & Roth, Citation1983), are gradually transferred from the EY into the embryonic circulation. This transfer occurs at basal level until embryonic day (ED) 14 and then increases, reaching the highest levels 2 to 3 days before hatch (Kowalczyk et al., Citation1985). In parallel, IgA and IgM are found in the albumen as a mucosal secretion from the oviduct during lay and are transmitted to the embryonic gut after oral absorption (Rose & Orlans, Citation1981; Kaspers et al., Citation1996).
MDA can provide passive protection against pathogens early in life (Al-Natour et al., Citation2004; Staszewski et al., Citation2007; Grindstaff, Citation2008), avoiding the energy required to combat infection with an immature immune system and retaining this saved energy for growth and further development of the immune system (Grindstaff, Citation2008). However, MDA is also responsible for some more adverse effects, inter alia interference with the induction of a specific active immune response by antigen capture, reducing antigen presentation, increasing clearance or reducing the immunological memory (Glezen, Citation2003; Zinkernagel, Citation2003; Lemke et al., Citation2009). The inhibition of the vaccine-induced immune response by MDA has been described for avian pathogens such as Newcastle disease virus (NDV) (Rauw et al., Citation2009), H5 avian influenza virus (AI-H5) (De Vriese et al., Citation2010; Maas et al., Citation2011) and infectious bursal disease virus (van den Berg et al., Citation2000; Ingrao et al.; Citation2013), and is of great concern to the poultry industry.
So far, the effects of MDA on immunological mechanisms have mostly been investigated in progeny from vaccinated dams (Glezen, Citation2003; Lemke et al., Citation2009). However, the levels of antibodies in such dams and their progeny are not consistent because of environmental factors such as the age, condition and diet of the dams (Blount et al., Citation2002), the season, the quality of the mate, the timing of egg collection after vaccination and the vaccination schedule applied (Alexander & Senne, Citation2008), complicating the interpretation and cross-linking of MDA interference studies.
To circumvent these external sources of variation and facilitate the study of the effects of MDA, a model for AI and NDV mimicking natural passive immunity was developed in the present study. Previous models used EY (Addison et al., Citation2010; Staszewski & Siitari, Citation2010) or intraperitoneal (IP) injection (Kim et al., Citation2010). By combining both techniques, an optimal model could be obtained. In this model for NDV, antibody levels similar to natural maternal antibodies were reached, allowing good reproducibility, easy control of the MDA level and high flexibility, as well as cost and time savings. In addition, the antibody persistence approaches the natural decline of NDV MDA. However, application of the model to an AI-H5 polyclonal serum induced lower and less sustainable MDA levels in the progeny. As a dose dependency was demonstrated for NDV, the injection of a higher dose of anti-H5 polyclonal serum could probably improve the AI-H5 model in the future.
This model gives new perspectives for research on the understanding of the mechanisms of action of MDA regarding passive protection, interference with vaccination and immune system development.
Materials and Methods
Virus
The A/crested_eagle/Belgium/01/2004 (Crested eagle 2004) highly pathogenic avian influenza (HPAI) clade 1 H5N1 virus (Van Borm et al., Citation2005) was chemically inactivated with β-propiolactone. The virus was dialysed overnight at 4°C after inactivation and two back-passages were performed in eggs to check the inactivation. The inactivated virus was then purified by centrifugation at 70,000×g for 2 h at 4°C. The precipitate was resuspended overnight under agitation and centrifuged again at 50,000×g for 2.5 h at 4°C through a discontinuous saccharose gradient (20 to 40%). The purified virus was dialysed again overnight in phosphate-buffered saline (PBS) at 4°C.
Chickens
Specific pathogen free (SPF) White Leghorn and commercial Isa Brown layer chickens were hatched from eggs provided by Lohmann Valo (Cuxhaven, Germany) and Wijverkens hatchery (Halle, Belgium), respectively. The NDV MDA-positive commercial Isa Brown layer eggs were obtained from a 28-week-old breeder flock that had been vaccinated following the Belgian standard vaccination schedule: dams were NDV vaccinated at 4 and 8 weeks of age with live NOBILIS Clone30® vaccine (Intervet International B.V., Boxmeer, the Netherlands) followed by a boost at 16 weeks of age with inactivated NOBILIS Newcavac® vaccine (Intervet International B.V). After hatch, all birds were kept in biosecurity level 3 isolators with water and food ad libitum. Bird experiments were conducted under the authorization and supervision of the Biosafety and Bioethics Committees at the Veterinary and Agrochemical Research Institute, following national and European regulations. At the end of each experiment the remaining birds were killed humanely according to national animal welfare regulations.
Production of specific NDV and AI-H5 chicken polyclonal sera
Production of specific NDV polyclonal serum (Pabs-NDV)
Sixty SPF chickens were inoculated oculonasally within the first 24 h of hatching (D1) and boosted at 2 weeks of age with 106 median egg infectious doses/100 µl (50 µl by eye and 50 µl intranasally) with an NDV La Sota strain. Two weeks after the second immunization, each bird was vaccinated intramuscularly with inactivated CEVAC® BROILER ND K vaccine (CEVA Santé Animale, Ceva-Phylaxia, Budapest, Hungary). Blood sampling was performed weekly and birds were killed humanely 3 weeks after the third immunization. The polyclonal serum obtained was sterilized by filtration through a 0.2µm filter and had a mean homologous NDV haemagglutination inhibition (HI) titre of log2 12/25 µl.
Production of specific AI-H5 polyclonal serum
Sixty SPF chickens were immunized intramuscularly at 6 weeks of age with 90 µg/dose of complete Freund's adjuvanted (Sigma-Aldrich, St Louis, Missouri, USA) inactivated purified HPAI clade 1 Crested eagle 2004 H5N1 virus. At 8 weeks of age, the birds were boosted intramuscularly with a 50 µg/dose of incomplete Freund's adjuvanted (Sigma-Aldrich) inactivated purified Crested eagle 2004 H5N1 virus. Three weeks after the last immunization, the birds were boosted by challenging with 105 median egg infectious doses of HPAI clade 1 Crested eagle 2004 H5N1 virus. Ten days after challenge, blood was collected and the birds were killed humanely. Subsequently, all sera were tested by HI test (World Organization of Animal Health (OIE, Paris, France), Citation2012) and samples with an HI titre > log2 7 were pooled. The polyclonal serum obtained was sterilized by filtration through a 0.2 µm filter and had an average homologous AI-H5 HI titre of log2 8/25 µl.
Bird experiments
Development of an NDV-specific passive immunity model following in ovo injection
Three different time points for in ovo injection were compared in three groups of 20 SPF embryonated eggs. Each group received log2 14 HI units (HIU) of Pabs-NDV in 100 µl, at ED10 and ED14 by EY injection and at ED17 by injection into the allantoic cavity. After hatch, all birds were bled and killed humanely for serological study.
In a second study, the dose effect was evaluated in four groups of 14-day-old SPF embryos. Each group received a different dose of Pabs-NDV by EY injection at ED14: 14 log2 HIU/inoculum in 100 µl (n = 10), 15 log2 HIU/inoculum in 200 µl (n = 10), 15.6 log2 HIU/inoculum in 300 µl (n = 10) or 16 log2 HIU/inoculum in 400 µl (n = 20). At hatch, birds were killed humanely for serological survey of the artificially-induced MDA (aMDA). Ten naïve non-immunized SPF birds were used as hatchability controls. In all in ovo studies, eggs were candled before injection to determine the position of the embryo and the yolk sac.
Development of an NDV-specific passive immunity model following intraperitoneal injection
Two groups of 20 SPF chickens were injected intraperitoneally at D1, within the first 24 h of hatch, with 13 and 15 log2 HIU/inoculum respectively of Pabs-NDV in 200 µl. For serological survey purposes, five birds were bled 1, 3, 6 and 9 h post injection (h.p.i.) in the group that received 13 log2 HIU/inoculum of Pabs-NDV and five birds were bled 1, 6, 9, 24 h.p.i. in the group that received 15 log2 HIU/inoculum.
Five SPF birds, immunized with serum collected from 6-week-old antibody-free SPF chickens, were used as negative controls and killed humanely at 24 h.p.i.
Development of an NDV-specific passive immunity model following a combination of egg yolk and intraperitoneal injection
Seventy-five 1-day-old chicks that had been EY injected (16 log2 HIU/400 µl) were divided into two groups. At hatch (D1), 10 birds were bled and killed humanely for serological studies. One group (n = 35) (EY + IP group) received 15 log2 HIU/200 µl of Pabs-NDV by IP injection at D1, while the second group of 14 birds was not injected intraperitoneally (EY group). In the EY + IP group, five birds were bled and killed humanely at D2 (24 h.p.i.), D8, D12, D18 and D21. The remaining 10 birds were killed humanely at D28. In the EY group, four birds were bled and killed humanely at D2, whilst blood sampling was performed on the 10 remaining birds at D7, D14 and D20 for comparative serology with the EY + IP group. An additional group comprising 10 non-immunized SPF birds was bled at hatch for confirmation of their negative antibody status.
NDV-positive progeny of commercial vaccinated dams (NDV(+)MDA)
Sixty commercial Isa Brown NDV-MDA birds were hatched in-house. Blood samples were collected at D2 (n = 10), D7 (n = 10), D14 (n = 40), D20 (n = 40) and D28 (n = 30) after hatch in order to follow MDA antibody levels.
AI H5 specific passive immunity model
Thirty-five SPF eggs at ED14 received log2 10 HIU/400 µl of PabsAI-H5 by EY injection. At hatch (D1), three birds were bled and all hatched birds received log2 11 HIU/200 µl of PabsAI-H5 by IP injection. Five birds were bled at D2, D6, D10 and D14, and the remaining birds were humanely killed at D17. Five non-immunized SPF birds were bled at hatch for confirmation of their SPF status.
Serological tests
Haemagglutination inhibition test
HI tests are considered the gold standard and were conducted following current standard procedures using 4 haemagglutinating units of A/crested-eagle/Belgium/01/2004 HPAI H5N1 or NDV La Sota strain virus per well as antigen (World Organization of Animal Health (OIE), Citation2012). The arithmetic mean HI titres of groups were expressed as log2 and were considered positive when the titre was > log2 3 (threshold). All birds presenting HI titre ≤ log2 3 received by default a value of 2 for statistical purposes.
NDV-specific and AI-H5-specific anti-IgY, anti-IgA and anti-IgM assays
NDV-specific anti-IgY, anti-IgA and anti-IgM enzyme-linked immunosorbent assays (ELISAs) were performed as described previously (Rauw et al., Citation2009). Sera were diluted 400-fold for the test.
For the AI-H5-specific assays, MaxiSorp Nunc-Immuno F96 microwell plates (Nunc, VWR, Leuven, Belgium) were coated overnight at 4°C with inactivated and purified H5N2 (A/Chicken/Belgium/150VB/99; Rauw et al., Citation2011) at 2 µg/ml in carbonate/bicarbonate buffer pH 9.6. Subsequently, the plates were washed three times with 0.1% Tween 80-supplemented PBS. Plates were blocked for 30 min at 37°C with PBS containing 2.5% casein. Plates were then incubated with 100-fold diluted samples in PBS containing 0.1% Tween 80, 5% NaCl and 4% BSA for 1 h at room temperature. After three washes, biotin-labelled mouse antibody directed against chicken IgY (Southern Biotech, Antwerpen, Belgium) at a dilution of 1:1000 was added for 1 h at room temperature for IgY assay. For AI IgA and IgM assays, biotin-labelled mouse antibody directed against chicken IgA at a dilution of 1:1000 (Southern Biotech) and goat antibody directed against chicken IgM at a dilution of 1:2000 (Nordic Immunological Labs, Susteren, The Netherlands) respectively, was added for 1 h at room temperature. After three washes, all plates were incubated with streptavidin–horseradish peroxidase conjugate (Biosource Europe, Nivelle, Belgium) for 1 h at room temperature. After six washes, peroxidase activity was revealed by adding 100 µl tetra-methyl benzidine peroxidase substrate (ThermoLabSystems, Erembodegem, Belgium) for 15 min in darkness before stopping the reaction with 1 M H3PO4 buffer. The optical density (OD) was determined at 450 to 560 nm with an ELISA reader. ELISA data were normalized and expressed as the sample/negative (S/N) ratio, which was calculated as follows:
Statistical analysis
Analysis of the serological data was carried out using Minitab 13 software (Minitab Ltd, Coventry, UK) and differences were considered significant at P < 0.05. After checking the validity hypotheses, one-way analysis of variance and Fisher's pairwise comparison tests were carried out on this criterion to compare different groups. When normality or homogeneity of variance failed, the non-parametric Kruskal–Wallis test was used. The humoral immune response by HI test was compared between NDV-MDA (NDV-aMDA) induced in this model and NDV(+)MDA-positive controls birds by the Student t test in order to underline the mimicking effect of the aMDA.
Results
Development of an NDV passive immunity model
In ovo injection
EY injection of Pabs-NDV at ED10 induced a seroconversion in 47% (8/17) of the birds (HI titre ≥ log2 3), although the average NDV seroconversion remained negative (log2 3.0 ± 1.1) (). EY injection at ED 14 resulted in 78% (22/28) of birds seroconverting, with a significantly higher mean NDV titre (log2 3.8 ± 1.1). In contrast, none of the birds seroconverted in the ED17 group inoculated in the allantoic cavity.
Table 1. Development of an NDV passive immunity model: comparison of routes of injection and doses.
None of the Pabs-NDV injections performed at three different time points (ED10, ED14 and ED17) adversely affected hatchability (85%, 93% and 90%, respectively) as compared with the non-inoculated controls (60%) ().
To further increase the passive NDV HI titres after injection at ED14, the dose of Pabs-NDV injected was increased up to log2 16. The highest dose induced a significantly higher HI titre and increased the number of birds seroconverting (log2 6.2 ± 0.9, 15/15 positive), compared with the log2 14, log2 15 and log2 15.6 HIU doses (log2 3.8 ± 1.1, 22/28 seroconverting; log2 5.0 ± 1.5, 5/6 seroconverting; log2 4.7 ± 1.7, 7/9 seroconverting, respectively).
The persistence of the artificially-induced NDV passive immunity after EY injection of 16 log2 HIU of Pabs-NDV was evaluated over a 4-week period () and was compared with the natural waning of NDV-MDA in the progeny of vaccinated dams. An average NDV seroconversion was detected at D2 (log2 4.8 ± 1.9) and D7 (log2 3.6 ± 1.2), with 75% and 70% of birds seroconverting, respectively. However, the average HI titres remained significantly lower than in the naturally vaccinated NDV(+)MDA controls at the corresponding time points (log2 8.4 ± 0.7 and log2 7.9 ± 1.0, respectively). The artificially-induced passive immunity became undetectable at 2 weeks of age, unlike antibody in the NDV(+)MDA controls. For the EY immunized group, only one bird remained positive at D14, resulting in no overall seroconversion (titre log2 2.2 ± 0.6), compared with more than one-half (20/30, 67%) of natural NDV(+)MDA controls remaining positive until D28 (titre log23.3 ± 1.2).
Table 2. Duration of artificially-induced NDV passive immunity as measured by HI test after individual EY or combined EY and IP injections at ED14 and D1, respectively, in comparison with natural passive immunity in conventional Isa Brown layers.
Intraperitoneal injection
The IP injection demonstrated a clear dose dependency (). Significantly higher antibody titres were observed at 24 h.p.i. when injecting log2 15 HI units (mean titre log2 5.6 ± 2.2; 4/5 positive) instead of log2 13 HI units (mean titre log2 3.2 ± 1.6; 2/5 positive). However, natural antibody levels (log2 8.4 ± 0.7) were not reached. No adverse effect of the IP injection itself was observed in the negative controls (data not shown).
Table 3. Duration of the artificially-induced NDV passive immunity as measured by HI test after IP injection at D1, in comparison with the natural passive immunity of conventional Isa Brown layers.
Combination of egg yolk and intraperitoneal injection
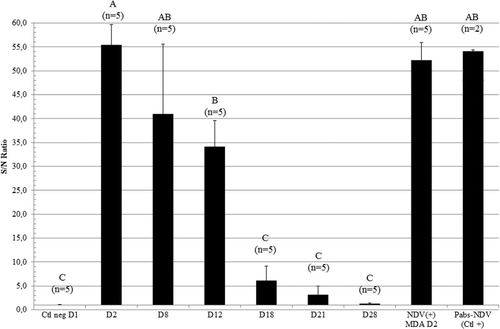
To further optimize the transfer of the artificially-induced passive immunity, a combination of EY and IP injections was attempted. This combination significantly increased the NDV HI titres (log2 8.6 ± 0.5; 5/5 positive) when compared with EY or IP injections alone (log2 4.8 ± 1.9, 3/4 positive and log2 5.6 ± 2.2, 4/5 positive, respectively) at D2. A statistically similar level of NDV seroconversion as compared with NDV(+)MDA controls was reached at D2 in the EY + IP inoculated birds ().
To determine the duration of the NDV passive immunity, the decay of NDV antibody was evaluated over a 4-week period (). When combining EY + IP routes of inoculation, the duration of the positive response was significantly extended to D18 compared with D14 for EY immunization alone. For the NDV(+)MDA controls, the majority (35/40) of birds were still seropositive (log2 4.6 ± 1.2) at D21, after which titres further declined to log2 3.3 ± 1.2 at D28 (20/30 positive).
In the EY + IP group, the same profile of the passively transferred NDV antibodies was shown using an NDV-specific anti-IgY ELISA (), with the highest levels of antibodies observed at D2 and a significant amount of anti-NDV IgY detected at D12. At D18, no difference could be observed from the negative control birds. No specific anti-NDV IgA or IgM were detected in either EY + IP birds or NDV(+)MDA control birds at D2 (data not shown).
AI-H5 passive immunity model
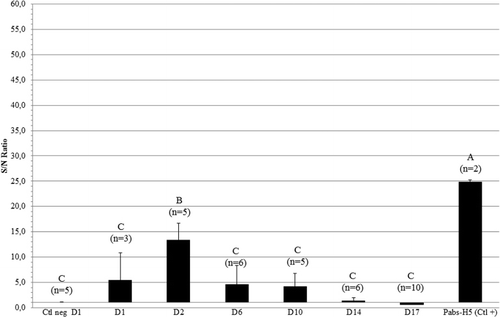
The applicability and duration of the above-described protocol for induction of passive immunity was also investigated for AI-H5 antibodies. To determine the moment of the fade-out of H5-aMDA, blood samples were collected more frequently between D2 and D14 (at D6 and D10) than in the NDV-aMDA study. EY injection at ED14 (log210 HIU/dose) did not allow detection of an AI-H5 seroconversion by HI test at D1. However, using a home-made AI-H5 ELISA, transmission of Pabs-AI-H5 from the EY to the embryo could be detected (). A mean AI-H5 seroconversion could be detected by HI test at D2, D6 and D10, with mean titres of log2 3.8 ± 1.1 (4/5 positive), log2 3.7 ± 1.4 (4/6 positive) and log2 3.6 ± 0.9 (4/5 positive) respectively (data not shown). At D14 and D17, no seroconversion could be detected (data not shown). As with the NDV study, no specific anti-AI-H5 IgA or IgM were detected in the Pabs-AI-H5.
Discussion
The progeny of vaccinated dams are most often used to study the effect of MDA (Naqi et al., Citation1983; Grindstaff, Citation2008; Rauw et al., Citation2009; Sarfati-Mizrahi et al., Citation2010). This approach is time-consuming, poorly flexible and expensive, and the antibody titres obtained are poorly reproducible as the level of passive immunity of the chicks is directly dependent on the immunity of the dams, which itself might be quite heterogeneous (Hamal et al., Citation2006). Moreover, the level of passive antibody transmission to progeny depends on numerous external factors (Blount et al., Citation2002; Hamal et al., Citation2006; Alexander & Senne, Citation2008). In order to obtain a better control of MDA levels, an alternative approach independent of dams’ vaccination and other variable factors is greatly desirable.
To this aim, we developed and optimized a model mimicking the transfer of natural passive NDV immunity in SPF chickens. Different modes, timing and dosage of injection were evaluated. The best results were obtained after EY injection at ED14, corresponding to the time when the transfer of MDA from the EY to the embryo accelerates (Kowalczyk et al., Citation1985). Injection at ED10 was probably too early and the antibodies could have been partially degraded before the peak of transmission. At ED17 the transfer of passive immunity did not occur, which could be explained by the fact that injection was performed into the allantoic cavity instead of the EY to prevent damage to the embryo. Most probably, the allantoic vein or intestinal epithelium do not possess functional IgY specific receptors (FcRY) allowing the transfer of MDA from the egg white into the embryonic circulation. FcRY are pH-dependent receptors, binding IgY and inducing endocytosis at acidic EY pH but not at neutral or basic pH (Tesar et al., Citation2008) as measured in the egg white (Van der Plancken et al., Citation2006) or in the intestinal tract (Herpol & Van Grembergen, Citation1967). Dose dependency was demonstrated at ED14 and resulted in an increase in NDV HI titre in the chicks without affecting hatchability. However, the NDV seroconversion was not comparable, either in HI titre or in duration of antibody detection to that found in NDV(+)MDA control birds hatched from vaccinated dams. Passive immunization against NDV has previously been performed in quail by EY injection of specific anti-NDV antibodies (Staszewski & Siitari, Citation2010). In that study, NDV seroconversion was obtained at D2 but was lower than that seen in progeny of vaccinated dams, and allowed the study of the blocking effect of passive antibodies on the B-cell immunological memory (Staszewski & Siitari, Citation2010). However, no detailed information on the duration of the immune response was provided in that study. Moreover, the EY technique was not rapid as it requires vaccination of the dams.
Another way to mimic the natural uptake of MDA from the yolk sac is by IP injection. At hatch, the yolk sac is not completely resorbed by the chick (Noy et al., Citation1996) and still fills a large part of the abdominal cavity. Therefore IP studies were performed in newly hatched 1-day-old SPF chicks in order to attempt to achieve MDA levels similar to the natural peak observed at D2 (Hamal et al., Citation2006). By increasing the dose, a significant increase in the artificially-induced NDV seroconversion could be detected, but titres did not reach the levels seen in the NDV(+)MDA controls. The IP injection of an anti-AI-H5 polyclonal serum at day 7 has been described previously to demonstrate the role of MDA in failures of commercial AI-H5 poultry vaccines in Egypt (Kim et al., Citation2010). However, no AI-H5 HI titres could be detected and, again, no data were given concerning the duration of the passive immune response.
To achieve a consistent level and durability of antibodies induced passively and in the progeny, similar to that seen in natural conditions, EY or IP injections alone were insufficient. To further increase the artificially-induced MDA titres, a combination of EY and IP injections was assessed and high MDA levels comparable with those seen in chicks with naturally acquired NDV(+)MDA were reached at the same age. Furthermore, by combining EY and IP injections, the duration of the passive immunity was prolonged up to 3 weeks (D18), approaching the duration of natural passive immunity, present for 3 to 4 weeks.
This NDV optimized protocol was also evaluated for AI immunity transfer. AI-H5 seroconversion could be detected at D2, D6 and D10. However, the AI-H5 antibody levels obtained remained lower than observed in progeny from AI-H5 vaccinated dams as described in the literature (De Vriese et al., Citation2010; Sarfati-Mizrahi et al., Citation2010). Moreover, the decline of the AI-H5 passive immunity was approaching (up to 10 days) that which had previously been shown under natural conditions (about 15 days) (De Vriese et al., Citation2010). But unlike NDV, the AI-H5 passive immunity obtained did not reach that found under natural conditions. This difference could, at least partly, be explained by the lower antibody dose used to immunize the progeny.
During the production of the polyclonal sera, IgY was shown to be the predominant antibody in the serum, while IgA and IgM were absent in the polyclonal sera and therefore in the passive serum. IgA and IgM in newly hatched birds offer mucosal immunity in the gut as primary defence of the intestinal tract (Hamal et al., Citation2006). This situation is similar to the natural situation in the EY or in NDV(+)MDA birds, where very limited quantities of IgA and IgM antibodies can be detected (Hamal et al., Citation2006). Therefore, natural local passive immunity induced by IgA and IgM in the intestinal tract cannot be mimicked by this model. Consequently, this model would not be appropriate to study the effect of local passive immunity.
In conclusion, in the present work a bird model reproducing systemic passive immunity was optimized in SPF chickens by a combination of EY injection at ED14 and IP injection at D1. This technique proved to be time saving and cost-effective, easy to perform and reproducible. For NDV, this resulted in good IgY passive antibody levels and persistence, approaching natural situations, while lower levels were obtained in the AI-H5 system. This can most probably be improved by increasing the dose applied.
This protocol appears to be a useful tool to allow a rapid evaluation of the potency of new commercial vaccines against avian pathogens. Moreover, it should allow a better standardization and improved reproducibility of future studies aiming at investigating the mechanisms behind the interference of MDA on early vaccination, as well as its impact on the development of the immune system.
Acknowledgements
This research was financially supported by the Veterinary and Agrochemical Research Center (Ukkel, Belgium) (project NewfluMDA). The authors gratefully acknowledge F. Rauw for the statistical analyses, M. Boschmans, S. Lemaire, M. Gonze and A. Ausloos for technical assistance, and C. Delgrange and M. Vandenbroeck for taking care of the birds.
References
- Addison, B., Ricklefs, R.E. & Klasing, K.C. (2010). Do maternally derived antibodies and early immune experience shape the adult immune response? Functional Ecology, 24, 824–829. 10.1111/j.1365-2435.2010.01706.x
- Alexander, D.J. & Senne, D.A. (2008). Newcastle disease, other avian paramyxoviruses, and pneumovirus infection. In Y.M. Saif, A.M. Fadly, J.R. Glisson, L.R. McDougald, L.K. Nolan, & D.E. Swayne (Eds.). Diseases of Poultry 12th edn (pp. 75–100). Ames: Iowa State Press.
- Al-Natour, M.Q., Ward, L.A., Saif, Y.M., Stewart-Brown, B. & Keck, L.D. (2004). Effect of diffferent levels of maternally derived antibodies on protection against infectious bursal disease virus. Avian Diseases, 48, 177–182. 10.1637/5319
- Baintner, K. (2007). Transmission of antibodies from mother to young: evolutionary strategies in a proteolytic environment. Veterinary Immunology and Immunopathology, 117, 153–161. 10.1016/j.vetimm.2007.03.001
- Blount, J.D., Surai, P.F., Nager, R.G., Houston, D.C., Møller, A.P., Trewby, M.L. & Kennedy, M.W. (2002). Carotenoids and egg quality in the lesser black backed gull Larus fuscus: a supplemental feeding study of maternal effects. Proceedings of the Royal Society of London B: Biological Sciences, 269, 29–36. 10.1098/rspb.2001.1840
- De Vriese, J., Steensels, M., Palya, V., Gardin, Y., Dorsey, K.M., Lambrecht, B., Van Borm, S. & van den Berg, T. (2010). Passive protection afforded by maternally-derived antibodies in chickens and the antibodies’ interference with the protection elicited by avian influenza-inactivated vaccines in progeny. Avian Diseases, 54, 246–252. 10.1637/8908-043009-Reg.1
- Glezen, W. (2003). Effect of maternal antibodies on the infant immune response. Vaccine, 21, 3389–3392. 10.1016/S0264-410X(03)00339-6
- Grindstaff, J.L. (2008). Maternal antibodies reduce costs of an immune response during development. The Journal of Experimental Biology, 211, 654–660. 10.1242/jeb.012344
- Hamal, K.R., Burgess, S.C., Pevzner, I.Y. & Erf, G.F. (2006). Maternal antibody transfer from dams to their egg yolks, egg whites, and chicks in meat lines of chickens. Poultry Science, 85, 1364–1372.
- Herpol, C. & Van Grembergen, G. (1967). La signification du pH dans le tube digestif de Gallus domesticus [An interpretation of pH values in the digestive tract of the domestic hen]. Annales de Biologie Animale Biochimie Biophysique, 7, 33–38. 10.1051/rnd:19670104
- Ingrao, F., Rauw, F., Lambrecht, B. & van den Berg, T. (2013). Infectious bursal disease: a complex host-pathogen interaction. Developmental and Comparative Immunology, 41, 429–438. 10.1016/j.dci.2013.03.017
- Kaspers, B., Bondl, H. & Göbel, T.W. (1996). Transfer of IgA from albumen into the yolk sac during embryonic development in the chicken. Zentralblatt für Veterinärmedizin. Reihe A, 43, 225–231. 10.1111/j.1439-0442.1996.tb00448.x
- Kim, J.-K., Kayali, G., Walker, D., Forrest, H.L., Ellerby, A.H., Griffin, Y.S., Rubrum, A., Bahgat, M.M., Kutkat, M.A, Ali, M.A.A., Aldridge, J.R., Negovetich, N.J., Krauss, S., Webby, R.J. & Webster, R.G. (2010). Puzzling inefficiency of H5N1 influenza vaccines in Egyptian poultry. Proceedings of the National Academy of Sciences of the United States of America, 107, 11044–11049. 10.1073/pnas.1006419107
- Kowalczyk, K., Diss, J., Halpern, J. & Roth, T.F. (1985). Quantitation of maternal-fetal IgG transport in the chicken. Immunology, 54, 755–762.
- Lemke, H., Tanasa, R.I., Trad, A. & Lange, H. (2009). Benefits and burden of the maternally-mediated immunological imprinting. Autoimmunity Reviews, 8, 394–399. 10.1016/j.autrev.2008.12.005
- Loeken, M.R. & Roth, T.F. (1983). Analysis of maternal IgG subpopulations which are transported into the chicken oocyte. Immunology, 49, 21–28.
- Maas, R., Rosema, S., van Zoelen, D. & Venema, S. (2011). Maternal immunity against avian influenza H5N1 in chickens: limited protection and interference with vaccine efficacy. Avian Pathology, 40, 87–92. 10.1080/03079457.2010.541226
- Naqi, S.A., Marquez, B. & Sahin, N. (1983). Maternal antibody and its effect on infectious bursal disease immunization. Avian Diseases, 27, 623–631. 10.2307/1590304
- Noy, Y., Uni, Z. & Sklan, D. (1996). Routes of yolk utilisation in the newly-hatched chick. British Poultry Science, 37, 987–996. 10.1080/00071669608417929
- Rauw, F., Anbari, S., van den Berg, T. & Lambrecht, B. (2011). Measurement of systemic and local respiratory cell-mediated immunity after influenza infection in chickens. Veterinary Immunology and Immunopathology, 143, 27–37. 10.1016/j.vetimm.2011.05.029
- Rauw, F., Gardin, Y., Palya, V., van Borm, S., Gonze, M., Lemaire, S., van den Berg, T. & Lambrecht, B. (2009). Humoral, cell-mediated and mucosal immunity induced by oculo-nasal vaccination of one-day-old SPF and conventional layer chicks with two different live Newcastle disease vaccines. Vaccine, 27, 3631–3642. 10.1016/j.vaccine.2009.03.068
- Rose, M.E. & Orlans, E. (1981). Immunoglobulins in the egg, embryo and young chick. Developmental and Comparative Immunology, 5, 15–20. 10.1016/S0145-305X(81)80003-1
- Sarfati-Mizrahi, D., Lozano-Dubernard, B., Soto-Priante, E., Castro-Peralta, F., Flores-Castro, R., Loza-Rubio, E. & Gay-Gutiérrez, M. (2010). Protective dose of a recombinant Newcastle disease LaSota-avian influenza virus H5 vaccine against H5N2 highly pathogenic avian influenza virus and velogenic viscerotropic Newcastle disease virus in broilers with high maternal antibody levels. Avian Diseases, 54, 239–241. 10.1637/8735-032509-Reg.1
- Staszewski, V., Gasparini, J., McCoy, K.D., Tveraa, T. & Boulinier, T. (2007). Evidence of an interannual effect of maternal immunization on the immune response of juveniles in a long-lived colonial bird. The Journal of Animal Ecology, 76, 1215–1223. 10.1111/j.1365-2656.2007.01293.x
- Staszewski, V. & Siitari, H. (2010). Antibody injection in the egg yolk: maternal antibodies affect humoral immune response of the offspring. Functional Ecology, 24, 1333–1341. 10.1111/j.1365-2435.2010.01745.x
- Tesar, D.B., Cheung, E.J. & Bjorkman, P.J. (2008). The chicken yolk sac IgY receptor, a mammalian mannose receptor family member, transcytoses IgY across polarized epithelial cells. Molecular Biology of the Cell, 19, 1587–1593. 10.1091/mbc.E07-09-0972
- Van Borm, S., Thomas, I., Hanquet, G., Lambrecht, B., Boschmans, M., Dupont, G., Decaestecker, M., Snacken, R. & van den Berg, T. (2005). Highly pathogenic H5N1 influenza virus in smuggled Thai eagles, Belgium. Emerging Infectious Diseases, 11, 702–705. 10.3201/eid1105.050211
- van den Berg, T. (2000). Acute infectious bursal disease in poultry: a review. Avian Pathology, 29, 175–194. 10.1080/03079450050045431
- Van der Plancken, I., Van Loey, A. & Hendrickx, M.E. (2006). Effect of heat-treatment on the physico-chemical properties of egg white proteins: a kinetic study. Journal of Food Engineering, 75, 316–326. 10.1016/j.jfoodeng.2005.04.019
- World organization of animal health (OIE). (2012). Chapter 2.3.4: Avian Influenza. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. ISBN 978-92-9044-878-5, 7th edn, pages 436-454.
- Zinkernagel, R.M. (2003). On natural and artificial vaccinations. Annual Review of Immunology, 21, 515–546. 10.1146/annurev.immunol.21.120601.141045
