Abstract
Infectious laryngotracheitis (ILT) is an economically important respiratory disease of poultry that affects the poultry industry worldwide. The disease is caused by gallid herpesvirus I (GaHV-1), a member of the genus Iltovirus, family Herpesviridae, subfamily Alphaherpesvirinae. The current incidence of the disease is heavily influenced by live attenuated vaccines, which have been used extensively since their introduction in the mid-twentieth century. The capability of current live attenuated vaccine viruses to revert to virulence and spread from bird to bird has shaped the molecular epidemiology of ILT. Because of the antigenic homogeneity among GaHV-1 strains, differentiation of strains has been achieved by targeting genomic differences between outbreak-related isolates and vaccine strains. Numerous genes and genomic regions have been utilized in the development of DNA-based diagnostic assays to differentiate outbreak-related isolates from vaccine strains in countries where ILT outbreaks have occurred. More recently, full genome sequences have allowed determination of the origin of some of the outbreak-related isolates circulating in some poultry production countries. Overall, molecular typing data collected worldwide have identified live attenuated vaccine-related isolates as the primary source for outbreaks of the disease.
Introduction
Infectious laryngotracheitis (ILT) is an upper respiratory disease of chickens, pheasants, and peafowl caused by the alphaherpesvirus gallid herpesvirus 1 (GaHV-1) (Guy & Garcia, Citation2008). The virus is shed in respiratory secretions, easily transmitted by inhalation or mechanically transmitted by people and fomites. ILT is characterized by acute respiratory disease with clinical signs including conjunctivitis, nasal discharge, and decrease in egg production. In severe forms of the disease, haemorrhagic tracheitis together with gasping, coughing, and expectoration of bloody mucus are common. ILT occurs worldwide, and morbidity and mortality rates vary depending on the virulence of the circulating strain (Kirkpatrick et al., Citation2006a; Oldoni et al., Citation2009), the levels of virus circulating in the field and infections with other respiratory diseases of poultry (Guy & Garcia, Citation2008). With an overall mortality due to ILT that can reach 70%, large economic losses usually occur in high-density poultry-producing regions (Bagust et al., Citation2000).
Since the identification of the disease in the 1920s, ILT continues to negatively impact the poultry industry across the globe. The disease is primarily controlled by vaccination with live attenuated vaccines, which have been attenuated either by sequential passages in cell culture (tissue culture origin [TCO]) or sequential passages in chicken embryos (chick embryo origin [CEO]) (Guy & Garcia, Citation2008). In particular, the CEO vaccines are largely associated with reversion to virulence (Guy et al., 1991) during vaccinal laryngotracheitis outbreaks (Dufour-Zavala, Citation2008). The use of modern molecular typing has been fundamental in understanding the epidemiology of the disease. The aim of this review is to define the impact that molecular typing has had in our understanding of ILT epidemiology and to describe the evolution of molecular assays utilized to differentiate GaHV-1 strains worldwide.
GaHV-1 live attenuated vaccines
A detailed review of ILT vaccines was published recently (Coppo et al., Citation2013) but in order to appreciate the epidemiology of ILT, a brief history of the development of ILT live attenuated vaccines is necessary. The disease was first reported in 1925 in Canada, followed by the USA in 1926, Australia and Great Britain in 1935, and Europe in 1940 (Cover, Citation1996). By 1962 the disease had been described in at least 40 countries (Pulsford, Citation1963). Gibbs described brush vent application as the first vaccination method for ILT (Gibbs, Citation1933, Citation1934). Live virulent virus isolated from tracheal scraping preparations was used for this. Response of birds to the vaccine involved inflammation of the cloacal mucosa 3 to 8 days post vaccination (Gibbs Citation1933, Citation1934). Although vaccination provided birds with protection against disease, sterilizing immunity was not achieved and vent-vaccinated birds were still able to shed live virulent virus.
Development of the chorioallantoic membrane virus propagation method by Brandly (Citation1935) gave way to efforts in the 1950s and 1960s to attenuate field strains by consecutive passages in chicken embryos. These vaccines exhibited reduced levels of virulence, increased safety, and improved efficacy. Worldwide adoption of this method, coupled with successive embryo passages of field viruses, gave rise to various attenuated strains. Of these, the Cover, Hudson, Samberg, SA2, A20, and Serva vaccine strains () are still in use, and are classified as CEO vaccines because of the common use of chicken embryos for their attenuation, even though the embryo inoculation routes varied slightly. In 1958 Cover and Benton first described the existence of a field strain with lower levels of virulence, capable of protecting birds against challenge 19 days post intratracheal inoculation (Benton et al., Citation1958); this strain came to be known in the USA as the CEO Cover strain vaccine. In 1969 the use of the CEO-attenuated Hudson strain was described for ocular, intranasal, or intratracheal vaccination (Hudson, Citation1969). In Israel, viruses from acute field cases of ILT were attenuated by chorioallantoic membrane passages and resulted in the CEO Samberg strain intended for intra-ocular or vent-brush application (Samberg & Aronovici, Citation1969a). In 1966, an Australian field isolate attenuated by serial passages in chick embryos resulted in the CEO SA2 vaccine strain. By 1983 the SA2 vaccine strain was further attenuated, in chicken embryonic cell cultures, to generate the A20 vaccine strain (Kirkpatrick et al., Citation2006a). The European Serva vaccine is also a chicken embryo passaged attenuated strain, but its exact origin is not known.
Table 1. Live attenuated ILT vaccines produced worldwide.Footnotea
Mass application of these CEO vaccines in drinking water provided producers with an economical option to control production losses due to the disease. However, as early as 1969 failures to vaccinate all birds uniformly by the drinking water vaccination method allowed vaccine strains to regain their virulence while circulating in unvaccinated birds (Samberg & Aronovici, Citation1969b; Samberg et al., Citation1971). Back passage of the CEO vaccine viruses through inadequately vaccinated flocks with concurrent reversion to virulence remains one of the main sources of GaHV-1 outbreaks in the USA and worldwide.
In 1964 the first tissue culture (TCO) modified vaccine was developed (Gelenczei & Marty, Citation1964); its attenuation was obtained by consecutive passages of the virulent ASL L-6 strain in primary tissue cultures of chicken cells. The TCO vaccine provided birds with immunity after ocular or intranasal application. In contrast to CEO, mass application of the TCO vaccine is not an option because eye-drop application is the sole delivery method (Gelenczei & Marty, Citation1964). Similar to CEO vaccines, the TCO vaccine can replicate in the trachea and conjunctiva, although to a lesser extent than the CEO vaccines, and both vaccines can transmit from bird to bird (Rodríguez-Avila et al., Citation2007).
Currently, vaccination with TCO or CEO vaccines remains common practice in the USA and worldwide (). Vaccination is performed either in a preventive manner, as is typically done in layers and breeding stock, or in the face of an outbreak in broilers (Guy & Garcia, Citation2008). Delivery of live attenuated GaHV-1 vaccines by the drinking water or spray methods are the most desirable modes for rapid mass applications. These methods can be effective, as long as vaccination is done in a manner that ensures the highest possible coverage of the flock using a high-titre vaccine and following established procedures for mass vaccination of poultry (Klausz, Citation2008).
Application of the CEO vaccine via drinking water relies on contact of the vaccine with the nasal cavity during the act of drinking (Robertson & Egerton, Citation1981; Loudovaris et al., Citation1991; Devlin et al., Citation2008). Uneven coverage of a flock allows the vaccine strains to circulate for prolonged periods within the flock. Consequently vaccine strains gain virulence, resulting in outbreaks of vaccinal laryngotracheitis. Clinical signs and losses from vaccinal laryngotracheitis outbreaks can range from mild to severe. However, most producers are willing to bear the production losses associated with vaccination as opposed to risking potential losses that would occur if the disease was not controlled (Zavala, Citation2008). In long-lived birds, a 2-week acute infection phase is followed by a latent infection that results in a cyclical pattern of outbreaks triggered by stress-induced reactivation of the latent alphaherpesvirus. In broiler production, vaccination coupled with shorter down-time between flocks results in a cyclical pattern that leads to increased viral loads, which can potentiate the spread to surrounding operations and result in the emergence of virulent viral strains, the cause of devastating outbreaks of the disease.
Studies in Taiwan (Chang et al., Citation1997), Northern Ireland (Graham et al., Citation2000), and Australia (Kirkpatrick et al., Citation2006b) suggest that, since their introduction, live attenuated ILT vaccines have displaced wild-type viruses and are responsible for many of the outbreaks occurring in these regions. Similarly, vaccinal laryngotracheitis is highly suspected in the US commercial poultry outbreaks, because circulating viruses are genetically closely related to CEO vaccines (Davison et al., Citation2005). A recent report from Australia presented convincing genome sequence data indicating that the dominant strains responsible for widespread outbreaks in Australia originated from recombination events between the widely-used Australian live attenuated vaccine strains, SA2 and A20, and the recently introduced European Serva CEO strain (Lee et al., Citation2012). These findings emphasize that the simultaneous use of multiple ILT live attenuated vaccines should be avoided because this practice increases chances and favours viral recombination.
In the face of an outbreak, the use of combined intervention strategies has proven most beneficial. These strategies included the implementation of enhanced biosecurity measures (Chin et al., Citation2009), establishment of vaccination zones (Davison et al., Citation2005), change of transportation route of infected birds to the processing plant, and establishment of extended down-time (Chin et al., Citation2009) However, efforts made to improve outbreak responses remain challenging. To circumvent the disadvantages associated with the use of live attenuated ILT vaccines, recombinant viral vector vaccines have been introduced recently as a safer alternative. Viral vector recombinant vaccines based on turkey herpesvirus and fowl poxviruses are currently available. They are increasingly safe due to their inability to transmit from bird to bird, revert to virulence, establish latent infections, or recombine. In addition, they can be administered in ovo without a negative impact on production performance (Johnson et al., Citation2010). Recent studies suggest that although these vaccines reduce clinical signs, they do not reduce challenge virus loads in the trachea as live attenuated vaccines do (Johnson et al., Citation2010; Vagnozzi et al., Citation2012).
Epidemiology of ILT worldwide
Based on data obtained from the World Organization for Animal Health (OIE) and World Animal Health Information Database disease time-lines (WAHID, Citation2013), and reports obtained from the Secretariat of the Pacific Community of the Food and Agricultural Organization (SPC-FAO, Citation2013), the Caribbean Animal Health Network (CaribVet, Citation2012), and extensive PubMed searches (Saepulloh & Rovira, Citation2003; Islam et al., Citation2010; Sadeghi et al., Citation2011) the current global distribution of ILT is shown in . Map regions shown in light purple indicate countries positive for ILT between 2000 and 2013. Dark purple regions with a grid pattern indicate countries where ILT cases have been documented for longer than 10 years. Countries that reported ILT outbreaks prior to, but not after 1999, are indicated in blue. Countries shown in yellow indicate that although no ILT reports have been made, these countries are considered suspects due to their proximity to GaHV-1-positive countries. Countries with no known reports or available data are marked red and green, respectively. Geographically, countries where the disease has been reported for longer than 10 years, or since 2000 (dark purple with grids and light purple), correspond to major poultry-producing regions in the world where the use of live attenuated vaccines is a common practice (). While licensed use of GaHV-1 live attenuated vaccines for both commercial and non-commercial poultry is determined at the country, state, or province level, the prevalence of these vaccine strains in the field has been confirmed even in countries that do not allow their use ().
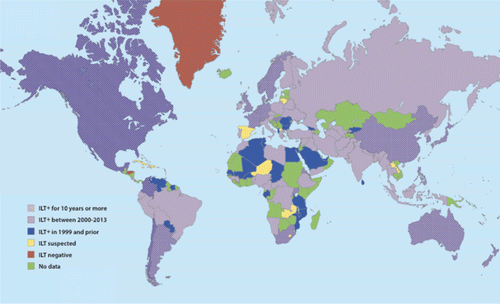
Table 2. Currently licensed live attenuated GaHV-1 vaccines.
Since GaHV-1 strains belong to a single serotype (Guy & Garcia, Citation2008), diagnostic assays based on antigenic differences are impossible. Thus, instead of serological methods, molecular assays have been adopted. Polymerase chain reaction (PCR) amplification of selected genome areas followed by restriction fragment length polymorphism (RFLP), or sequencing of PCR products are widely used. Initially, both PCR-RFLP and sequencing were critical in strain differentiation of GaHV-1 strains from the USA (Oldoni & Garcia, Citation2007; Oldoni et al., Citation2008), while complete genome comparisons of outbreak-related isolates and vaccine strains allowed further insight into the origin of GaHV-1 US isolates (Garcia et al., Citation2013). In the USA, the majority of commercial poultry outbreak-related strains are either indistinguishable from or closely related to vaccine strains, while outbreaks caused by wild-type strains occur to a much lesser extent than those attributed to live attenuated vaccine strains (Oldoni et al., Citation2008). In Australia, the introduction of the European-origin Serva CEO vaccine to a population of birds previously vaccinated with native vaccines SA2 and A20 resulted in the devastating emergence of novel virulent strains responsible for numerous outbreaks of ILT. Full genome sequencing clearly indicated that a recombination event between the native vaccine strains and the CEO Serva strain resulted in the emergence of new virulent genotypes identified as classes 8 and 9 (Lee et al., Citation2012).
Assays to differentiate field isolates from vaccine strains vary depending on the region of the world, with optimal target genes for detection and strain differentiation dependent on the strains circulating and the vaccination programme utilized in the region. Each region of the world uses its own optimal set of genes for detection and differentiation of strains. outlines target genes for GaHV-1 PCR-RFLP and PCR-sequencing analysis of GaHV-1 strains based on regions. However, these gene specifications are not concrete and, as made apparent by the recent recombination of vaccine viruses in Australia, changes in these targets may occur with time and as vaccine strategies evolve.
Table 3. Differentiation of infectious laryngotracheitis virus by PCR-RFLP and PCR-sequencing: targeted genes and enzymes.
Historically, PCR-RFLP was the method of choice for strain differentiation and involves differentiation of virus strains by restriction enzyme cleavage patterns of targeted genes. Within the USA, nine groups with unique PCR-RFLP patterns have been identified using genes ORFB-TK, gM, ICP4, and gG (Oldoni & Garcia, Citation2007). The resulting groupings consist of the USDA reference strain in group I, the TCO vaccine strain in group II, field isolates closely related to the TCO vaccine in group III, CEO vaccine strains and CEO identical commercial poultry isolates in group IV, commercial poultry isolates closely related to the CEO vaccine in group V, vaccine-unlike commercial poultry isolates in group VI, and unique backyard flock isolates in groups VII, VIII, and IX (Oldoni & Garcia, Citation2007).
Alternatively, in Australia, five classes of strains were originally compiled based on PCR-RFLP differentiation using a combination of gG, TK, ICP4, and ICP18.5 target genes (Kirkpatrick et al., Citation2006b). Class 1 consisted of the SA2 and A20 CEO vaccines as well as related strains, classes 2 and 3 of vaccine-unlike field strains, class 4 of the Australian CSW virulent field strain, and class 5 of vaccine-like and vaccine-unlike field strains (Kirkpatrick et al., Citation2006b). In 2011, four new classes were identified, including class 6 strains isolated from the region of Victoria, the Nobilis (Serva) ILT vaccine in class 7, and the new recombinant isolates in classes 8 and 9 (Blacker et al., Citation2011; Lee et al., Citation2012).
In South America, based on PCR-RFLP of the TK and gG genes, five patterns were identified among Brazilian and Peruvian field isolates (Chacon & Ferreira, Citation2009). Pattern A consisted of isolates from the Brazilian State of Sao Paulo, pattern B isolates originated in southern Brazil, pattern C isolates originated in Peru, pattern D corresponded to the TCO vaccine, and pattern E corresponded to the CEO vaccine.
In Taiwan, based on PCR-RFLP of gG, TK, and ICP4, three groups of strains were identified (Chang et al., Citation1997). Group 1 consisted of the TCO vaccine and TCO-like field strains, Group 2 includes the CEO vaccine and CEO-like field strains, and Group 3 includes vaccine-unlike field strains. In Korea, three groups of field strains were differentiated using the TK gene alone (Han & Kim, Citation2001). Group 1 consisted of virulent strains, group 2 of low-virulence strains, and group 3 of vaccine strains. In this example, the level of differentiation did not match that of other studies because only one target gene was utilized, outlining the importance of using multiple genes in PCR-RFLP differentiation. However, in a recent study using multiple PCR-RFLP analysis, Korean outbreak-related isolates showed similar PCR-RFLP patterns to the CEO vaccine produced in the region (Kim et al., 2013). Isolates from Israel (, Middle East region) were genotyped using the PCR-RFLP target TK digested with HaeIII and this assay allowed the differentiation of vaccines and field isolates (Davidson et al., 2009).
Despite the level of differentiation, the PCR-RFLP method initially revealed the presence of circulating vaccine-like strains as a source of outbreaks in the USA, South America, Asia, and Australia. Although PCR-RFLP of multiple genes remains a less costly option for differentiation of GaHV-1 strains, recently it has been steadily replaced by DNA sequencing. One main advantage of sequencing over PCR-RFLP is that the data are easier to document, analyse, and maintain, whereas PCR-RFLP can be highly subjective. Sequencing is also more precise when compared with PCR-RFLP, especially when multiple target genes are utilized for differentiation.
Like PCR-RFLP, sequencing of PCR products for strain differentiation is regionally dependent (). In North America, target genes sequenced either in their entirety or partially include ICP4, UL47, gB, gG and gM (Ojkic et al., Citation2006; Callison et al., Citation2009). In South America the ICP4 gene has been sequenced (Chacon & Ferreira, Citation2009; Chacon et al., Citation2010), and in the UK the TK and ICP4 genes are the targets (Creelan et al., Citation2006). In Europe, larger scale sequencing investigation has been performed using the target genes TK, ICP4, gG, gE, ORFB-TK as well as the gene blocks containing ORFB, ORFC, ORFD, ORFE, gH, TK, and ICP18.5 (Neff et al., Citation2008; Moreno et al., Citation2010). The partial sequencing of the ICP4 has been used successfully in both Africa and the Middle East (Sadeghi et al., Citation2011; Shehata et al., Citation2013). Strains in East Asia have been successfully differentiated by sequencing the target genes TK, ICP4, gC, gG, gE, and gJ (Han & Kim, Citation2001), and in Australia the TK and gG genes have been used (Diallo et al., Citation2010). No sequence data have been published on GaHV-1 isolates from Russia or Southeast Asia.
The use of multi-locus PCR-RFLP and sequencing of more than two genes produced the discriminatory power necessary to identify CEO-related isolates and differentiate these from non-vaccine-related isolates, particularly in North America, Europe, South America and East Asia (Chang et al., Citation1997; Han & Kim, Citation2001; Kirkpatrick et al., Citation2006b; Ojkic et al., Citation2006; Oldoni & Garcia, Citation2007; Oldoni et al., Citation2008; Neff et al., Citation2008; Chacon et al., Citation2010; Moreno et al., Citation2010). In some cases a combination of PCR-RFLP and sequencing was necessary to achieve a greater degree of discrimination. For instance, to differentiate Canadian field isolates from vaccine strains a combination of PCR-RFLP analysis of the ICP4, UL47, gE, and gG genes with sequencing of UL47 and gG genes was utilized (Ojkic et al., Citation2006). Differentiation of field isolates and vaccine strains from Brazil was achieved using PCR-RFLP of the TK, ICP4, UL47/gG, and gE genes combined with sequencing of the TK and ICP4 genes (Chacon & Ferreira, Citation2009; Chacon et al., Citation2010). Differentiation of field isolates and vaccine strains in the UK combined PCR-RFLP of the TK and ICP4 genes with partial sequencing analysis of the ICP4 gene (Creelan et al., Citation2006). Differentiation of isolates from Europe generally involved PCR-RFLP of the gE, gG, ICP18.5, ORFB-TK and TK genes in combination with sequencing of the ICP4, TK, gE, gG, ORFB-TK, and ICP18.5 to UL43 genes (Neff et al., Citation2008; Moreno et al., Citation2010). For differentiation of isolates from Korea, a combination of PCR-RFLP and sequencing of the TK and gG genes was sufficient (Han & Kim, Citation2001). PCR-RFLP and sequencing of only the TK gene was successful in differentiating field isolates from the SA2 Australian vaccine (Diallo et al., Citation2010).
Typing methods that distinguish between GaHV-1 isolates and vaccine strains of the USA, Australia, Europe and Asia (Chang et al., Citation1997; Ojkic et al., Citation2006; Oldoni & Garcia, Citation2007; Chacon et al., Citation2010; Moreno et al., Citation2010) utilized varied combinations of genes (multilocus genotyping) (). Most of the multilocus genotyping schemes target common genes (TK, ICP4, UL47 and gG). In addition to these common targets, genotyping of US isolates included RFLP of the gM and gB genes, genotyping of European and Asian isolates included the sequencing or RFLP of ORF-TK genome fragment, and genotyping of Australian isolates included sequencing of the ICP18.5 gene. It is relevant to note that amplification and partial sequencing of either the TK gene or the ICP4 gene had been utilized frequently in the differentiation of GaHV-1 strains from South America, the UK, Africa, Asia and the Middle East. Either one of these two genes have been fundamental in the development of fast, cost-efficient methods to identify and differentiate GaHV-1 isolates (Creelan et al., Citation2006; Chacon & Ferreira, Citation2009; Chakma et al., Citation2010; Sadeghi et al., Citation2011; Shehata et al., Citation2013).
In addition to PCR-RFLP and PCR-sequencing assays for the differentiation of field isolates and vaccine strains of GaHV-1, development of very sensitive GaHV-1 nucleic acid detection assays have advanced our understanding of the disease epidemiology. presents a list of nucleic acid amplification assays and target genes that have been pivotal in the rapid detection of infected flocks. PCR assays targeting the gC, gE, ICP4, TK and gB genes have been the most widely used method for amplification of GaHV-1 nucleic acid. GaHV-1 PCR assays utilize either nested primers for increased sensitivity (Humberd et al., Citation2002; Chacon & Ferreira, Citation2008; Davidson et al., Citation2009), SYBR Green (Creelan et al., Citation2006; Callison et al., Citation2009; Mahmoudian et al., Citation2011) or Taqman probes (Callison et al., Citation2007; Ou et al., Citation2012; Zhao et al., Citation2013) for real-time detection. Loop-mediated isothermal amplification assays targeting the ICP4 and TK genes have also been utilized in the detection of GaHV-1 nucleic acid (Xie et al., Citation2010; Ou et al., Citation2012).
Table 4. Targeted genes and methods for ILT nucleic acid detection.
Complete genome analysis
Although there is some evidence indicating that unique nucleotide changes in the TK gene may be related to virulence of Korean isolates (Han & Kim, Citation2001), none of the genotyping methods outlined above has been successful in relating strain genotype to pathotype. In an attempt to identify genetic determinants of attenuation in vaccine strains and determinants of virulence in field isolates, comparison of full genome sequences was performed among US vaccines and field isolates. Comparison of the European Serva vaccine strain with four virulent GaHV-1 strains from the USA revealed non-synonymous amino acid changes exclusive to the vaccine strain. While some changes occurred among structural glycoproteins, which were suspected to account for geographical differences between strains, those found in the non-structural proteins UL28, UL5, and ICP4 are believed to be related to virulence or attenuation (Spatz et al., Citation2012). Comparative genome analysis of US CEO and TCO “revertants” and their vaccine counterparts revealed single nucleotide polymorphisms within open reading frame (ORF) C, gB, UL28, UL39 and the UL41, which may be related to regaining virulence in the field (Garcia et al., 2013). Full genome analysis of the Australian vaccine strains, vaccine SA2 being the parental strain of A20, indicated that non-synonymous changes in the ORF B and UL15 non-structural proteins were introduced after tissue culture passages of the SA2 strain and are related to the greater attenuation characteristics of the A20 vaccine (Kirkpatrick et al., Citation2006a; Lee et al., Citation2011a).
Full genome phylogeny of GaHV-1 virulent isolates and vaccine strains
Twenty full genome sequences were aligned and used to generate a phylogenetic tree. These strains include: the US CEO vaccine strains (Trachivax, Fowl Laryngo, LT-Blen, Laryngo-Vac); chicken passage 1 (Fowl Laryngotracheitis low passage [LP]) and passage 20 (Fowl Laryngotracheitis high passage [HP]) of the Fowl Laryngo vaccine; US TCO vaccine (LT-Ivax) chicken passage 1 (LT-Ivax LP) and passage 20 (LT-Ivax HP); European CEO vaccine strain Serva; and Australian vaccine strains (SA2 and A20). The virulent strains include the US virulent isolates (63140, 81658, 1874C5, USDA reference strain), the Australian virulent isolates (CSW-I, VI-99, CL9, ACC78), and the Chinese virulent strain (LJS09). The phylogenetic relationship among attenuated live vaccine strains and virulent isolates of GaHV-1 are shown in . Eighteen of the 20 strains are distributed into three clades. Clade I includes all of the US CEO vaccines that originated from the Hudson strain: Trachivax, Fowl Laryngo, Fowl Laryngotracheitis LP and Fowl Laryngotracheitis HP in chickens; LT-Blen; the CEO vaccine that originated from the Cover strain Laryngo-Vac; the European CEO vaccine Serva strain; and the Australian CEO-like ACC78 (CL8) virulent isolate that originated from the Serva strain. Clade II includes the LT-Ivax US vaccine, LT-Ivax LP and LT-Ivax HP in chickens, the virulent USDA reference strain, and the US virulent broiler breeder isolate 81658. Clade III is composed of the two Australian vaccines (SA2 and A20), two virulent Australian isolates (CSW-1 and VI-99) and virulent isolate LJS09 from China (Kong et al., Citation2013).
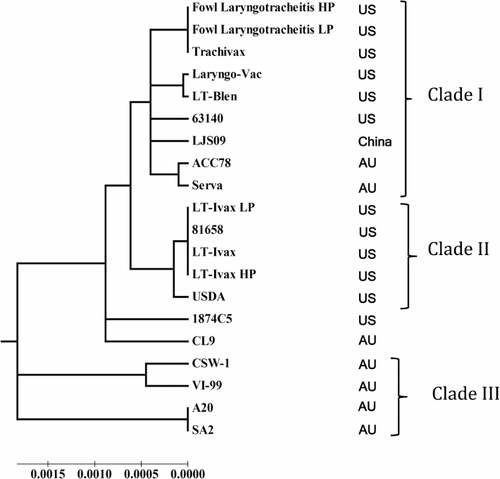
In addition to the US virulent isolate 63140, the Australian virulent isolate ACC78 genome was included in Clade I and is closely related to the Serva CEO European vaccine strain. Strikingly, the Australian isolate CL9 grouped outside Clade III, which encompasses isolates and vaccine strains from Australia. This result confirms a recent report that isolate ACC78 (CL8 classification) emerged after a recombination event between the Serva European CEO vaccine with either the SA2 or A20 Australian vaccines, with the Serva vaccine strain as the main contributor. The CL9 isolate emerged as the result of recombination between two vaccine strains, the Australian A20 vaccine strain and the European Serva vaccine strain, with the A20 vaccine strain as the main sequence contributor (Lee et al., Citation2012). The genomic sequence of the virulent US isolate1874C5 also failed to group within the three main clades.
In a recent published study, full and partial genome analysis of Australian, US, and European isolates revealed that the Australian field strains V1-99 and CSW-1 and the US strain 1874C5 shared a distinct sequence segment within the unique long region of their genomes. The authors speculated that the Australian field strains (V1-99 and CSW-1) and the US isolate 1874C5 emerged as the result of recombination events, and may share the same (or very similar) parent strain(s), or alternatively these strains obtained the distinctive unique long sequence segment from some other strain during independent recombination events (Lee et al., 2013). It is possible that Clade III should be subdivided into a virulent clade containing 1874C5, CL 9, CSW-1, and VI-99 and a vaccine clade containing vaccine strains A20 and SA2. Future genome analysis of historical isolates from the USA, Europe and elsewhere may provide additional information on the role that recombination has played in the evolution of GaHV-1 strains.
Conclusions
GaHV-1 has been a significant pathogen since the dawn of the modern poultry industry. While implementation of biosecurity methods remains the optimal mode to control ILT, gaps in biosecurity consistently occur, forcing the poultry industry to rely on vaccination in order to control the disease. Live attenuated vaccines (CEO and TCO) are capable of reducing mortality rates and drops in egg production (Guy & Garcia, Citation2008), and zone vaccination, particularly with the CEO vaccine, has been a successful approach in curtailing large outbreaks of the disease (Dufour-Zavala, Citation2008). However, the residual virulence associated with live attenuated vaccines exacerbated by poor mass administration of these vaccines, together with lax biosecurity and the presence of latent carriers, results in the emergence of virulent strains that will cause future devastating outbreaks of ILT.
Genotyping of GaHV-1 strain was initially performed by digestion of viral genomes with restriction endonucleases followed with Southern blot analysis with labelled probes. Molecular epidemiological studies carried out in the early 1990s had already suggested that GaHV-1 isolates circulating in dense poultry production areas were related to vaccine strains (Guy & Garcia, Citation2008). With the advent of the PCR, most of the GaHV-1 genotyping is now performed either by RFLP or by sequencing of multiple PCR products. However, recently available and relatively inexpensive deep sequencing technologies are permitting sequence-based genotyping of GaHV-1 vaccine strains and field isolates to become mainstream (Lee et al., Citation2011b; Chandra et al., 2011; Spatz et al., Citation2012; Garcia et al., 2013; Kong et al., Citation2013). Full genome sequence analysis has revealed that the emergence of current virulent GaHV-1 viruses is due to the accumulation of point mutations scattered across the genomes of vaccine strains, and recombination events between vaccine strains. Although incomplete, the sequencing of the genomes from some historical strains has already contributed to our current understanding of the molecular epidemiology of ILT and has led investigators to establish the origin of GaHV-1 strains responsible for the majority of outbreaks around the world. Comparative genomics data strongly suggest that, over time, circulating GaHV-1 strains worldwide have shifted from wild type to virulent vaccine-related strains.
Despite the high discrimination of PCR-RFLP, PCR-sequencing and full genome analysis, these methods lack the ability to precisely identify the probable multigenic determinants responsible for virulence. In order to identify determinants of virulence precisely, specific mutations need to be introduced within the GaHV-1 genome to match genotype with phenotype. Full genome analysis has provided a starting point to narrow down determinants of virulence and to identify sites in the GaHV-1 genome that may trigger recombination events, which will help in the development of more effective and safer live attenuated vaccines. Ultimately it is also important to improve current vaccine administration protocols and to develop new protocols in order to enhance flock immunity and improve the control of disease.
CAVP_886004_supplemental_material_global_distribution_of_ILT_as_of_2013.pdf
Download PDF (111.3 KB)References
- Bagust, T.J., Jones, R.C. & Guy, J.S. (2000). Avian infectious laryngotracheitis. Revue Scientifique et Technique, Office International Epizootics, 19, 483–492.
- Benton, W.J., Cover, M.S. & Greene, L.M. (1958). The clinical and serological response of chickens to certain laryngotracheitis viruses. Avian Diseases, 2, 383–396. 10.2307/1587478
- Blacker, H.P., Kirkpatrick, N.C., Rubite, A., O'Rourke, D. & Noormohammadi, A.H. (2011). Epidemiology of recent outbreaks of infectious laryngotracheitis in poultry in Australia. Australian Veterinary Journal, 89, 89–94. 10.1111/j.1751-0813.2010.00665.x
- Brandly, C.A. (1935). Some studies of infectious laryngotracheitis—The continued propagation of the virus upon the chorioallantoic membrane of the hen's egg. The Journal of Infectious Disease, 57, 201–206. 10.1093/infdis/57.2.201
- Callison, S.A., Riblet, S.M., Oldoni, I., Sun, S., Zavala, G., Williams, S., Resurreccion, R.S., Spackman, E. & Garcia, M. (2007). Development and validation of a real-time Taqman PCR assay for the detection and quantification of infectious laryngotracheitis virus in poultry. Journal of Virological Methods, 139, 31–38. 10.1016/j.jviromet.2006.09.001
- Callison, S.A., Riblet, S.M., Rodriguez-Avila, A. & Garcia, M. (2009). Reverse restriction fragment length polymorphism (RRFLP): a novel technique for genotyping infectious laryngotracheitis virus (ILTV) live attenuated vaccines. Journal of Virological Methods, 160, 119–124. 10.1016/j.jviromet.2009.04.036
- Caribbean Animal Health Network (CaribVet). (2012). Infectious Laryngotracheitis Monograph. Retrieved July 20, 2012, from http://www.caribvet.net/en/diseases/infectious-laryngotracheitis/monograph.
- Chacon, J.L. & Ferreira, A.J.P. (2008). Development and validation of nested-PCR for the diagnosis of clinical and subclinical infectious laryngotracheitis. Journal of Virological Methods, 151, 188–193. 10.1016/j.jviromet.2008.05.012
- Chacon, J.L. & Ferreira, A.J. (2009). Differentiation of field isolates and vaccine strains of infectious laryngotracheitis virus by DNA sequencing. Vaccine, 27, 6731–6738. 10.1016/j.vaccine.2009.08.083
- Chacon, J.L., Mizuma, M.Y. & Piantion Ferreira, A.J. (2010). Characterization by restriction fragment length polymorphism and sequence analysis of field and vaccine strains of infectious laryngotracheitis virus involved in severe outbreaks. Avian Pathology, 39, 425–433. 10.1080/03079457.2010.516386
- Chakma, S., Sarker, S., Talukder, S., Haque, M.H., Chowdhury, E.H. & Bari, A.S.M. (2010). Rapid detection of infectious laryngotracheitis virus by standardization of polymerase chain reaction targeting a relatively conserved region of the thymidine kinase gene. University Journal of Zoology, Rajshahi University, 29, 61–64.
- Chandra, Y.G., Lee, J. & Kong, B.W. (2012). Genome sequence comparison of two United States live attenuated vaccines of infectious laryngotracheitis virus (ILTV). Virus Genes 44, 470–474.
- Chang, P.C., Lee, Y.L., Shien, J.H. & Shieh, H.K. (1997). Rapid differentiation of vaccine strains and field isolates of infectious laryngotracheitis virus by restriction fragment length polymorphism of PCR products. Journal of Virological Methods, 66, 179–186. 10.1016/S0166-0934%2897%2900050-5
- Chin, R.P., García, M., Corsiglia, C., Riblet, S., Crespo, R., Shivaprasad, H.L., Rodriguez-Avila, A., Woolcock P. & França M. (2009). Intervention strategies for laryngotracheitis: impact of extended downtime. Avian Diseases, 53, 574–577. 10.1637/8873-041309-Reg.1
- Coppo, M.J.C., Noormohammadi, A.H., Browning, G.F. & Devlin, J.M. (2013). Challenges and recent advancements in infectious laryngotracheitis virus vaccines. Avian Pathology, 42, 195–205. 10.1080/03079457.2013.800634
- Cover, M.S. (1996). The early history of infectious laryngotracheitis. Avian Diseases, 40, 494–500. 10.2307/1592256
- Creelan, J.L., Calvert, V.M., Graham, D.A. & McCullough, S.J. (2006). Rapid detection and characterization from field cases of infectious laryngotracheitis virus by real-time polymerase chain reaction and restriction fragment length polymorphism. Avian Pathology, 35, 173–179. 10.1080/03079450600598244
- Davison, S., Dufour-Zavala, L., García, M., Ghorin, H., Hoerr, F., Hopkins, B., Smith, J. & Waldrip, D. (2005). Vaccinal laryngotracheitis—overview in the United States. Proceedings of the 109th Annual Meeting of the United States Animal Health Association (p. 580).
- Davidson, I., Nagar, S., Ribshtein, I., Shkoda, I., Perk, S. & García, M. (2009). Detection of wild-and vaccine-type infectious laryngotracheitis virus in clinical samples and feather shafts of commercial chickens. Avian Diseases, 53, 618–23. 10.1637/8668-022709-ResNote.1
- Devlin, J.M., Browning, G.F., Gilkerson, J.R., Fenton, S.P. & Hartley, C.A. (2008). Comparison of the safety and protective efficacy of vaccination with glycoprotein-G-deficient infectious laryngotracheitis virus delivered via eye-drop, drinking water or aerosol. Avian Pathology, 37, 83–88. 10.1080/03079450701802214
- Diallo, I.S., Taylor, J., Gibson, J., Hoad, J., De Jong, A., Hewitson, G., Corney, B.G. & Rodwell, B.J. (2010). Diagnosis of a naturally occurring dual infection of layer chickens with fowlpox virus and gallid herpesvirus 1 (infectious laryngotracheitis virus). Avian Pathology, 39, 25–30. 10.1080/03079450903447412
- Dufour-Zavala, L. (2008). Epizootiology of infectious laryngotracheitis and presentation of an industry control program. Avian Diseases, 52, 1–7. 10.1637/8018-051007-Review
- Elkin, N. (2012). Infectious laryngotracheitis live vaccines. PoultryMed. Retrieved December, 5 2012, from http://www.poultrymed.com/Poultry/Templates/showpage.asp?DBID=1&LNGID=1&TMID=652&FID=661&PID=2901.
- García, M, Volkening, V., Riblet, S.M. & Spatz, S. (2013). Genomic sequence analysis of the United States infectious laryngotracheitis vaccine strains chicken embryo origin (CEO) and tissue culture origin (TCO). Virology, 440, 64–74.
- Gelenczei, E.F. & Marty, E.W. (1964). Studies on a tissue-culture-modified infectious laryngotracheitis virus. Avian Diseases, 8, 105–122. 10.2307/1587827
- Gibbs, C.S. (1933). The immunology of infectious laryngotracheitis. Massachusetts Agricultural Experimental Station Bulletin, 295, 1–20.
- Gibbs, C.S. (1934). Infectious laryngotracheitis vaccination. Massachusetts Agricultural Experimental Station Bulletin, 311, 1–20.
- Graham, D.A., McLaren, I.E., Calvert, V., Torrens D. & Meehan B.M. (2000). RFLP analysis of recent Northern Ireland isolates of infectious laryngotracheitis virus: comparison with vaccine virus and field isolates from England, Scotland and the Republic of Ireland. Avian Pathology, 29, 57–62. 10.1080/03079450094298
- Guy, J.S. & Garcia, M. (2008). Laryngotracheitis. In Y.M. Saif, A.M. Fadly, J.R. Glisson, L.R. McDougald, L.K. Nolan & D.E. Swayne (Eds.), Diseases of Poultry 12th edn (pp. 137–152). Ames, IA: Blackwell Publishing Professional.
- Han, M.G. & Kim, S.J. (2001). Analysis of Korean strains of infectious laryngotracheitis virus by nucleotide sequences and restriction fragment length polymorphism. Veterinary Microbiology, 83, 321–331. 10.1016/S0378-1135%2801%2900423-0
- Hudson, C.B. (1969). United States Patent No. 3,444,293. Vineland Poultry Laboratories. Vineland, NJ, USA.
- Humberd, J., Garcia, M., Riblet, S.M., Resurreccion, R.S. & Brown, T.P. (2002). Detection of infectious laryngotracheitis virus in formalin-fixed, paraffin-embedded tissues by nested polymerase chain reaction. Avian Diseases, 46, 64–74.
- Islam, M.S., Khan, M.S.R, Islam, M.A., Hassan, J., Affroze, S. & Islam, M.A. (2010). Isolation and characterization of infectious laryngotracheitis virus in layer chickens. Bangladesh Journal of Veterinary Medicine, 8, 123–130.
- Johnson, D.I., Vagnozzi, A., Dorea, F., Riblet, S.M., Mundt, A., Zavala, G. & Garcia, M. (2010). Protection against infectious laryngotracheitis by in ovo vaccination with commercially available viral vector recombinant vaccines. Avian Diseases, 54, 1251–1259. 10.1637/9401-052310-Reg.1
- Kim, H.R., Kang, M.S., Kim, M.J., Lee, H.S. & Kwon, Y.K. (2013). Restriction fragment length polymorphism analysis of multiple genome regions of Korean isolates of infectious laryngotracheitis virus collected from chickens. Poultry Science, 92, 2053–2058.
- Kirkpatrick, N.C., Mahmoudian, A., Colson, C.A., Devlin, J.M. & Noormohammadi, A.H. (2006a). Relationship between mortality, clinical signs and tracheal pathology in infectious laryngotracheitis. Avian Pathology, 35, 449–453. 10.1080/03079450601028803
- Kirkpatrick, N.C., Mahmoudian, A., O'Rourke, D. & Noormohammadi, A.H. (2006b). Differentiation of infectious laryngotracheitis virus isolates by restriction fragment length polymorphic analysis of polymerase chain reaction products amplified from multiple genes. Avian Diseases, 50, 28–34. 10.1637/7414-072205R.1
- Klausz, A. (2008). Mass application of live vaccines. ZOOTECNICA World's Poultry Journal. http://www.zootecnicainternational.com/article-archive/veterinary/217-mass-application-of-live-vaccines-.html
- Kong, C., Zhao, Y., Cui, X., Zhang, X., Cui, H., Xue, M. & Wang, Y. (2013). Complete genome sequence of the first Chinese virulent infectious laryngotracheitis virus. PLoS ONE, 8, e70154. 10.1371/journal.pone.0070154
- Lee, S.W., Devlin, J.M., Markham, J.F., Noormohammadi, A.H., Browning, G.F., Ficorilli, N.P., Hartley, C.A. & Markham, P.F. (2011a). Comparative analysis of the complete genome sequences of two Australian origin live attenuated vaccines of infectious laryngotracheitis virus. Vaccine, 29, 9583–9587. 10.1016/j.vaccine.2011.10.055
- Lee, S.W., Markham, P.F., Coppo, M.J., Legione, A.R., Markham, J.F., Noormohammadi, A.H., Browning, G.F., Ficorilli, N., Hartley, C.A. & Devlin, J.M. (2012). Attenuated vaccines can recombine to form virulent field viruses. Science, 337, 188. 10.1126/science.1217134
- Lee, S.W., Markham, P.F., Markham, J.F., Petermann, I., Noormohammadi, A.H., Browning, G.F., Ficorilli, N.P., Hartley, C.A. & Devlin, J.M. (2011b). First complete genome sequence of infectious laryngotracheitis virus. BMC Genomics, 12, 197.10.1186/1471-2164-12-197
- Lee, S-W., Devlin, J.M., Markham, J.F., Nooormohammadi, A.H., Browning, G.F., Ficotilli, N.P., Hartley, C.A. & Markham, P. F. (2013). Phylogenetic and molecular epidemiological studies reveal evidence of multiple past recombination events between infectious laryngotracheitis viruses. PLoS ONE 8(2), e55121.
- Loudovaris, T., Yoo, B.H. & Fahey, K.J. (1991). Genetic resistance to infectious laryngotracheitis in inbred lines of white Leghorn chickens. Avian Pathology, 20, 357–361. 10.1080/03079459108418771
- Mahmoudian, A., Kirkpatrick, N.C., Coppo, M., Lee, S., Devlin, J.M., Markham, P.F., Browning, G.F. & Noormohammadi, A.H. (2011). Development of a SYBR Green quantitative polymerase chain reaction assay for rapid detection and quantification of infectious laryngotracheitis virus. Avian Pathology, 40, 237–242. 10.1080/03079457.2011.553582
- Moreno, A., Piccirillo, A., Mondin, A., Morandini, E., Gavazzi, L. & Cordioli, P. (2010). Epidemic of infectious laryngotracheitis in Italy: characterization of virus isolates by PCR-restriction fragment length polymorphism and sequence analysis. Avian Diseases, 54, 1172–1177. 10.1637/9398-051910-Reg.1
- Neff, C., Sudler, C. & Hoop, R.K. (2008). Characterization of western European field isolates and vaccine strains of avian infectious laryngotracheitis virus by restriction fragment length polymorphism and sequence analysis. Avian Diseases, 52, 278–283. 10.1637/8168-110107-Reg.1
- Ojkic, D., Swinton, J., Vallieres, M., Martin, E., Shapiro, J., Sanei, B. & Binnington, B. (2006). Characterization of field isolates of infectious laryngotracheitis virus from Ontario. Avian Pathology, 35, 286–292. 10.1080/03079450600815481
- Oldoni, I. & Garcia, M. (2007). Characterization of infectious laryngotracheitis virus isolates from the Unites States by polymerase chain reaction and restriction fragment length polymorphism of multiple genome regions. Avian Pathology, 36, 167–176. 10.1080/03079450701216654
- Oldoni, I., Rodríguez-Avila, A., Riblet, S. & García, M. (2008). Characterization of infectious laryngotracheitis virus (ILTV) isolates from commercial poultry by polymerase chain reaction and restriction fragment length polymorphism (PCR-RFLP). Avian Diseases, 52, 59–63. 10.1637/8054-070607-Reg
- Oldoni, I., Rodriguez-Avila, A., Riblet, S.M., Zavala, G. & Garcia, M. (2009). Pathogenicity and growth characteristics of selected infectious laryngotracheitis virus strains from the United States. Avian Pathology, 38, 47–53. 10.1080/03079450802632031
- Ou, S.C., Gaimbrone, J.J. & Macklin, K.S. (2012). Comparison of a Taqman real-time polymerase chain reaction assay with a loop-mediated isothermal amplification for detection of Gallid herpesvirus 1. Journal of Veterinary Diagnostic Investigation, 21, 138–141. 10.1177/1040638711427578
- Pulsford, M.F. (1963). Infectious laryngotracheitis in poultry. The Veterinary Bulletin, 33, 477–483.
- Robertson, G.M. & Egerton, J.R. (1981). Replication of infectious laryngotracheitis virus in chickens following vaccination. Australian Veterinary Journal, 57, 119–123. 10.1111/j.1751-0813.1981.tb00472.x
- Rodríguez-Avila, A., Oldoni, I., Riblet, S. & García, M. (2007). Replication and transmission of live attenuated infectious laryngotracheitis virus (ILTV) vaccines. Avian Diseases, 51, 905–911. 10.1637/8011-041907-REGR.1
- Sadeghi, M., Bozorgemehrifard, M.H., Keyvanfar, H., Momtaz, H., Shooshtari, A. & Charkhkar, S. (2011). Differentiation of field isolates and vaccine strains of infectious laryngotracheitis virus by DNA sequencing. African Journal of Microbiology Research, 5, 4112–4117.
- Saepulloh, M. & Rovira, H.G. (2003). Isolation and identification of infectious laryngotracheitis virus from outbreaks at Lipa City, Batangas, the Philippines. Indonesian Journal of Animal and Veterinary Sciences, 8, 122–133.
- Samberg, Y. & Aronovici, I. (1969a). The development of a vaccine against avian infectious laryngotracheitis. I. Modification of a laryngotracheitis virus. Refuah Veterinarith, 26, 54–59.
- Samberg, Y. & Aronovici, I. (1969b). The development of a vaccine against avian infectious laryngotracheitis. III. Characteristics of a modified virus. Refuah Veterinarith, 26, 158–167.
- Samberg, Y., Cuperstein, E., Bendheim, U. & Aronvici, I. (1971). The development of a vaccine against avian infectious laryngotracheitis IV. Immunization of chickens with a modified laryngotracheitis vaccine in the drinking water. Avian Diseases, 15, 413–417. 10.2307/1588714
- Secretariat of the Pacific Community (SPC) Food and Agriculture Organization FAO. (2013). B302—avian infectious laryngotracheitis. AHP Disease Manual. Retrieved January 29, 2013, from http://www.spc.int/lrd/ext/Disease_Manual_Final/b302__avian_infectious_laryngotracheitis.html#auto_top.
- Shehata, A.A., Halami, M.Y., Sultan, H.H., Abd El-Razik, A.G. & Vahlenkamp, T.W. (2013). Chicken embryo origin-like strains are responsible for infectious laryngotracheitis virus outbreaks in Egyptian cross-breed chickens. Virus Genes, 46, 423–430.
- Spatz, S.J., Volkening, J.D., Keeler, C.L., Kutish, G.F., Riblet, S.M., Boettger, C.M., Clark, K.F., Zsak, L., Afonso, C.L., Mundt, E.S., Rock, D.L. & Garcia, M. (2012). Comparative full genome analysis of four infectious laryngotracheitis virus (Gallid herpesvirus-1) virulent isolates from the United States. Virus Genes, 44, 273–285. 10.1007/s11262-011-0696-3
- Vagnozzi, A., Zavala, G., Riblet, S.M., Mundt, A. & Garcia, M. (2012). Protection induced by commercially available live attenuated and recombinant viral vector vaccines against infectious laryngotracheitis virus in broiler chickens. Avian Pathology, 41, 21–31. 10.1080/03079457.2011.631983
- World Animal Health Information Database (WAHID). (2013). Avian infectious laryngotracheitis disease timelines. Retrieved August 21, 2013, from http://www.oie.int/wahis_2/public/wahid.php/Diseaseinformation/Diseasetimelines.
- Xie, Q.M., Ji, J., Pickens, T.T., Du, L.Q., Cao, Y.C., Li, H.M., Wang, L.G., Ma, J.Y. & Bi, Y.Z. (2010). Rapid detection of infectious laryngotracheitis virus isolates by loop-mediated isothermal amplification. Journal of Virological Methods, 165, 71–75. 10.1016/j.jviromet.2010.01.006
- Zavala, G. (2008). The old and new landscapes of infectious laryngotracheitis virus. The Poultry Informed Professional, 118, 1–7.
- Zhao, Y., Kong, C., Cui, X., Cui, H., Shi, X., Zhang, X., Hu, S., Hao, L. & Wang, Y. (2013). Detection of infectious laryngotracheitis virus by real-time PCR in naturally and experimentally infected chickens. PLoS ONE, 8, e67598. 10.1371/journal.pone.0067598
