Abstract
The objective of this study was to investigate the effect of feed restriction on the intestinal ecosystem and on the pathogenesis of experimental necrotic enteritis in broiler chicks. To induce subclinical necrotic enteritis, an experimental challenge model using a specific diet formulation, Gumboro vaccination, oral inoculation of broilers with a 10-fold dose of attenuated anticoccidial vaccine and multiple oral inoculations with a specific strain of Clostridium perfringens was adopted. Two hundred and forty 1-day-old Cobb 500 broilers were randomly allocated to four groups: feed restricted, challenged, both feed restricted and challenged, and negative control. At 21, 22, 23 and 24 days of age, the intestines, gizzard and liver were collected from 15 birds in each group and scored for gross lesions. The intestinal digesta was collected for pH and viscosity determination. One caecum from each bird was taken for microbiological analysis. The application of feed restriction in birds challenged with C. perfringens reduced the necrotic enteritis lesion score significantly (P ≤ 0.05) and feed restriction significantly reduced (P ≤ 0.05) pH in the small intestine, the viscosity of the jejunum digesta as well as the C. perfringens counts in the caeca compared with the controls. In conclusion, feed restriction of broilers has a positive effect on the intestinal ecosystem and a significant protective effect against necrotic enteritis in the subclinical experimental model.
Introduction
The broiler chicken has been intensely selected for higher growth rates, increased feed conversion, meat yield and breast percentage. Market weight can be obtained in 60% less time than broilers of 40 years ago (Baghbanzadeh & Decuypere, Citation2008). This successful selection has had a positive effect on the ecological footprint of broiler meat production and at the same time has made poultry meat affordable.
Several qualitative and quantitative feed restriction programmes have been tried in attempts to reduce the feed intake, limit the early growth and decrease the incidence of infectious, metabolic and skeletal disorders (Zulkifli et al., Citation1993; Zhan et al., Citation2007; Thompson et al., Citation2008; Benyi et al., Citation2010). The main target of feed restriction is to reduce the nutrients in the gastrointestinal tract, so that they can be utilized by neither birds nor intestinal microbiota (Tsiouris, Citation2010). The reduction of nutrients in the intestinal tract alters the intestinal microbiota, changes the physico-chemical properties of the intestinal digesta and disturbs the balance of the intestinal ecosystem (Hinton et al., Citation2000; Thompson et al., Citation2008).
Necrotic enteritis is an infectious disease typically associated with the imbalance of the intestinal ecosystem. Although, netB-positive Clostridium perfringens type A strain is the causal agent of the disease, a number of co-factors are usually required to precipitate an outbreak of the disease, such as damage to the intestinal mucosa and increase of pH and viscosity of intestinal digesta (Kaldhusdal et al., Citation1999; McReynolds et al., Citation2007; Tsiouris et al., Citation2013). Various preventive and curative measures have been proposed to control necrotic enteritis (Lanckriet et al., Citation2010; Timbermont et al., Citation2010), including changes in feeding management (Mateos et al., Citation2002).
The effect of feed restriction on the growth rate, body weight and feed conversion ratio has been well documented (Zhan et al., Citation2007; Baghbanzadeh & Decuypere, Citation2008; Benyi et al., Citation2010). However, to the best of our knowledge, the feed restriction has not been evaluated as a specific control measure for necrotic enteritis. Hence, the objective of this study was to evaluate the effect of temporary feed restriction on the physicochemical properties of intestinal digesta and on the pathogenesis of necrotic enteritis in broiler chicks.
Materials and Methods
C. perfringens strain and cultivation
C. perfringens strain 56 was isolated from the intestine of a broiler chicken with severe necrotic enteritis lesions. This strain belongs to toxinotype A (no β2 or enterotoxin genes) and, in vitro, produces moderate amounts of alpha-toxin. The strain carries the netB gene and has been used previously to induce necrotic enteritis (Lanckriet et al., Citation2010). To facilitate the detection of the inoculated strain in experimental birds, rifampicin-resistant mutants were isolated with the gradient technique as described by Pedersen et al. (Citation2008) using brain heart infusion broth (02-599; Scharlau Chemie S.A., Barcelona, Spain) containing rifampicin in a gradient concentration from 0 to 100 µg/ml (R 3501-1G; Sigma Aldrich Chemie GmbH, Steinheim, Germany). The bacterium was cultivated for 24 h at 37°C in brain heart infusion broth in an anaerobic atmosphere (Anaerocult A, 1.13829.0001; Merck KGaA, Darmstadt, Germany) before inoculation of the chicken. The attenuated anticoccidial vaccine (Paracox-5; MSD Animal Health, Summit, NJ, USA) was used at 10-fold dose on the 18th day of age. The vaccine contains live, attenuated oocysts of Eimeria acervulina, Eimeria maxima (two lines), Eimeria mitis and Eimeria tenella. The Gumboro vaccine (Nobilis Gumboro D78; MSD Animal Health) was used as an additional predisposing factor to necrotic enteritis.
Birds and housing
Two hundred and forty 1-day-old Cobb 500 broiler chicks were obtained from a local commercial hatchery and were randomly allocated into four treatment groups of 60 birds. The birds in each group were placed in a pen with deep litter of wood shavings, which were previously sterilized in an autoclave at 121°C for 20 min.
Each group was kept in a specially designed experimental room (Unit of Avian Medicine, Faculty of Veterinary Medicine, Aristotle University of Thessaloniki, Greece), where temperature, relative humidity and lighting were controlled, following the recommendations of the breeding company. The stocking density for each group was 15 birds/m2 or 33 kg/m2 (European Commission, Citation2007). The experimental rooms, prior to bird allocation, were cleaned and disinfected with broad-spectrum disinfectants (CID 20 and VIROCID; CID LINES, Ieper, Belgium) and specific disinfectants (Neopredisan 135-1; Menno Chemie-Vertrieb Gmbh, Norderstedt, Germany) against Eimeria spp. and C. perfringens, respectively.
Experimental diets
Broilers in all groups were fed a specially formulated three-phase ration (starter day 1 to 9, grower day 10 to 16 and finisher day 17 to 24), which included large quantities of wheat and rye (). From the 17th day of age onwards, the same isocaloric ration (finisher) was used with the exception that fishmeal replaced the soybean meal as the protein source, in order to predispose to necrotic enteritis. No antibiotic growth promoters or anticoccidial drugs were used. Feed and water were available ad libitum throughout the trial, except for groups where feed restriction was applied.
Table 1. Feed formulation and chemical analysis of rations used in the experiment of feed restriction.
Experimental design
The bird experimentation received the approval of the Veterinary Directorate of Thessaloniki and was conducted in compliance with the National Institutes of Health guidelines, Greek Government guidelines, and the local ethics committee. All birds were vaccinated against Gumboro disease on the 16th day of age via drinking water, after removing the water for 2 h. For the experimental induction of subclinical necrotic enteritis, the birds were challenged orally, using an insulin syringe with a small plastic catheter adapted to its opening, with 1 ml C. perfringens with 4 × 108 cell-forming units three times daily (at 09:00, 13:00 and 17:00 h) on four consecutive days when birds were between 17 and 20 days of age, and with a 10-fold dose of attenuated anticoccidial vaccine on the 18th day of age. The feed restriction of birds was applied temporarily by taking out the feeders for 12 h (21:00 to 09:00 h) each day starting from day 16 and until day 19 of age. The four groups were: a negative control group (group N), a feed-restricted group (group S), a group challenged with C. perfringens and with a 10-fold dose of attenuated anticoccidial vaccine (group P), and a group where birds were both challenged and feed restricted (group SP). From each experimental group, 15 birds per sampling day were removed on the 21st, 22nd, 23rd and 24th day of age. The birds were euthanized by exposure to a rising concentration of carbon dioxide in an air-tight container and were subject to necropsy. The gastrointestinal tract was removed immediately and divided into its anatomical parts (gizzard, duodenum, jejunum, ileum, caeca).
Performance evaluation
The body weight was measured on a pen basis when birds were 1, 10, 16, 17 and 21 days of age, while the feed consumption, average daily weight gain and feed conversion ratio were calculated for the periods from day 10 to 16 and again from days 17 to 21 of age. Mortality was recorded daily.
Gross lesion scoring system
The intestine, gizzard and liver were examined microscopically and scored for gross lesions (). In particular, the intestines were scored for necrotic enteritis lesions following a 0 to 6 scoring system described by Keyburn et al. (Citation2008). Birds with intestinal lesion scores greater than 1 were classified as necrotic enteritis positive. The gizzards were scored for gross lesions by the use of a 0 to 2 scale, described by Novoa-Garrido et al. (Citation2006). The livers were scored using a 0 to 2 scale, described by Tsiouris et al. (Citation2013), giving a score 0 when no gross lesions were observed, a score 1 when liver congestion and/or gall bladder distension and wall thickening and/or bile discoloration were observed, and a score 2 when necrotic lesions in liver were observed.
Table 2. Scoring system for gross lesions of the intestine, gizzard and liver.
pH values
After euthanasia, the digesta of the duodenum, jejunum, ileum and one caecum from each bird was immediately collected in separate tubes (10 ml), and were vortexed, individually, in order to obtain a homogeneous content from each anatomical part of the intestine per bird. The pH of the duodenum, jejunum, ileum and caecum from each bird was measured using a digital pH-meter (pH 315i; WTW Wissenschaftlich-Technische Werkstatten, Weilheim, Germany).
Viscosity
The homogeneous content of the jejunum and the ileum from each bird was transferred to separate Eppendorf tubes (1.5 ml). The tubes were centrifuged at 3000 × g for 45 min in order to separate the feed particles from the liquid phase. Supernatants (0.5 ml) from each tube were taken and the viscosity was measured in a Brookfield DV-II+ PRO Digital Viscometer (Brookfield Engineering Laboratories, Stoughton, Massachusetts, USA). Two readings were taken from each tube and were presented in units of centipoise.
Bacteriologic culture
The other caecum from each bird was used for microbiological analysis. The quantification of C. perfringens counts in the caecum was carried out according to Kaldhusdal et al. (Citation1999). The samples were cultured anaerobically on a 5% sheep blood agar plate (Columbia blood agar, 01-034; Scharlau Chemie S.A.) and C. perfringens selective supplement (SR0093; Oxoid Ltd, Cambridge, UK) for 24 h. The samples from challenged groups were plated on agar containing an addition of 100 µg/ml rifampicin. Circular, transparent colonies, 2 to 4 mm wide, with typical “target” haemolysis (an inner zone of L-haemolysis and an outer zone of partial haemolysis) were diagnosed as presumptive C. perfringens. In cases of doubt, aerobic and anaerobic secondary cultivation on blood agar and microscopy of Gram-stained smears were used. The counts of C. perfringens per gram of caecal content in each sample were calculated. The figures from the bacterial counts were recorded as cell-forming units and were transformed to the common logarithm.
Parasitological examination
To confirm the absence or presence of coccidia in non-vaccinated and vaccinated groups, respectively, parasitological examination of faeces with the Faust method was applied on the 10th, 17th and 20th day of age and microscopic examination of smears from intestinal mucosa on the 20th day of age.
Histopathological examination
Tissue samples from the duodenum, jejunum (proximal to Meckel's diverticulum) and ileum (proximal to the ileo-caecal junction) were fixed in 4% buffered formaldehyde for 48 to 72 h. Coronal sections from the samples were embedded in paraffin by a routine procedure. Dewaxed sections 3 to 5 µm thick were stained with haematoxylin and eosin.
Statistical analysis
Both parametric and non-parametric statistical methods were applied for the statistical evaluation of the experimental results. All analyses were made to the cumulative values received from all sampling days. For accessing the assumptions of normality and stability of variances, data were transformed to log10 or square root. In the case of normality and variance homogeneity, a one-way analysis of variance was performed to evaluate possible significant effects of treatment on gross lesions in the intestine, gizzard and liver, on the population of C. perfringens in caeca, as well as on the pH values of the duodenum, jejunum, ileum and caeca and the viscosity values of the jejunum and ileum. Differences between mean values of specific treatments were evaluated using Duncan's new multiple-range test. Where assumptions about either variability or the form of the populations distribution were seriously violated, with or without transformed data, the Kruskal–Wallis non-parametric test was applied to evaluate treatment-dependent differences, while differences between mean values of specific treatments were evaluated using the non-parametric Wilcoxon rank-sum test (Mann–Whitney U test). All analyses were conducted using the statistical software program SPSS for Windows (v. 15.0; IBM Corporation, Armonk, NY, USA). Significance was declared at P ≤ 0.05. Back-transformed mean values are reported in the results.
Results
The body weight of birds in all experimental groups as well as the average daily weight gain, the feed consumption and the feed conversion ratio prior to any treatment was similar amongst experimental groups (). The feed restriction, the challenge and its combination reduced the body weight and the feed consumption. The challenge of birds had no effect on the feed conversion ratio, while the feed restriction improved it. The data were collected and calculated on a pen basis and variations between treatments could not be statistically analysed.
Table 3. Effect of feed restriction, necrotic enteritis challenge model or its combination on body weight, feed consumption, average daily weight gain and feed conversion ratio according to age.
The effects of challenge and feed restriction on the numbers of birds with macroscopic necrotic enteritis lesions and overall mean scores of necrotic enteritis gross lesions in the intestine, gizzard and liver are presented in and , respectively. None of the birds of groups N and S developed necrotic enteritis gross lesions in the intestine, while 50% of birds in group SP and 70% of birds in group P developed necrotic enteritis gross lesions. The overall mean score of necrotic enteritis gross lesions in the intestine in non-challenged groups was below 1. On the other hand, the overall mean score of necrotic enteritis gross lesions in the intestine in challenged groups was above 1, indicating that the experimental model that was used for the reproduction of necrotic enteritis was efficient. In particular, the overall mean score of necrotic enteritis gross lesions in the intestine was significantly higher (P ≤ 0.05) in group P compared with group SP. The overall mean score of gross lesions in the liver was not significantly different (P ≤ 0.05) amongst experiential groups. In contrast, the overall mean score of gross lesions in the gizzard was significantly lower (P ≤ 0.05) in feed restricted groups (groups S and SP), compared with the non-feed restricted groups (groups N and P) ().
Table 4. Number of birds with macroscopic necrotic enteritis lesions in the feed restriction experiment.
Table 5. Effect of feed restriction, necrotic enteritis challenge model or its combination on the overall means gross lesions score of the intestine, liver and gizzard.
The feed restriction and challenge of birds had a significant (P ≤ 0.05) effect on the pH and viscosity values of the intestinal digesta as well as on the C. perfringens caecal counts (). The pH values of the duodenum, jejunum and ileum digesta in group S were significantly lower (P ≤ 0.05) compared with group N. Moreover, the pH values of the duodenum, jejunum and ileum digesta in group P were significantly lower (P ≤ 0.05) compared with group N, while the pH values of the caecum digesta in group P were significantly higher (P ≤ 0.05). The combination of feed restriction and challenge of birds decreased the pH of the duodenum and jejunum digesta significantly (P ≤ 0.05), compared with group N and the pH of the ileum digesta compared with all groups. The feed restriction and challenge of birds reduced the viscosity of the jejunum digesta significantly (P ≤ 0.05) compared with the control group, while its combination reduced the viscosity of the jejunum digesta significantly (P ≤ 0.05) compared with all treatment groups. The caecal C. perfringens counts in group S were significantly lower (P ≤ 0.05) compared with group N. In contrast, the caecal C. perfringens counts in groups P and SP were significantly higher (P ≤ 0.05) compared with groups N or S.
Table 6. Effect of feed restriction, necrotic enteritis challenge model or its combination on the pH and viscosity values of intestinal contents and caecal C. perfringens.
Results of the faecal parasitological examination on the 10th and 17th day of age were negative in all groups, indicating the absence of Eimeria spp. infection. Furthermore, the faecal parasitological examination and microscopic examination of smears from intestinal mucosa on the 20th day of age were negative in groups N and S and were positive in groups P and SP.
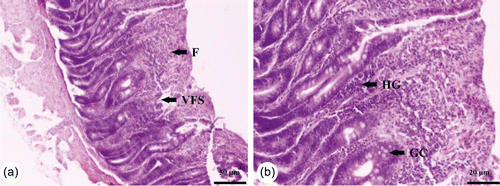
The histopathological lesions of the duodenum, jejunum and ileum in birds challenged with C. perfringens and 10-fold dose of attenuated anticoccidial vaccine were compatible with necrotic enteritis lesions. Lesions were located mainly in the jejunum and ileum, and to a limited extent in the duodenum. There was severe necrosis of the intestinal mucosa, with an abundance of fibrin admixed with cellular debris adherent to the necrotic mucosa, where large clusters of bacteria were detected. Villus fusion and shortening as well as marked infiltration of heterophilic granulocytes were also observed. Finally, the presence of goblet cells was clearly detected ().
Discussion
Feed restriction is a management strategy, which is applied in the poultry industry in order to reduce the feed intake, to limit the early growth, to decrease the incidence of infectious, metabolic and skeletal disorders and to improve the nutrient utilization via compensatory growth (Zulkifli et al., Citation1993; Zhan et al., Citation2007; Thompson et al., Citation2008; Benyi et al., Citation2010). In the present study, the reduction in body weight as well as the change of feed conversion ratio in experimental groups where feed restriction was applied, is in agreement with the results of the study of Lee & Leeson (Citation2001). Feed restriction is applied in broiler chicks in order to take advantage of the growth compensation of birds and to reduce the feed conversion ratio. The growth compensation of birds defines the increased growth rate, when growth has been retarded by nutritional restriction and followed by ad libitum feeding (Shariatmadari & Torshizi, Citation2004; Zhan et al., Citation2007). In the beginning, feed restriction reduces the body weight, as a result of reduced feed consumption. Later on, when feed consumption returns to a normal level and birds become older, the body weight increases at a higher rate as a result of the growth compensation of birds, and finally reaches the target body weight with a lower feed conversion ratio. However, in our study the body weight was lower in feed restricted groups of birds. This reduction could be attributed to the fact that the period between feeding replacement at a normal level (ad libitum) and sampling was too short and growth compensation could not be completed.
The feed conversion ratio was relatively high in all experimental groups prior to any treatment. This was attributed to the specific feed formulation, and especially to the high percentage of wheat and rye, which are rich in non-starch polysaccharides. However, the replacement of soya with fishmeal in the finisher ration improved the feed conversion ratio.
The feed restriction alleviated the severity of lesions in the gizzard and reduced the C. perfringens counts in the caeca. Moreover, the application of feed restriction in challenged birds reduced the number of necrotic enteritis positive birds as well as the necrotic enteritis score, indicating a protective effect against necrotic enteritis in broiler chicks.
The protective effect of the feed restriction against necrotic enteritis could be attributed to stimulation of the immune system and to the influence of the endocrine system, which are both affected by feed restriction (Bruggeman et al., Citation1997; Goddeeris et al., Citation2002). It is known that feed restriction increases the serum concentration of glucocorticoids (Zhan et al., Citation2007), which leads to the detachment of heterophils from the endothelium of the blood vessel and migration to the tissues (Gross & Siegel, Citation1988; Zulkifli et al., Citation1995). Moreover, the feed restriction in poultry increases the heterophil/lymphocyte ratio, so the number of migrant heterophils is even higher (Zulkifli et al., Citation2000; Jones et al., Citation2005). The lower growth rate, the reduction of haematocrit value and the beneficial effect of feed restriction on the cardiovascular system facilitate the migration of heterophils to the tissues, such as the gastrointestinal tract (Zubair & Leeson, Citation1994; Balog et al., Citation2000). These effects may favour blood circulation and innate immune response of the intestinal mucosa against pathogens, such as C.perfringens, and could be another plausible explanation for the protective effect of feed restriction against necrotic enteritis.
The glucocorticoids influence the expression of genes, which are responsible for the production of cytokines and other factors that play a significant role in the immunity system. For instance, a high concentration of glucocorticoids reduces the concentration of phospholipases, and subsequently the concentration of arachidonic acid, prostaglandins and leukotriens, and as a consequence suppresses inflammation (Goddeeris et al., Citation2002). According to Novoa-Garrido et al. (Citation2006), there is an association between gizzard lesions and increased caecal C. perfringens counts in broiler chicks. In the present study, the feed restriction alleviated the severity of gizzard gross lesions and reduced the caecal C. perfringens counts significantly (P ≤ 0.05). The decrease of caecal C. perfringens counts in the feed restriction group may be a direct effect of the nutrient reduction in the intestinal tract. Since the minimal requirements for growth of C. perfringens include more than 11 amino acids, as well as many growth factors and vitamins, the specific ration, with high levels of animal protein and non-starch polysaccharides, provide the necessary growth substrate for the extensive proliferation of these bacteria (Van Immerseel et al., Citation2004). Moreover, the feed restriction causes qualitative and quantitative alterations in intestinal microbiota, in particular in the anaerobic bacteria Clostridium spp., Bacteroides spp. and Prevotella spp. (Thompson et al., Citation2008), which could be the result of quiescent state of bacterial cells (Gasch & Werner-Washburne, Citation2002). C. perfringens belongs to the mucolytic bacteria, which are able to grow within the mucous layer by degrading and fermenting mucins (Deplancke et al., Citation2002). Mucogenesis in the small intestinal mucosa, while being appropriate for restoring barrier function, may lead indirectly to secondary complications associated with bacterial mucolysis, such as C. perfringens proliferation (Collier et al., Citation2008). On the other hand, any factor that reduces the production of intestinal mucus—for example, feed restriction (Smirnov et al., Citation2004)—could have a protective effect against necrotic enteritis in broiler chicks.
The feed restriction caused a significant reduction (P ≤ 0.05) in the pH of small intestine digesta, but had no effect on the pH of caeca, which is in accordance with Hinton et al. (Citation2000). The environment of the caeca is more stable than the rest of the intestine, because caeca are periodically filled with contents from the colon, and caecal contents are evacuated once or twice per day and passed as faeces along with faecal material from the colon. The reduction in the pH in the small intestine could be attributed to the proliferation of lactic acid bacteria, which produce lactic acid from carbohydrates in the broiler feed (Hinton et al., Citation2000). The increased concentration of lactic acid produced by the bacteria can reduce the pH to levels that are low enough to inhibit the growth of pathogens, such as C. perfringens (McReynolds et al., Citation2007). The reduction in the pH of small intestinal digesta may further contribute to the protective effect of feed restriction against necrotic enteritis.
The viscosity of the jejunum digesta was lower than the ileum digesta in all experimental groups. This is physiologically normal and consistent with the findings described by Petersen et al. (Citation1999). As the intestinal content moves from the gizzard to the caecum, the nutrients are digested and absorbed from intestinal mucosa together with water, resulting in the concentration of intestinal digesta. The viscosity values of the intestinal content in all experimental groups were relatively high compared with results reported in the literature (Bedford & Classen, Citation1992). This could be attributed to the specific feed composition, and, in particular to the large amount of wheat and rye. These cereals are rich in water-soluble non-starch polysaccharides, which cannot be digested by the digestive enzymes of the birds (Juskiewicz et al., Citation2004). A higher intestinal viscosity increases the average retention time of the intestinal content and creates a favourable environment for bacterial activity. To be more specific, the flow of digesta is reduced and the amount of undigested material in the intestinal tract increased, which gives C. perfringens more time and substrate to colonize the small intestine (Waldenstedt et al., Citation2000). However, the feed restriction reduced the viscosity values of intestinal digesta significantly (P ≤ 0.05) and thus might contribute to the protective effect of feed restriction.
In conclusion, the feed restriction had a significant (P ≤ 0.05) effect on the pH and viscosity values as well as on the C. perfringens counts in caeca. Moreover, it is known that feed restriction influences the endocrine and the immune system. All of these actions of feed restriction suggest a positive effect on the intestinal ecosystem and a significant protective effect against necrotic enteritis. Further studies are required in order to elucidate the mechanisms underlying these actions as well as the effect of feed restriction against other intestinal pathogens.
Acknowledgements
The authors express their gratitude to Professor F. Haesebrouck (Department of Pathology, Bacteriology and Avian Diseases, Faculty of Veterinary Medicine, Ghent University, Belgium) for generously and kindly providing the C. perfringens. Moreover, the authors are also grateful to veterinarian Deligeorgi Ioanni and to Mr Moulto Serafim for their excellent assistance and technical support. The feed mill “Stravaridis S.A.” (Thessaloniki, Macedonia, Greece) is thanked for feed formulation, while the hatchery “Tzotzas-Koutsou S.A.” (Thessaloniki, Macedonia, Greece) is thanked for the provision of 1-day-old chicks.
References
- Baghbanzadeh, A. & Decuypere, E. (2008). Ascites syndrome in broilers: physiological and nutritional perspectives. Avian Pathology, 37, 117–126. 10.1080/03079450801902062
- Balog, J., Anthony, N., Cooper, M., Kidd, B., Huff, G., Huff, W. & Rath, N. (2000). Ascites syndrome and related pathologies in feed restricted broilers raised in a hypobaric chamber. Poultry Science, 79, 318–323. 10.1093/ps/79.3.318
- Bedford, M. & Classen, H. (1992). Reduction of intestinal viscosity through manipulation of dietary rye and pentosanase concentration is effected through changes in the carbohydrate composition of the intestinal aqueous phase and results in improved growth rate and food conversion efficiency of broiler chicks. Journal of Nutrition, 122, 560–569.
- Benyi, K., Acheampong-Boateng, O., Norris, D. & Ligaraba, T. (2010). Response of Ross 308 and Hubbard broiler chickens to feed removal for different durations during the day. Tropical Animal Health and Production, 42, 1421–1426. 10.1007/s11250-010-9568-4
- Bruggeman, V., Vanmontfort, D., Renaville, R., Portetelle, D. & Decuypere, E. (1997). The effect of food intake from two weeks of age to sexual maturity on plasma growth hormone, insulin-like growth factor-I, insulin-like growth factor-binding proteins, and thyroid hormones in female broiler breeder chickens. General and Comparative Endocrinology, 107, 212–220. 10.1006/gcen.1997.6917
- Collier, C., Hofacre, C., Payne, A., Anderson, D., Kaiser, P., Mackie, R. & Gaskins, H. (2008). Coccidia-induced mucogenesis promotes the onset of necrotic enteritis by supporting Clostridium perfringens growth. Veterinary Immunology and Immunopathology, 122, 104–115. 10.1016/j.vetimm.2007.10.014
- Deplancke, B., Vidal, O., Ganessunker, D., Donovan, C.M., Mackie, R.I. & Gaskins, H.R. (2002). Selective growth of mucolytic bacteria including Clostridium perfringens in a neonatal piglet model of total parenteral nutrition. American Journal of Clinical Nutrition, 76, 1117–1125.
- European Commission. (2007). Council Directive 2007/43/EC of 28 June 2007 laying down minimum rules for the protection of chickens kept for meat production. Official Journal of the European Union, 182, 19–28.
- Gasch, A. & Werner-Washburne, M. (2002). The genomics of yeast responses to environmental stress and starvation. Functional and Integrative Genomics, 2, 181–192. 10.1007/s10142-002-0058-2
- Goddeeris, B., Boersma, W., Cox, E., Van der Stede, Y., Koenen, M., Vancaeneghem, S., Mast, J. & Wan den Broeck, W. (2002). The porcine and avian intestinal immune system and its nutritional modulation. In M. Blok, H. Vahl, L. Lange, A. Braak, G. Hemke, & M. Hessing (Eds.). Nutrition and Health of the Gastrointestinal Tract (pp. 97–134). The Netherlands: Wageningen Academic Publishers.
- Gross, W. & Siegel, P. (1988). Environment-genetic influences on immunocompetence. Journal of Animal Science, 66, 2091–2094.
- Hinton, A., Buhr, R. & Ingram, K. (2000). Physical, chemical, and microbiological changes in the caeca of broiler chickens subjected to incremental feed withdrawal. Poultry Science, 79, 483–488. 10.1093/ps/79.4.483
- Jones, T., Donnelly, C. & Dawkins, M. (2005). Environmental and management factors affecting the welfare of chickens on commercial farms in the United Kingdom and Denmark stocked at five densities. Poultry Science, 84, 1155–1165. 10.1093/ps/84.8.1155
- Juskiewicz, J., Zdunczyck, Z. & Jankowski, J. (2004). Selected parameters of gastrointestinal tract metabolism of turkeys fed diets with flavomycin and different inulin content. World's Poultry Science Journal, 60, 177–185. 10.1079/WPS20040013
- Kaldhusdal, M., Hofshagen, M., Lovland, A., Langstrand, H. & Redhead, K. (1999). Necrotic enteritis challenge models with broiler chickens raised on litter: evaluation of preconditions, Clostridium perfringens strains and outcome variables. FEMS Immunology and Medical Microbiology, 24, 337–343. 10.1111/j.1574-695X.1999.tb01303.x
- Keyburn, A., Boyce, J., Vaz, P., Bannam, T., Ford, M., Parker, D., Rubbo, A., Rood, J. & Moore, R. (2008). NetB, a new toxin that is associated with avian necrotic enteritis caused by Clostridium perfringens. PLoS Pathogens, 4, e26. 10.1371/journal.ppat.0040026
- Lanckriet, A., Timbermont, L., De Gussem, M., Marien, M., Vancraeynest, D., Haesebrouck, F., Ducatelle, R. & Van Immerseel, F. (2010). The effect of commonly used anticoccidials and antibiotics in a subclinical necrotic enteritis model. Avian Pathology, 39, 63–68. 10.1080/03079450903505771
- Lee, K. & Leeson, S. (2001). Performance of broilers fed limited quantities of feed or nutrients during seven to fourteen days of age. Poultry Science, 80, 446–454. 10.1093/ps/80.4.446
- Mateos, G.G., Lazaro, R. & Gracia, M.I. (2002). The feasibility of using nutritional modifications to replace drugs in poultry feeds. Journal of Applied Poultry Research, 11, 437–452.
- McReynolds, J., Byrd, J., Genovese, K., Poole, T., Duke, S., Farnell, M. & Nisbet, D. (2007). Dietary lactose and its effect on the disease condition of necrotic enteritis. Poultry Science, 86, 1656–1661.
- Novoa-Garrido, M., Larsen, S. & Kaldhusdal, M. (2006). Association between gizzard lesions and increased caecal Clostridium perfringens counts in broiler chickens. Avian Pathology, 35, 367–372. 10.1080/03079450600924150
- Pedersen, K., Bjerrum, L., Heuer, O., Wong, D. & Nauerby, B. (2008). Reproducible infection model for Clostridium perfringens in broiler chickens. Avian Diseases, 52, 34–39. 10.1637/7955-022307-Reg
- Petersen, S., Wiseman, J. & Bedford, M. (1999). Effects of age and diet on the viscosity of intestinal contents in broiler chicks. British Poultry Science, 40, 364–370. 10.1080/00071669987467
- Shariatmadari, F. & Torshizi, R. (2004). Feed restriction and compensatory growth in chicks: effects of breed, sex, initial body weight and level of feeding. Spring Meeting of the WPSA UK Branch. British Poultry Science, 45, S52. 10.1080/00071660410001698290
- Smirnov, A., Sklan, D. & Uni, Z. (2004). Mucin dynamics in the chick small intestine are altered by starvation. Journal of Nutrition, 134, 736–742.
- Thompson, K., Burkholder, K., Patterson, J. & Applegate, T. (2008). Microbial ecology shifts in the ileum of broilers during feed withdrawal and dietary manipulations. Poultry Science, 87, 1624–1632. 10.3382/ps.2007-00324
- Timbermont, L., Lanckriet, A., Dewulf, J., Nollet, N., Schwarzer, K., Haesebrouck, F., Ducatelle, R. & Van Immerseel, F. (2010). Control of Clostridium perfringens-induced necrotic enteritis in broilers by target-released butyric acid, fatty acids and essential oils. Avian Pathology, 39, 117–121. 10.1080/03079451003610586
- Tsiouris, V. (2010). The effect of stress management factors on the pathogenesis of necrotic enteritis in broiler chickens. PhD Thesis. Clinic of Avian Diseases, Faculty of Veterinary Medicine, Aristotle University of Thessaloniki, Greece.
- Tsiouris, V., Georgopoulou, I., Batzios, Chr., Pappaioannou, N., Diakou, A., Petridou, E., Ducatelle, R. & Fortomaris, P. (2013). The role of an attenuated anticoccidial vaccine on the intestinal ecosystem and on the pathogenesis of experimental necrotic enteritis in broiler chickens. Avian Pathology, 42, 163–170. 10.1080/03079457.2013.776161
- Van Immerseel, F., De Buck, J., Pasmans, F., Huyghebaert, G., Haesebrouck, F. & Ducatelle, R. (2004). Clostridium perfringens in poultry: an emerging threat for animal and public health. Avian Pathology, 33, 537–549. 10.1080/03079450400013162
- Waldenstedt, L., Elwinger, K., Lunden, A., Thebo, P., Bedford, M.R. & Uggla, A. (2000). Intestinal digesta viscosity decreases during coccidial infection in broilers. British Poultry Science, 41, 459–464. 10.1080/713654959
- Zhan, X., Wang, M., Ren, H., Zhao, R., Li, J. & Tan, Z. (2007). Effect of early feed restriction on metabolic programming and compensatory growth in broiler chickens. Poultry Science, 86, 654–660.
- Zubair, A. & Leeson, S. (1994). Effect of early feed restriction and realimentation on heat production and changes in sizes of digestive organs of male broilers. Poultry Science, 73, 529–538. 10.3382/ps.0730529
- Zulkifli, I., Dunnington, E., Gross, B., Larsen, S., Martin, A. & Siegel, B. (1993). Responses of dwarf and normal chickens to feed restriction, Eimeria tenella infection, and sheep red blood cell antigen. Poultry Science, 72, 1630–1640. 10.3382/ps.0721630
- Zulkifli, I., Norma, M., Israf, D. & Omar, A. (2000). The effect of early age feed restriction on subsequent response to high environmental temperatures in female broiler chickens. Poultry Science, 79, 1401–1407. 10.1093/ps/79.10.1401
- Zulkifli, I., Siegel, H., Mashaly, M., Dunnington, E. & Siegel, P. (1995). Inhibition of adrenal steroidogenesis, neonatal feed restriction, and pituitary-adrenal axis response to subsequent fasting in chickens. General and Comparative Endocrinology, 97, 49–56. 10.1006/gcen.1995.1005
