Abstract
The aim of this study was to investigate the resistance mechanisms of quinolones, macrolides and tetracycline in campylobacter isolates from grandparent and parent broiler breeders in Spain. Twenty-six isolates were investigated for quinolone resistance, three isolates for macrolide resistance and 39 for tetracycline resistance. All of the quinolone-resistant isolates possessed the mutation Thr86Ile in the quinolone resistance-determining region of gyrA and one isolate possessed the mutation Pro104Ser. Only one Campylobacter coli population (defined by restriction fragment length polymorphism–polymerase chain reaction of flaA and pulsed field gel electrophoresis) was resistant to erythromycin, and the mutation A2075G (23S rDNA) was responsible for macrolide resistance. The tetO gene was found in all of the tetracycline-resistant isolates. Twenty-two out of the 39 isolates investigated by Southern blot possessed chromosomic location of tetO and 17 were located on plasmids. Most of the plasmids with tetO were of around 60 kb and conjugation was demonstrated in a selection of them. In conclusion, we showed that Thr86Ile is highly prevalent in quinolone-resistant isolates as well as mutation A2075G in macrolide-resistant isolates of poultry origin. More variability was found for tetO. The possibility of horizontal transmission of tetO among campylobacter isolates is also an issue of concern in public health.
Introduction
Campylobacter jejuni is the most common zoonotic pathogen in the European Union (EFSA & ECDC, Citation2013). Moreover, it is the most important bacterial cause of gastroenteritis worldwide (Scallan et al., Citation2011; On, Citation2013), even when one considers that data for human infection are underestimated (Toljander et al., Citation2012).
There are several identified sources of C. jejuni (and its closely related species Campylobacter coli) infection, such as consumption of raw milk, untreated water, red meat, and contact with pets and farm animals, among others (Whiley et al., Citation2013). Different studies of risk factors, however, point to the handling or consumption of chicken meat as the main source of infection in humans (Lee & Newell, Citation2006). Most cases of campylobacteriosis are usually self-limiting and do not require hospitalization. Antibiotic treatment is required, however, when severe enteritis or complications are present, as well as when very young or elderly patients and pregnant women are infected. Macrolides and quinolones are the drugs of choice, with tetracycline as an alternative treatment for campylobacteriosis (Moore et al., Citation2006). Resistance against quinolones is of concern in many countries (Ge et al., Citation2013). In Spain, for example, during the 1980s quinolone resistance was 11% in human C. jejuni isolates, while at the end of the 1990s the resistance rates reached 85% (Prats et al., Citation2000). Most variability is found among macrolides and tetracycline resistances, and the monitoring and the susceptibility of these two groups of antimicrobials are an issue of interest in public health (Ge et al., Citation2013).
For zoonotic bacteria it is of importance both to study the antimicrobial resistances as well as the resistance determinants and their genetic composition. This might be very useful to prevent the spread of resistance and for understanding the relationships in antibiotic resistance between bacterial populations (Wirz et al., Citation2010). In Campylobacter spp., it is well known that mutations affecting the quinolone resistance-determining region (QRDR) of the gyrA gene are responsible for quinolone resistance (Piddock et al., Citation2003). Among the described mutations, Thr86Ile is the most prevalent (Bachoual et al., Citation2001), conferring high minimum inhibitory concentration (MIC) values. In addition to this mutation, several other point mutations at gyrA have been described at the same codon—Thr86Val (Piddock et al., Citation2003), Thr86Lys and Thr86Ala (Wang et al., Citation1993)—as well as in other codons: Ala87Pro, Asp90His, and Asp90Tyr. These other mutations confer resistance against quinolones with different MICs values. Polymorphisms in the gyrB gene have been disregarded as the cause of resistance to quinolones in Campylobacter spp. (Payot et al., Citation2006). Other mechanisms such as qnr or aac(6′)-Ib-cr genes have not been described in Campylobacter spp.
There are two main mechanisms conferring resistance against macrolides in Campylobacter spp. The most prevalent are the mutations at positions 2074 and 2075 (domain V) in the rrn gene, which encodes for 23S rRNA (Gibreel et al., Citation2005). Transversion A2075G is the most commonly found, in most cases, in homozygosis where the three copies of rrn gene possess the mutation (Gibreel et al., Citation2005). This mutation is responsible for the high MIC values against macrolides. Other described mechanisms are the mutations in rplD and rplV genes, which encode for L4 and L22 ribosomal proteins, respectively. Only the mutations in these genes affecting the regions comprising amino acids 55 to 77 in L4 and 109 to 142 in L22 have been associated with macrolide resistance (Caldwell et al., Citation2008).
Among the different known mechanisms of tetracycline resistance, protection of the ribosomal site of 16S rRNA mediated by the TetO protein is the most important in C. jejuni and C. coli (Pratt & Korolik, Citation2005). This protein is coded by the tetO gene and can be located on the chromosome and plasmids (Avrain et al., Citation2004). The presence of conjugative plasmids containing tetO gene is likely to play a substantial role in dissemination of the resistance (Wieczorek & Osek, Citation2013).
In a previous study (Pérez-Boto et al., Citation2013), we monitored the antimicrobial resistance of 697 C. jejuni and 108 C. coli isolates recovered from poultry in two early stages of broiler production in Spain. Both grandparent breeder broiler farms (GPBF) and the parent breeder broiler farms (PBF) were monitored during a 4-year period (2002 to 2005). The total percentages of resistance were 74.9%, 73.9%, 48.2%, 19.8% and 3.1%, respectively for nalidixic acid, ciprofloxacin, tetracycline, amoxicillin and erythromycin, and 10.3% were pan-susceptible. With these previous data, the objective of our study was to characterize the mechanisms of quinolone, macrolide, and tetracycline resistance among the avian Campylobacter isolates obtained in two early stages of broiler production in Spain.
Materials and Methods
Origin of campylobacter isolates and MIC determination
Campylobacter isolates representing the different resistotypes that had been determined in the previous study by the disk-diffusion method (Pérez-Boto et al., Citation2013) were chosen for investigation of mechanisms of resistance to quinolones, macrolides and tetracycline ().
Table 1. Origin of Campylobacter isolates, species relationships and resistotypes with respect to stage of production.
One isolate of each resistotype found in each flock was selected for MIC determination. When the same resistotype in a flock could be detected, one isolate per species of C. jejuni and C. coli, respectively was chosen. In total, 51 isolates of C. jejuni and 10 of C. coli were selected for MIC determination.
In the selected isolates, MICs were investigated by the E-test method (AB Biodisk, Solna, Sweden) according to the manufacturer's instructions. We followed the Clinical and Laboratory Standards Institute (CLSI) guidelines and recommendations for testing the isolates. The MIC values were compared with cut-off values provided by the CLSI (Citation2010) to ensure the resistance profile of the strains. Mueller–Hinton agar supplemented with 5% defibrinated sheep blood (Becton & Dickinson, Madrid, Spain) was used as culture medium in all the susceptibility tests.
Typing of isolates
Two different subtyping methods were carried out for comparison purposes between the isolates and for the characterization of populations within a flock. Restriction fragment length polymorphism-polymerase chain reaction (RFLP-PCR) of the flaA gene was performed following a previously published protocol (Nachamkin et al., Citation1993) using DdeI as restriction enzyme (FastDigest®; Fermentas Thermo Fisher, Madrid, Spain). Pulsed field gel electrophoresis (PFGE) using KpnI (Takara Bio Inc., Madrid, Spain) was carried out following the conditions described previously (On et al., Citation1998), including an initial time of 4 sec and a final time pulse of 20 sec for 22 h in 0.9% w/v agarose (Seakem Gold; Lonza, Madrid, Spain) in 0.5× TBE buffer.
Comparison of restriction profiles was performed using Infoquest Software (BioRad, Madrid, Spain) by employing the Dice index algorithm with an optimization and tolerance of 1%. Dendrograms were constructed using the unweighted pair group method with arithmetic mean.
Selection of resistant isolates of campylobacter
For quinolone resistance, one isolate per flock within the most frequent resistotype was selected, which resulted in 22 C. jejuni and four C. coli isolates (). This selection was made because MICs against ciprofloxacin and nalidixic acid were observed as equal among the different resistotypes and populations of Campylobacter isolates (populations defined by RFLP-PCR of flaA and PFGE).
The macrolide resistance had been detected only in a C. coli population from the single flock P-H (). The flock was distributed in three different sheds, and hence three isolates, one per shed, were selected for the investigation of the mechanisms of resistance.
Tetracycline-resistant isolates had been detected in all but five flocks, and in this case one isolate by resistotype was investigated (30 C. jejuni and nine C. coli isolates) (), because, unlike quinolone resistance, there were differences in the MIC for tetracycline within populations and resistotypes from the same flocks.
Table 2. Mechanisms of tetracycline resistance in Campylobacter isolates with indication of location of tetO investigated by Southern blotting, MICs, sizes of isolated plasmids and recipient strains (Rec1 and Rec2) in mating experiments.
Isolation, amplification and sequencing of DNA
DNA templates of all strains examined in the study were obtained using InstaGene Matrix (BioRad Spain). All PCR amplifications were carried out using the Ready-to-Go system (Ge Healthcare Life Sciences, Buckinghamshire, UK), following the manufacturer's instructions.
For the investigation of quinolone resistance, a region of 1081 base pairs (bp) from the gyrA gene including the QRDR region (210 bp) was amplified using the primers GyrAF1 (5′-CAACTGGTTCTAGCCTTTTG-3′) and GyrAR1 (5′-AATTTCACTCATAGCCTCACG-3′), as described previously (Luo et al., Citation2005). Another PCR was carried out to determine the presence of the tetO gene responsible for tetracycline resistance in Campylobacter spp. The primers tetOF (5′-GTGACATCTTTTCAGTGGGAGG-3′) and tetOR (5′-CTTCCATCTGCACATTCCCC-3′) designed by us were used for the detection of a region of 1014 bp of the tetO gene.
For the investigation of macrolide resistance, a 852-bp fragment of 23S rDNA was amplified by PCR in each resistant isolate using the pair of primers 23Suniv + 41783 (5′-GCTCGAAGGTTAATTGATG-3′) and 23Suniv-42635 (5′-GCTCTTGGCAGAACAAC-3′) as described previously (Pérez-Boto et al., Citation2010). In addition to this, ribosomal genes rplD and rplV were amplified due to their role in macrolide resistance using the primers and PCR conditions presented earlier (Cagliero et al., Citation2006).
For sequencing, the PCR products were purified using the GFX PCR DNA and the Gel Band Purification Kit (GE Healthcare Life Sciences) following the manufacturer's instructions. The sequences were obtained with ABI PRISM 377 equipment (Perkin-Elmer, Applied Biosystems Division, Foster City, CA, USA) using Big Dye Ready Reaction Mix version 3.1 (Applied Biosystems), following the manufacturer's protocol.
The sequences were assembled using Lasergene Seqman II software (DNA Star Inc., Madison, WI, USA) and the amino acid sequences were investigated using the CLUSTAL W routine of MegAlign software (v. 6.1; DNA Star, Inc.).
Plasmid isolation and detection of the tetO gene
Plasmids from isolates of Campylobacter spp. resistant to tetracycline were obtained from bacteria cultivated on Columbia agar supplemented with 5% sheep blood. Plasmid extraction was carried out using the GeneJet™ Plasmid Miniprep Kit (Fermentas Thermo Fisher). Escherichia coli strain V517 was used as a positive control for plasmid extraction and the molecular weight ladder. The presence and size of the plasmids in the eluted purified liquid obtained with the commercial kit were checked by 1.2% agarose gel electrophoresis (MS8 agarose; Conda, Madrid, Spain). Determination of molecular weights of the plasmids from Campylobacter isolates was carried out with Quantity One software (BioRad Laboratories Inc., Hercules, CA, USA).
The location of tetO was investigated using Southern blot experiments. A tetO probe was generated and labelled with digoxigenin-11-dUTP by PCR (DIG probe synthesis kit; Roche Applied Science, Barcelona, Spain) using the same pair of primers that was used in PCR for tetO amplification. As a template, all of the DNA templates from the tetracycline-resistant isolates of our study were mixed and used in the labelling of the probe. Another probe, labelled with digoxigenin-11-dUTP, was constructed using hexanucleotide random primers and V517 E. coli plasmid as a template for PCR. It was used to detect the molecular marker used in plasmid gel electrophoresis.
The plasmids were transferred into positively charged nylon membranes (Roche Applied Science, Madrid, Spain) overnight, and fixed to the membrane using ultraviolet light exposure. In the tetracycline-resistant strains, in which no plasmids were isolated, DNA from KpnI-PFGE gels was also transferred to nylon membranes. The membranes were washed twice for 10 min in 2× SSC and 0.1% SDS at 65°C and once for 15 min in 1× SSC and 0.1% SDS at 65°C. The tetO probes were immunodetected with anti-digoxigenin-AP and Fab fragments (Roche Applied Sciences. Barcelona, Spain) and then visualized by colorimetric detection using NBT/BCIP (Roche Applied Sciences, Barcelona, Spain).
Conjugation experiments
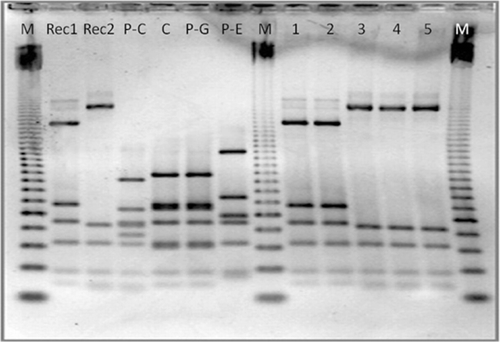
Conjugation experiments were carried out as described by Schmidt-Ott et al. (Citation2005) with the modification that erythromycin was used as the antimicrobial selected by recipient strains instead of nalidixic acid. As recipient strains, two erythromycin-resistant C. jejuni strains of clinical origin were selected and named as Rec1 and Rec2 (). In these strains, a substitution A2075G in the three alleles of 23S rDNA was responsible for the macrolide resistance. Erythromycin resistance was chosen due to their chromosomal resistance location, thereby avoiding transfer of this resistance from recipient strains to donor strains by plasmid conjugation. Furthermore, the recipient strains were confirmed plasmid free.
Four C. jejuni tetracycline-resistant strains containing plasmids were selected as donors in the conjugation experiment (). They varied in their MIC value against tetracycline (32 mg/l, 96 mg/l, and two isolates with >256 mg/l). Muller–Hinton agar with 5% sheep blood, supplemented with tetracycline (50 mg/l) and erythromycin (50 mg/l), was used to select the possible transconjugants. The transconjugant colonies were selected and analysed for the MIC for tetracycline, tetO sequence analysis, and PCR-RFLP of flaA, to verify the profile of the recipient strains and their resistance to tetracycline.
Sequence data
All of the DNA sequences described in this study have been deposited in the GenBank database with the accession numbers KF845970 to KF846002 for gyrA and KF845959 to KF845968 for tetO, and the 23S rDNA sequence from erythromycin-resistant population with the accession number KF845969.
Results
Quinolone resistance
As shown in , only three out of 29 flocks (flocks B, I, and P-J) were colonized by campylobacter isolates susceptible to quinolones. In all of the investigated resistant isolates, the MICs against nalidixic acid were >256 mg/l, while those against ciprofloxacin were >32 mg/l, with the exception of isolates from flocks F and P–M (both with 16 mg/l), and flock P–F (12 mg/l). As previously explained, the QRDR of gyrA gene was sequenced in one isolate from a resistant flock, which showed, in all cases, the mutation Thr86Ile. Moreover, high homology among the amino acid sequences was found independent of the flock, stage of production, or even subtype of the strains (data not shown). One isolate, from flock P-C, possessed an additional mutation (Pro104Ser) in QRDR, with MICs of >256 mg/l and >32 mg/l for nalidixic acid and ciprofloxacin, respectively (the highest values in E-test strips).
Macrolide resistance
The mechanisms of macrolide resistance were investigated in the only macrolide-resistant population of C. coli isolated from flock P-H. They formed a single population as evidenced by subtyping methods (RFLP-PCR of flaA and KpnI-PFGE; data not shown). As explained previously, three isolates within the flock were chosen, all them with MIC >256 mg/l against erythromycin.
In these isolates, mutation A2075G was present and the electropherogram of the 23S rDNA sequence showed a single peak of guanidine at position 2075, which suggested that the three copies of the gene were mutated. None of the known mutations in rplD and rplV responsible for macrolide resistance were found in any of the investigated isolates.
Tetracycline resistance
Tetracycline resistance showed a variety of MICs in the isolates of the study (from 16 to >256 mg/l) independent of the production stage, species of Campylobacter, or location of tetO gene (). A fragment of tetO was detected by Southern blot only in plasmids from isolates from eight flocks (three GPBF and five PBF) and apparently only in the chromosome of isolates from 10 flocks (six GPBF and four PBF); on the other hand, in six flocks (four GPBF and two PBF), there were isolates in which tetO appeared in both locations. For this group with two locations, a comparison of the KpnI-PFGE profiles of tetracycline resistant isolates was carried out. As shown in , the maximum similarity between isolates within a flock with different tetO location was 62%, between isolates of flock P-L.
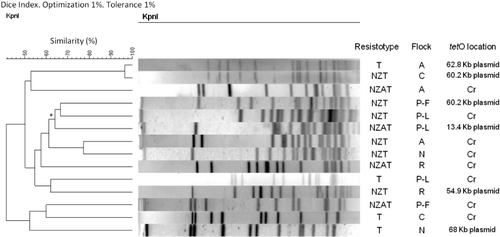
Regarding the isolation of plasmids, the sizes varied between 3.3 and 78.3 kb, but most of them were around 60 kb.
With regard to the conjugation experiments, Rec1 was able to acquire resistance to tetracycline through the plasmids from the isolates of flocks P-C and P-G. The recipient strain Rec2 acquired the plasmid from isolates of flocks C, P-C, P-E, and P-G. MICs of transconjugants were calculated and values of 16 mg/l for flock P-C (Rec-1), 16 mg/l for flock P-G (Rec-1), 32 mg/l for flock C (Rec-2), 16 mg/l for flock P-C (Rec-2), 32 mg/l for flock P-E (Rec-2), and 48 mg/l for flock P-G (Rec-2) were obtained.
The RFLP-PCR of flaA of transconjugants was carried out, which showed the same restriction pattern as that of the recipient strains ().
Discussion
The objective of this study was to describe the molecular mechanisms responsible for antimicrobial resistance in C. jejuni and C. coli isolated from two early stages of poultry production in Spain sampled during a 4-year period. During that time, resistance against nalidixic acid, ciprofloxacin, erythromycin, tetracycline and amoxicillin was found.
With regard to the quinolones, mutation Thr86Ile was found in all of the quinolone-resistant isolates analysed, even when they were of different species or subtypes. These data are consistent with published data (Piddock et al., Citation2003; Luo et al., Citation2005). This mutation is the most prevalent in clinical and veterinary isolates, among other described mutations (Thr86Ala, Thr86Lys, Ala87Pro, Asp90His, Asp90Tyr, etc.) (Wang et al., Citation1993; Bachoual et al., Citation2001; Piddock et al., Citation2003) mostly of in vitro origin (Bachoual et al., Citation2001). One isolate possessed another mutation in QRDR, Pro104Ser, previously described in isolates of animal and human origin (Piddock et al., Citation2003; Jesse et al., Citation2006). Pro104Ser has always been detected together with Thr86Ile, and its function in resistance is not known and more studies are necessary to ascertain its role.
Very high MIC values were found in all isolates, showing that Thr86Ile, as described previously (Piddock et al., Citation2003; Alfredson & Korolik, Citation2007), provides by itself high resistance to quinolones.
Macrolide resistance in Campylobacter spp. has been linked mostly with C. coli isolates of swine origin. Poultry isolates seem to be more susceptible to this family of antimicrobials, because macrolides are not commonly used in antibiotic treatment of broilers as in swine (Belanger & Shryock, Citation2007). In our study, only a population of C. coli from a PBF was resistant to this antimicrobial. The mechanism of resistance described in this population was the mutation A2075G in rrn gene, which is the most prevalent in Campylobacter spp., providing a high MIC value. It has been shown that at least two out of the three copies of rrn gene have to be mutated to show high MIC values (Gibreel et al., Citation2005). Our isolates seem to have been mutated in all three copies resulting in the high MIC values observed (>256 mg/l).
Regarding tetracycline resistance, lack of association has been described between the polymorphisms along the sequence of tetO and the chromosomal or plasmid location of this gene with MIC values (Pratt & Korolik, Citation2005; Piddock et al., Citation2008). In our study, we also found an apparent lack of association between the location of tetO (chromosome or plasmid) and the MIC value. Furthermore, on a flock level, different populations of Campylobacter spp., either with tetO located in the plasmid or chromosome, could coexist. Within the same population of Campylobacter defined by comparison of PFGE profiles, the same location of tetO in both the plasmid and the chromosome was never found. The chromosomal location of tetO may represent mobilization and integration of part of the resistance plasmid in the chromosome after conjugation between isolates from different populations within the same flock.
Recombination and integration of tetracycline resistance plasmids have been documented previously in Campylobacter spp. (Pratt & Korolik, Citation2005). As previously demonstrated, plasmids with tetracycline resistance genes are very conserved, with high homology in size and sequence (Ge et al., Citation2013). Most of the tetracycline resistance plasmids reported in different studies are 30 to 58 kb in size (Pratt & Korolik, Citation2005; Alfredson & Korolik, Citation2007). In our study, we noted plasmids between 3.3 and 78.3 kb, but the tetO gene conferring resistance against tetracycline could be found in plasmids of around 60 kb. This observation indicates the observed conservative nature of the tetracycline resistance plasmids.
Conjugation of tetracycline resistance plasmids was demonstrated by transmission of the resistance against this antimicrobial, although the MICs for transconjugants were less than for the original strains. Therefore, the transmission of tetracycline resistance among the populations of Campylobacter spp. in the farms from our study is plausible.
Our results confirm the high prevalence of mutation Thr86Ile in the gyrA gene among quinolone-resistant isolates (without previous quinolone treatment) of poultry origin. This might indicate, as suggested previously, an advantage in colonization and persistence provided by that mutation in the absence of antimicrobial treatment. In contrast, macrolide resistance is of low occurrence and is acquired by mutation at 23S rDNA, a mechanism commonly found in Campylobacter spp. Tetracycline resistance located in conjugative plasmids might be a risk factor in disseminating resistance among other Campylobacter populations of avian origin or even among other species commonly found in avian gut.
Acknowledgements
The authors thank the Genomic Unit from the “Centro Nacional de Microbiología” for conducting the sequencing reactions in the study. They also thank Mathieu Bangert for his critical review of the manuscript. This work was supported by the Instituto de Salud Carlos III [Grant MPY-1179/02].
References
- Alfredson, D.A. & Korolik, V. (2007). Antibiotic resistance and resistance mechanisms in Campylobacter jejuni and Campylobacter coli. FEMS Microbiology Letters, 277, 123–132. 10.1111/j.1574-6968.2007.00935.x
- Avrain, L., Vernozy-Rozand, C. & Kempf, I. (2004). Evidence for natural horizontal transfer of tetO gene between Campylobacter jejuni strains in chickens. Journal of Applied Microbiology, 97, 134–140. 10.1111/j.1365-2672.2004.02306.x
- Bachoual, R., Ouabdesselam, S., Mory, F., Lascols, C., Soussy, C.-J. & Tankovic, J. (2001). Single or double mutational alterations of gyrA associated with fluoroquinolone resistance in Campylobacter jejuni and Campylobacter coli. Microbial Drug Resistance, 7, 257–261. 10.1089/10766290152652800
- Belanger, A.E. & Shryock, T.R. (2007). Macrolide-resistant Campylobacter: the meat of the matter. Journal of Antimicrobial Chemotherapy, 60, 715–723. 10.1093/jac/dkm300
- Cagliero, C., Mouline, C., Cloeckaert, A. & Payot, S. (2006). Synergy between efflux pump CmeABC and modifications in ribosomal proteins L4 and L22 in conferring macrolide resistance in Campylobacter jejuni and Campylobacter coli. Antimicrobial Agents & Chemotherapy, 50, 3893–3896. 10.1128/AAC.00616-06
- Caldwell, D.B., Wang, Y. & Lin, J. (2008). Development, stability, and molecular mechanisms of macrolide resistance in Campylobacter jejuni. Antimicrobial Agents & Chemotherapy, 52, 3947–3954. 10.1128/AAC.00450-08
- Clinical and Laboratory Standards Institute. (2010). Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria; Approved guideline-Second Edition. CLSI Document M45-A2 Vol. 30 No. 18. Wayne, PA: Clinical and Laboratory Standards Institute.
- EFSA & ECDC (2013). The European union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2011. EFSA Journal, 11, 3129. Retrieved from http://www.efsa.europa.eu/en/efsajournal/doc/3129.pdf
- Ge, B., Wang, F., Sjolund-Karlsson, M. & McDermott, P.F. (2013). Antimicrobial resistance in Campylobacter: susceptibility testing methods and resistance trends. Journal of Microbiological Methods, 95, 57–67. 10.1016/j.mimet.2013.06.021
- Gibreel, A., Kos, V.N., Keelan, M., Trieber, C.A., Levesque, S., Michaud, S. & Taylor, D. E. (2005). Macrolide resistance in Campylobacter jejuni and Campylobacter coli: molecular mechanism and stability of the resistance phenotype. Antimicrobial Agents & Chemotherapy, 49, 2753–2759. 10.1128/AAC.49.7.2753-2759.2005
- Jesse, T.W., Englen, M.D., Pittenger-Alley, L.G. & Fedorka-Cray, P.J. (2006). Two distinct mutations in gyrA lead to ciprofloxacin and nalidixic acid resistance in Campylobacter coli and Campylobacter jejuni isolated from chickens and beef cattle. Journal of Applied Microbiology, 100, 682–688. 10.1111/j.1365-2672.2005.02796.x
- Lee, M.D. & Newell, D.G. (2006). Campylobacter in poultry: filling an ecological niche. Avian Diseases, 50, 1–9. 10.1637/7474-111605R.1
- Luo, N., Pereira, S., Sahin, O., Lin, J., Huang, S., Michel, L. & Zhang, Q. (2005). Enhanced in vivo fitness of fluoroquinolone-resistant Campylobacter jejuni in the absence of antibiotic selection pressure. Proceedings of the National Academy of Sciences of the USA, 102, 541–546. 10.1073/pnas.0408966102
- Moore, J.E., Barton, M.D., Blair, I.S., Corcoran, D., Dooley, J.S.G., Fanning, S., Kempf, I., Lastovica, A.J., Lowery, C.J., Matsuda, M., McDowell, D.A., McMahon, A., Millar, B.C., Rao, J.R., Rooney, P.J., Seal, B.S., Snelling, W.J. & Tolba, O. (2006). The epidemiology of antibiotic resistance in Campylobacter. Microbes & Infection, 8, 1955–1966. 10.1016/j.micinf.2005.12.030
- Nachamkin, I., Bohachick, K. & Patton, C.M. (1993). Flagellin gene typing of Campylobacter jejuni by restriction fragment length polymorphism analysis. Journal of Clinical Microbiology, 31, 1531–1536.
- On, S.L. (2013). Isolation, identification and subtyping of Campylobacter: where to from here? Journal Microbiological Methods, 95, 3–7. 10.1016/j.mimet.2013.06.011
- On, S.L., Nielsen, E.M., Engberg, J. & Madsen, M. (1998). Validity of SmaI-defined genotypes of Campylobacter jejuni examined by Sal I, KpnI, and Bam HI polymorphisms: evidence of identical clones infecting humans, poultry, and cattle. Epidemiology & Infection, 120, 231–237. 10.1017/S0950268898008668
- Payot, S., Bolla, J.-M., Corcoran, D., Fanning, S., Mégraud, F. & Zhang, Q. (2006). Mechanisms of fluoroquinolone and macrolide resistance in Campylobacter spp. Microbes & Infection, 8, 1967–1971. 10.1016/j.micinf.2005.12.032
- Pérez-Boto, D., Lopez-Portoles, J.A., Simon, C., Valdezate, S. & Echeita, M.A. (2010). Study of the molecular mechanisms involved in high-level macrolide resistance of Spanish Campylobacter jejuni and Campylobacter coli strains. Journal of Antimicrobial Chemotherapy, 65, 2083–2088. 10.1093/jac/dkq268
- Pérez-Boto, D., García-Peña, F.J., Abad-Moreno, J.C. & Echeita, M.A. (2013). Antimicrobial susceptibilities of Campylobacter jejuni and Campylobacter coli strains isolated from two early stages of poultry production. Microbial Drug Resistance, 19, 323–330. 10.1089/mdr.2012.0160
- Piddock, L.J., Ricci, V., Pumbwe, L., Everett, M.J. & Griggs, D.J. (2003). Fluoroquinolone resistance in Campylobacter species from man and animals: detection of mutations in topoisomerase genes. Journal of Antimicrobial Chemotherapy, 51, 19–26. 10.1093/jac/dkg033
- Piddock, L.J.V., Griggs, D., Johnson, M.M., Ricci, V., Elviss, N.C., Williams, L.K., Jorgensen, F., Chisholm, S.A., Lawson, A.J., Swift, C., Humphrey, T.J. & Owen, R.J. (2008). Persistence of Campylobacter species, strain types, antibiotic resistance and mechanisms of tetracycline resistance in poultry flocks treated with chlortetracycline. Journal of Antimicrobial Chemotherapy, 62, 303–315. 10.1093/jac/dkn190
- Prats, G., Mirelis, B., Llovet, T., Munoz, C., Miro, E. & Navarro, F. (2000). Antibiotic resistance trends in enteropathogenic bacteria isolated in 1985–1987 and 1995–1998 in Barcelona. Antimicrobial Agents & Chemotherapy, 44, 1140–1145. 10.1128/AAC.44.5.1140-1145.2000
- Pratt, A. & Korolik, V. (2005). Tetracycline resistance of Australian Campylobacter jejuni and Campylobacter coli isolates. Journal of Antimicrobial Chemotherapy, 55, 452–460. 10.1093/jac/dki040
- Scallan, E., Hoekstra, R.M., Angulo, F.J., Tauxe, R.V., Widdowson, M.-A., Roy, S.L., Jones, J.L. & Griffin, P.M. (2011). Foodborne illness acquired in the United States–major pathogens. Emerging Infectious Diseases, 17, 7–15. 10.3201/eid1701.P11101
- Schmidt-Ott, R., Pohl, S., Burghard, S., Weig, M. & Groß, U. (2005). Identification and characterization of a major subgroup of conjugative Campylobacter jejuni plasmids. Journal of Infection, 50, 12–21. 10.1016/j.jinf.2004.02.013
- Toljander, J., Dovarn, A., Andersson, Y., Ivarsson, S. & Lindqvist, R. (2012). Public health burden due to infections by verocytotoxin-producing Escherichia coli (VTEC) and Campylobacter spp. as estimated by cost of illness and different approaches to model disability-adjusted life years. Scandinavian Journal of Public Health, 40, 294–302. 10.1177/1403494811435495
- Wang, Y., Huang, W.M. & Taylor, D.E. (1993). Cloning and nucleotide sequence of the Campylobacter jejuni gyrA gene and characterization of quinolone resistance mutations. Antimicrobial Agents and Chemotherapy, 37, 457–463. 10.1128/AAC.37.3.457
- Whiley, H., van den Akker, B., Giglio, S. & Bentham, R. (2013). The role of environmental reservoirs in human campylobacteriosis. International Journal of Environmental Research and Public Health, 10, 5886–5907. 10.3390/ijerph10115886
- Wieczorek, K. & Osek, J. (2013). Antimicrobial resistance mechanisms among Campylobacter. BioMed Research International, 2013, 340605.
- Wirz, S.E., Overesch, G., Kuhnert, P. & Korczak, B.M. (2010). Genotype and antibiotic resistance analyses of Campylobacter isolates from ceca and carcasses of slaughtered broiler flocks. Applied and Environmental Microbiology, 76, 6377–6386. 10.1128/AEM.00813-10
