Abstract
Infectious bronchitis virus (IBV) was detected in 185 samples originating from chicken flocks of various commodity groups in Canada. Flocks with clinical signs such as respiratory challenge, sudden death, egg production problems, or nephropathogenic conditions, and randomly selected flocks sampled at slaughter as part of an Ontario broiler surveillance project, were included. Most samples were from Ontario and Québec; however, a small number from British Columbia, Nova Scotia, and Newfoundland and Labrador were also analysed. The nucleotide sequence of the spike (S) protein gene was compared with sequences available in GenBank. Based on their S gene sequence similarities, Canadian IBVs could be divided into nine genotypes belonging to four groups: Canadian variant virus, strain Qu_mv; the classic, vaccine-like viruses, Connecticut and Massachusetts; US variant-like virus strains, California 1734/04, California 99, CU_82792, Pennsylvania 1220/98 and Pennsylvania Wolg/98; and non-Canadian, non-US virus, strain 4/91. Based on the field situation, the effectiveness of current vaccination practices mostly based on Massachusetts and Connecticut-type vaccines appeared generally satisfactory for minimizing the damage due to infection with Canadian variant and US variant-like viruses. However, the recent outbreaks of severe respiratory disease and production problems in Ontario chicken flocks related to the incursion of IBV strain 4/91 were not prevented by standard vaccination protocols. It appears that IBV strain 4/91 has now become endemic in Ontario and the need for 4/91-type vaccines must be evaluated.
Introduction
Infectious bronchitis virus (IBV) has a worldwide distribution and affects both broilers and egg-laying chickens. Diseases caused by or associated with IBV infection have significant economic importance. Infectious bronchitis is an acute disease transmitted via the respiratory tract by inhalation or by direct contact with contaminated poultry, litter, or equipment. The incubation period is short, with clinical signs developing 24 to 48 hours post exposure. In young birds, the clinical signs can range from reduced weight gain and feed efficiency to respiratory disease and depression. Secondary infections include bacterial septicaemia, airsacculitis, pericarditis and perihepatitis. Although primarily described as a respiratory agent, many IBV strains are nephropathogenic (de Wit et al., Citation2011). IBV can also infect reproductive organs and cause egg production problems (Cavanagh, Citation2007). A possible role for IBV in proventriculitis has been investigated (Pantin-Jackwood et al., Citation2005). However, enteric IBV infections are typically not considered to be associated with a particular disease (Raj & Jones, Citation1997).
Pleomorphic IBV particles are enveloped and have large surface projections called the spike (S) protein, which play a role in virus attachment and fusion of the virus with the host cell. The S protein is cleaved into two subunits, S1 and S2. Neutralizing antibody epitopes are located within the S1 subunit (Moore et al., Citation1997). Involvement of IBV in clinical disease is not always overt and diagnosis often involves compatible clinical history coupled with histopathology, increase in antibody titres, virus isolation, and/or detection of viral RNA by polymerase chain reaction (PCR). In recent years, genotyping carried out by S1 gene sequencing (Kingham et al., Citation2000; Jackwood et al., Citation2005) has become the method of choice for differentiation between field and vaccine viruses.
Historically, outbreaks of IBV in Ontario have been sporadic. However, both respiratory and nephropathogenic strains have been described in the field (Grgic et al., Citation2008). A relatively small number of IBVs obtained from disease outbreaks in the field in Ontario have been genotyped, and, based on S1 gene sequencing, these viruses were highly related to variant IBVs circulating in the USA (Ojkic & Binnington, Citation2002). Since early 2012, an increased number of IBV-associated cases in chicken flocks of various commodities have been reported. The objective of this study was to genotype IBVs in samples submitted to the Animal Health Laboratory at the University of Guelph in Ontario from 2000 to 2013 in order to better understand the sudden increase in IBV outbreaks in commercial chicken flocks in Ontario in 2012 and 2013.
Materials and Methods
Samples and testing
A total of 185 samples were included in this study: 148 samples (80%) from case submissions associated with clinical problems involving various commodities from 2000 to 2013, and 37 samples (20%) collected at slaughter from randomly selected broiler flocks during an Ontario surveillance project between July 2010 and January 2012 (Eregae et al., Citation2014). Each sample represented one flock; when more than one positive sample per flock was available, the strongest positive was selected for genotyping. Testing was carried out at the Animal Health Laboratory, which provides full-service veterinary diagnostic laboratory support primarily for Ontario and which also accepts samples from other Canadian provinces. In this study, samples from Québec, Nova Scotia, British Columbia, and Newfoundland and Labrador were also included.
Virus isolation
From 2000 to 2011, virus isolation in specific pathogen free embryonated chicken eggs was used as a primary test for the detection of IBV in clinical samples. Nine-day-old to 11-day-old specific pathogen free embryonated chicken eggs (Charles River, North Franklin, CT, USA or Sunrise Farms, Cauterskill, CT, USA) were inoculated with 200 µl samples that had been filtered through a 0.45 µm filter. Eggs were candled daily to detect embryo mortality and were passaged weekly up to three times if mortality was not observed earlier.
Polymerase chain reaction and sequencing
Since January 2012, real-time PCR has been used as a primary screening test for IBV detection in clinical samples (Callison et al., Citation2006). All samples collected during the surveillance project were tested by real-time PCR. The PCR genotyping was carried out as described previously (Kingham et al., Citation2000), or with a primer set modified to better match IBVs prevalent in Ontario (IBV_For4_130129, 5′-GTK TAC TAC TAY CAA AGT GCC TT-3′; and IBV_Rev4_130129, 5′-GCA TGC WAR CAA RCC TCT AGG-3′). Primers were designed to amplify a 621 base pair fragment of S gene from nucleotides 20,392 to 21,012, based on the IBV 4/91 vaccine sequence (GenBank accession number: KF377577). Nucleotide sequences of PCR products were determined at the University of Guelph Laboratory Services sequencing facility. The GenBank accession numbers of the sequences are KJ196087 to KJ196271. Sequence comparison of the 505 base pair fragment from IBV 4/91 vaccine nucleotides 20,452 to 20,956 was performed using the LaserGene software (DNAStar Inc., Madison, WI, USA).
Results
Based on their partial S protein gene sequences, IBVs identified in Canada during the study period could be divided into nine genotypes (). Based on their presumptive origins, these genotypes could be placed into four general groups (). More than one-third (35.7%) of the genotyped samples were Canadian variants, 66 viruses being 90.9 to 99.2% identical to strain Qu_mv. Classic, vaccine-like viruses were the second largest group, representing approximately one-third (28.6%) of genotyped samples since 27 viruses were 95.9 to 100.0% identical to Connecticut and 26 viruses were 96.0 to 100.0% identical to Massachusetts.
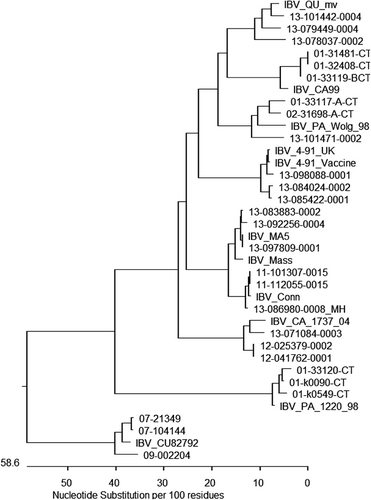
Table 1. Genotypes of 185 IBVs detected in Canada between 2000 and 2013.
US variant-like viruses represented one-fifth (20.5%) of the genotyped samples, and 14 viruses were 91.1 to 100.0% identical to CU_82792. Seven viruses showed the best match, 95.4 to 95.8%, to California 99 and four viruses were 95.2 to 95.6% identical to California 1734/04. Nine viruses were 98.1 to 99.8% identical to Pennsylvania 1220/98 and four viruses were 88.3 to 91.2% identical to Pennsylvania Wolg/98. Twenty-eight viruses showed 94.1 to 99.4% identity to the non-Canadian, non-US strain 4/91. Vaccine-like viruses were detected in 19.6% of the samples from clinical cases and 64.9% of the samples collected at slaughter.
In 2012 and 2013, strain 4/91 became the most frequently detected IBV, representing 56.7% of clinical cases, while all other strains together were present in 43.3% of genotyped cases ().
Table 2. Summary of the genotype of 185 IBV strains typed in Canada by year from 2000 to 2013.
Discussion
The most common IBV detected in this study was strain Qu_mv, a strain that so far has not been described anywhere other than in eastern Canada. It has been suggested that this virus was generated by recombination between the Massachusetts and Arkansas-like strains of IBV (Smati et al., Citation2002). Interestingly, we did not find Arkansas-type viruses; however, it is possible that they had been present at some point in the past. First isolated in 1996, strain Qu_mv has become the most common genotype in Québec and Nova Scotia, and has also spread to Ontario (Ojkic & Binnington, Citation2002). Among the samples that originated from Québec, Qu_mv viruses represented 81.0% of all genotyped viruses, while among the samples that originated from Ontario, involvement of Qu_mv in IBV-associated cases was much lower at 11.5%.
Around 20% of the IBVs detected in this study appeared to be related to variants previously described in the USA, specifically in Pennsylvania, New York State, and California. Geographical proximity and trade patterns could explain these findings. However, it appears that most of these viruses have not established themselves to a significant extent in Canada. Detection of nephropathogenic Pennsylvania strains 1220/98 and Wolg/98 (Ziegler et al., Citation2002) in Ontario from 2000 to 2002 caused considerable concern at the time. As a response, adjustments of Massachusetts-based vaccination protocols were applied in the field and apparently have been successful in preventing these viruses becoming widespread in Ontario. California 99-like viruses, presumably generated by recombination between Connecticut, Massachusetts, and Arkansas vaccines (Mondal & Cardona, Citation2007), were also detected in Ontario, but only in 2001. Based on field experiences in large layer establishments, these viruses were successfully controlled by introducing additional live boosters in Massachusetts strain-based vaccination programmes (unpublished data). CU_82792-like isolates, classified as IBV Delaware 072, were first recovered from sentinel chickens placed with layer flocks in New York State (Mondal et al., Citation2001). The last time CU_82792 was detected in Canada was in 2009. Since March 2012, another California variant, 1734/04 (Jackwood et al., Citation2007), has been detected in Ontario. Massachusetts strain-based vaccination also appears effective in controlling the California 1734/04 variant (Wood et al., Citation2009).
There was a marked difference in the proportion of vaccine-like viruses detected between samples from clinical cases and those collected at slaughter. Almost two-thirds of IBVs genotyped from samples collected at slaughter were vaccine-like, compared with less than one-fifth of those from clinical cases. Only Massachusetts-based and Connecticut-based live IBV vaccines are licensed for use in Canada. Thus, the higher proportion of vaccine-like IBVs in slaughter samples was not surprising. However, it was somewhat unexpected to detect a higher proportion of Connecticut strains (45.9%) among the slaughter samples compared with Massachusetts strains (18.9%), because all 1-day-old broilers in Ontario receive Massachusetts-type vaccines. When field vaccination is practiced, Connecticut strains are typically used and they may persist longer, until slaughter. Because the clinical significance of Connecticut IBVs has been low (i.e. they have rarely been associated with disease problems to date), the probable explanation for the higher proportion of Connecticut strains at slaughter is field vaccination.
Since early 2012, increased numbers of IBV-associated cases in chicken flocks of various commodities have been reported in Ontario. Clinical findings included acute respiratory disease and increased mortality, urate nephrosis and, less often, egg production issues. Genotyping showed that 43.2% of IBVs from these cases were related to vaccine-like, Canadian variant, or US variant-like viruses (nine cases had the best match to Massachusetts, one case to Pennsylvania Wolg/98, four cases with urate nephrosis to California 1734/04, and four respiratory cases were associated with Qu_mv) (). Surprisingly, viruses from 21 cases with predominantly respiratory disease and/or egg production problems, representing 56.8% of genotyped samples in 2012 and 2013, were related to IBV strain 4/91. Although this strain was first detected in Europe more than 20 years ago (Gough et al., Citation1992), and later detected in Asia and South America (Cook et al., Citation1996), it has not been reported in Canada. Based on the genotyping results of banked samples collected as part of the Ontario broiler surveillance project, IBV strain 4/91 was first present in Ontario as early as June 2011. Although most other variant viruses are presumably of USA origin, detection of strain 4/91 has not yet been reported in the USA; therefore, the more likely source of this virus was Europe or Asia where strain 4/91 is endemic. The initial introduction appears to have occurred from the same source, since seven S1 sequences from 4/91 viruses detected in 2011 were 99.2 to 100% identical amongst themselves and 99.2 to 99.6% identical to the IBV 4/91 vaccine strain (GenBank accession number: KF377577). Based on partial sequencing of the S1 gene it appears that IBV strain 4/91 can change rapidly or that multiple introductions have occurred. In 2012 the identity to the IBV 4/91 vaccine was 97.8 to 98.6%, and in 2013 the identity was 94.3 to 98.8%.
Field reports and experimental studies in Ontario have shown that Massachusetts-type vaccines can provide solid cross-protection against various IBV variants (Grgic et al., Citation2009). This can be attributed to cross-reactivity involving cytotoxic T lymphocytes (Collisson et al., Citation2000). However, the 4/91 challenge cannot be controlled by Massachusetts-type vaccines alone, but effective protection can be provided by the combination of Massachusetts-type vaccination of 1-day-old chicks followed by 4/91-type vaccination 2 weeks later (Cook et al., Citation1999). In 2012 and 2013, IBV 4/91 was the most frequently detected IBV strain in Ontario in clinical cases processed at the Animal Health Laboratory and it has not yet been detected in other Canadian provinces. Strain 4/91 now appears endemic in Ontario and use of 4/91 protective vaccines will need to be considered.
Acknowledgements
The broiler surveillance project was financially supported by the Poultry Industry Council, Ontario Ministry of Agriculture, Food and Rural Affairs (OMAFRA)/University of Guelph Partnership, the OMAFRA–University of Guelph Agreement through the Animal Health Strategic Investment fund managed by the Animal Health Laboratory of the University of Guelph, and the Chicken Farmers of Ontario. The authors thank Dr Rachel Ouckama, Dr Michael Eregae, Hind Kasab-Bachi, Eric Nham, Elise Myers, Thelma Martinez, Heather McFarlane, Stephanie Wong, Veronique Gulde, and Chanelle Taylor for their contributions to the surveillance project. The cooperation of the Chicken Farmers of Ontario, broiler farmers, and broiler processing plants is greatly appreciated. Stipend support for Michael Eregae was provided through an OMAFRA Highly Qualified Personnel scholarship. Stipend support for Hind Kasab-Bachi was provided through an Ontario Veterinary College MSc Scholarship and through the Ontario Veterinary College incentive fund for transferring to a PhD programme.
References
- Callison, S.A., Hilt, D.A., Boynton, T.O., Sample, B.F., Robison, R., Swayne, D.E. & Jackwood, M.W. (2006). Development and evaluation of a real-time Taqman RT-PCR assay for the detection of infectious bronchitis virus from infected chickens. Journal of Virological Methods, 138, 60–65. 10.1016/j.jviromet.2006.07.018
- Cavanagh, D. (2007). Coronavirus avian infectious bronchitis virus. Veterinary Research, 38, 281–297. 10.1051/vetres:2006055
- Collisson, E.W., Pei, J., Dzielawa, J. & Seo, S.H. (2000). Cytotoxic T lymphocytes are critical in the control of infectious bronchitis virus in poultry. Developmental and Comparative Immunology, 24, 187–200. 10.1016/S0145-305X(99)00072-5
- Cook, J.K.A., Orbell, S.J., Woods, M.A. & Huggins, M.B. (1996). A survey of the presence of a new infectious bronchitis virus designated 4/91 (793B). The Veterinary Record, 138, 178–180. 10.1136/vr.138.8.178
- Cook, J.K.A., Orbell, S.J., Woods, M.A. & Huggins, M.B. (1999). Breadth of protection of the respiratory tract provided by different live-attenuated infectious bronchitis vaccines against challenge with infectious bronchitis viruses of heterologous serotypes. Avian Pathology, 28, 477–485. 10.1080/03079459994506
- de Wit, J.J., Cook, J.K.A. & van der Heijden, H.M.J.F. (2011). Infectious bronchitis virus variants: a review of the history, current situation and control measures. Avian Pathology, 40, 223–235. 10.1080/03079457.2011.566260
- Eregae, M.E., Dewey, C.E., McEwen, S.A., Ouckama, R., Ojkić, D. & Guerin, M.T. (2014). Flock prevalence of exposure to avian adeno-associated virus, chicken anemia virus, fowl adenovirus, and infectious bursal disease virus among Ontario broiler chicken flocks. Avian Diseases, 58, 71–77.
- Gough, R.E., Randall, C.J., Dagless, M., Alexander, D.J., Cox, W.J. & Pearson, D. (1992). A ‘new’ strain of infectious bronchitis virus infecting domestic fowl in Great Britain. The Veterinary Record, 130, 493–494. 10.1136/vr.130.22.493
- Grgic, H., Hunter, D.B., Hunton, P. & Nagy, E. (2008). Pathogenicity of infectious bronchitis virus isolates from Ontario chickens. Canadian Journal of Veterinary Research, 72, 403–410.
- Grgic, H., Hunter, D.B., Hunton, P. & Nagy, E. (2009). Vaccine efficacy against Ontario isolates of infectious bronchitis virus. Canadian Journal of Veterinary Research, 73, 212–216.
- Jackwood, M.W., Hilt, D.A., Lee, C.-W., Kwon, H.M., Callison, S.A., Moore, K.M., Moscoso, H., Sellers, H. & Thayer, S. (2005). Data from 11 years of molecular typing infectious bronchitis virus field isolates. Avian Diseases, 49, 614–618. 10.1637/7389-052905R.1
- Jackwood, M.W., Hilt, D.A., Williams, S.M., Woolcock, P., Cardona, C. & O'Connor, R. (2007). Molecular and serologic characterization, pathogenicity, and protection studies with infectious bronchitis virus field isolates from California. Avian Diseases, 51, 527–533. 10.1637/0005-2086(2007)51[527:MASCPA]2.0.CO;2
- Kingham, B.F., Keeler, C.L., Jr, Nix, W.A., Ladman, B.S. & Gelb, J., Jr. (2000). Identification of avian infectious bronchitis virus by direct automated cycle sequencing of the S-1 gene. Avian Diseases, 44, 325–335. 10.2307/1592547
- Mondal, S.P. & Cardona, C.J. (2007). Genotypic and phenotypic characterization of the California 99 (Cal99) variant of infectious bronchitis virus. Virus Genes, 34, 327–341. 10.1007/s11262-006-0014-7
- Mondal, S.P., Lucio-Martinez, B. & Naqi, S.A. (2001). Isolation and characterization of a novel antigenic subtype of infectious bronchitis virus serotype DE072. Avian Diseases, 45, 1054–1059. 10.2307/1592888
- Moore, K.M., Jackwood, M.W. & Hilt, D.A. (1997). Identification of amino acids involved in a serotype and neutralization specific epitope within the S1 subunit of avian infectious bronchitis virus. Archives of Virology, 142, 2249–2256. 10.1007/s007050050239
- Ojkic, D. & Binnington, B. (2002). Phylogenetic analysis of Ontario infectious bronchitis virus isolates. In D.D. Frame (Ed.). Proceedings of 51st Western Poultry Disease Conference (p. 73). Sacramento, California.
- Pantin-Jackwood, M.J., Brown, T.P. & Huff, G.R. (2005). Reproduction of proventriculitis in commercial and specific-pathogen-free broiler chickens. Avian Diseases, 49, 352–360. 10.1637/7326-011305R.1
- Raj, G.D. & Jones, R.C. (1997). Infectious bronchitis virus: immunopathogenesis of infection in the chicken. Avian Pathology, 26, 677–706. 10.1080/03079459708419246
- Smati, R., Silim, A., Guertin, C., Henrichon, M., Marandi, M., Arella, M. & Merzouki, A. (2002). Molecular characterization of three new avian infectious bronchitis virus (IBV) strains isolated in Québec. Virus Genes, 25, 85–93. 10.1023/A:1020178326531
- Wood, M.K., Ladman, B.S., Preskenis, L.A., Pope, C.R., Bautista, D.A. & Gelb, J., Jr. (2009). Massachusetts live vaccination protects against a novel infectious bronchitis virus S1 genotype DMV/5642/06. Avian Diseases, 53, 119–123. 10.1637/8454-082108-ResNote.1
- Ziegler, A.F., Ladman, B.S., Dunn, P.A., Schneider, A., Davison, S., Miller, P.G., Lu, H., Weinstock, D., Salem, M., Eckroade, R.J. & Gelb, J., Jr. (2002). Nephropathogenic infectious bronchitis in Pennsylvania chickens 1997–2000. Avian Diseases, 46, 847–858. 10.1637/0005-2086(2002)046[0847:NIBIPC]2.0.CO;2
