Abstract
Mycotoxins are unavoidable contaminants of animal and human feed and food respectively. This study was designed to investigate the protective activity of vitamin E (Vit E) in White Leghorn breeder hens and their progeny against aflatoxin B1 (AFB1)-induced damage. The results indicated a significant decrease in egg production and quality in the groups exposed to dietary AFB1. A detectable amount of AFB1 residue appeared in the eggs during the first week of mycotoxin exposure at levels ≥ 2.5 mg kg−1, which reached its peak (0.403 ± 0.04 ng/g [mean ± standard deviation]) during the second week of the experiment (in the group fed 10 mg kg−1). Feeding Vit E + AFB1 resulted in higher AFB1 residues (0.467 ± 0.03) when compared with the hens fed AFB1 alone. The resistance of red blood cells to oxidative damage was decreased, while embryonic mortalities and deformities were increased in the AFB1-fed groups. The protective effect of Vit E on these parameters was noted in the groups fed lower doses of AFB1. After the withdrawal of mycotoxin-contaminated feed, most of the parameters returned towards normal within 2 weeks, except AFB1 residues that were still detectable. From the findings of this study one can conclude that the addition of Vit E in the diet of hens provided only partial protection against AFB1-induced damage.
Introduction
Mycotoxins, the secondary metabolites of some toxigenic fungi, are major contaminants of animal feed. Aflatoxins (AF) produced by Aspergillus flavus and Aspergillus parasiticus are reported in poultry feeds and its raw materials practically worldwide (Binder et al., Citation2007; Ahmad et al., Citation2012; Saleemi et al., Citation2012; Streit et al., Citation2012; Zaki et al., Citation2012). Among the various types of AF, aflatoxin B1 (AFB1) is an established hepatocarcinogen that causes liver lesions in both mammals and birds (Wogan, Citation1973), and has been placed in category A of human carcinogens by the International Agency for Research on Cancer (IARC). Losses associated with the consumption of aflatoxin-contaminated feedstuff in the poultry industry include growth retardation, decreased feed intake, increased mortality, decreased egg production, and carcass condemnation (Agag, Citation2004; Hussain et al., Citation2008; Khan et al., Citation2010).
Apart from the direct losses there is concern about the transfer of AFB1 to eggs as a result of dietary intake by hens (Micco et al., Citation1988; Hassan et al., Citation2012a) as well as its effect on the hatching egg and progeny chicks.
The finding that feeding mycotoxin-contaminated diets adversely affected the immune system of developing embryos/chicks (Todd & Bloom, Citation1980; Potchinsky & Bloom, Citation1993) was supported by the findings of Qureshi et al. (Citation1998). In that particular study, feeding 1 and 5 mg AFB1 kg−1 to broiler breeder hens resulted in a severe depression in antibody titres of the progeny chicks against sheep red blood cells (RBC) and Brucella abortus antigen. Recently, Hassan et al. (Citation2012a, Citationb) monitored the carry-over of AFB1 and ochratoxin A to eggs, and the immunological status of the progeny chicks obtained from the breeder hens kept on mycotoxin-contaminated feed. Significant damage to the DNA in B lymphocytes and T lymphocytes in 18-day-old embryos has also been observed as a result of hens' exposure to AF (Potchinsky & Bloom, Citation1993).
As AF are a potent toxin and of great importance, many studies have sought out natural/man-made compounds that could be employed to mitigate the toxic effects. Among these, vitamin E (Vit E) was reported to ameliorate the toxicity induced by AFB1 both in mammals and birds (Choi et al., Citation1995; Verma & Nair, Citation2001; Mubarak et al., Citation2009). A “carry-over” of Vit E to eggs from hens has also been reported (Meluzzi et al., Citation2000; Mori et al., Citation2003). Therefore, it would not be unreasonable to suspect that chicks from hens maintained on a feed containing both AFB1 and Vit E might be protected from the harmful effects of the mycotoxin. Accordingly, the objectives of the present study were to determine whether, in fact, there was any protective effect afforded to the progeny of hens fed AFB1 by the co-consumption of dietary Vit E.
Materials and Methods
Aflatoxin and feed
AFB1 was produced by A. flavus (NRRL 6540; CECT 2687) using the method described by Shotwell et al. (Citation1966) on rice. Briefly, colonies of A. flavus were grown on potato-dextrose agar slants that were incubated at 28°C for 7 days. On day 7, 3 ml sterile water containing 0.005% Triton X-100 was added on top of each slant to prepare the inoculum. For the production of AFB1, 100 g rice was soaked in 50 ml distilled water in a 1-l Ehrlen-Meyer flask and held at room temperature (with frequent shaking) for 2 h. The flask was then autoclaved at 121°C for 15 min. After the flask cooled, the inoculum was introduced and the flask was placed on an orbital shaker (200 r.p.m.) and incubated at 28°C. After 6 days, the flask was autoclaved for 3 min to destroy mycelial growth and the AFB1 content was confirmed by high-performance liquid chromatography (HPLC) (AOAC, Citation2000).
Basal feed (16% total protein and 2900 kcal kg−1 metabolizable energy) was prepared without the addition of a toxin binder, vitamins, minerals, and antibiotics. Prior to use, each batch of the basal feed was analysed for ochratoxin, aflatoxin, and zearalenone to ensure that the levels of each were <1.0 µg kg−1. Mycotoxin-contaminated feed was prepared by the incorporation of known quantities of AFB1. For this purpose, fermented rice was extracted by soaking in a three-fold quantity of chloroform (1:3) overnight and then filtered through cotton cloth. All of the chloroform was then evaporated and the concentrated residues re-suspended into polyethylene glycol. This suspension was then evenly mixed in the required quantity of basal feed to prepare the experimental feeds containing the desired concentration of AFB1. Prior to being used for feeding, the AFB1 concentration in each experimental diet was verified by HPLC.
Induction of aflatoxicosis in White Leghorn breeder hens
All of the bird experiments were conducted according to the rules and regulations of the Animal Ethics Committee, Faculty of Veterinary Science, at the University of Agriculture, Faisalabad, Pakistan. A total of 192 laying hens, free from Salmonella and Mycoplasma, were procured from a layer farm. The birds were acclimatized for 1 week on basal layer feed having AFB1 levels < 1.0 µg kg−1. The experimental birds were then divided into 12 groups (A to L, n = 16), kept in layer battery cages, and offered feed contaminated with different doses of AFB1 alone and in combination with Vit E for a period of 3 weeks. Group A was kept on basal feed and served as the control, while Group B was offered feed supplemented with Vit E (0.1 mg kg−1). Groups C to G were offered feed containing 0.1, 0.5, 2.5, 5.0, or 10.0 µg AFB1 kg−1, respectively, whereas Groups H to L were offered the same dietary levels of AFB1 along with additional supplementation of Vit E (0.1 mg kg−1). After 3 weeks, all the birds were kept on the basal diet for another 2 weeks.
Forty healthy mature male White Leghorn breeders were maintained for semen collection. Semen was collected by the abdominal massage method and mixed (1:1) with semen extender (Ovodyl Poultry Semen Extender; IMV, L'Aigle, France). The laying hens were then inseminated on alternate days using an artificial insemination gun (IMV) and straws (IMV) starting at day 7 before initiation of the exposures to the test diets. Hatching eggs were then collected on a daily basis and set for incubation on a weekly basis. Prior to setting, eggs were stored at 15°C.
Egg production and shell thickness
The egg production of the hens in the different groups was recorded on a daily basis and the shell thickness was recorded in millimetres with the help of a pressure-sensitive micrometre screw gauge. The shell membrane was removed manually and measurements were taken at four different areas of the shell. The final reading was calculated by taking the mean of all four measurements.
Residue of aflatoxin B1 in eggs
The residue of AFB1 in the eggs was determined on a weekly basis. Egg samples from each group were thoroughly mixed and prepared for residue analysis by a method validated in our laboratory (Hassan et al., Citation2012a, Citationb).
Immunoaffinity column clean-up
Extracted samples were passed through immunoaffinity clean-up columns (AflaTestWB; VICAM, Watertown, Massachusetts, USA) at a rate of 1 or 2 drops sec−1 under gentle pressure using a vacuum clean-up assembly. After washing the column with 10 ml water-methanol (90:10, v/v), the column was dried under nitrogen gas for 5 min, and the toxins were eluted from the column by passing 2.0 ml methanol.
High-performance liquid chromatography
For determination of the mycotoxin content, 20 µl sample was loaded into the HPLC system (Prominence™; Shimadzu, Tokyo, Japan) coupled with spectrofluorometric detector RF-10AXL® (Shimadzu). Acetonitrile, methanol and double-distilled deionized water were used as the mobile phase in the ratio 22.5:22.5:55 at 30°C column temperature.
Assessment of red blood cell resistance to oxidative damage
The assessment of RBC resistance to oxidative stress was performed according to Bertrand et al. (Citation2006). Blood samples were taken from 10 birds from each group to assess the resistance of their red blood cells to the oxidant 2,2′-azobis (2-amidino-propane) hydrochloride (AAPH) (Cayman Chemical Co., Ann Arbor, MI, USA). This assay provided a quantitative measurement of overall antioxidant status of a hen's whole blood as it assessed the time required to haemolyse 50% (HT50) of the red blood cells in the sample when exposed to a controlled free radical attack. Briefly, 20 ml hen whole blood was immediately diluted in 730 ml modified Ringer's solution (pH 7.4). Thereafter, 80 ml of the diluted blood was added to each well of a 96-well micro plate. The micro plate was kept in an incubator at 40°C for 30 min before 136 ml of a 150 mM (646 mg AAPH in 20 ml modified Ringer's solution) AAPH (free radical generator) was added to each well (Rojas et al., Citation1998). The micro plate was maintained at 40°C and then read (Universal Microplate Reader ELX 800; Bio-Tek, Winooski, VT, USA). The rate of haemolysis was determined by the change in optical density at 540 nm.
Incubation of hatching eggs
Eggs from each group were incubated separately on a weekly basis at 38°C and 60% relative humidity in the Park Hatchery, Faisalabad, Pakistan. On day 11 of incubation all of the eggs were candled and those showing dead embryos were broken to study the stage of death. This mortality was designated as early embryonic mortality. Twenty eggs showing live embryos from each group were opened, weighed, washed with normal saline and observed under stereomicroscope for developmental deformities. All of the embryonic mortalities occurring during days 11 to 21 of incubation were designated as late deaths. Hatchability (percentage) on the basis of fertile eggs set in the different groups was calculated at the end of the incubation period.
Statistical analysis
All data were subjected to an analysis of variance test. The means of the different groups were compared using Duncan's multiple-range test within the MSTATC statistical package (Department of Crop and Soil Sciences, Michigan State University, East Lansing, MI, USA). Data were considered significantly different at P ≤ 0.05.
Results
Egg production
Egg production (percentage) of the hens fed different levels of AFB1 alone or together with Vit E is presented in . During week 1, the mean value of the egg production of hens in Group G (fed AFB1 at 10 mg kg−1 alone) and Group L (fed AFB1 at 10 mg kg−1 along with Vit E) was significantly lower than the hens in the control group, while the egg production in all the other groups was not significantly affected. During week 2 of the experiment, a significant decrease in the egg production was also noted at lower doses (i.e. 2.5 and 5.0 mg kg−1) when compared with the control. This decrease in egg production was not prevented by the addition of Vit E to the diet. During week 3, a decrease in egg production was also noted at 0.5 mg kg−1 AFB1 in the feed. At higher levels of AFB1, the supplementation of Vit E did not prevent the AFB1-induced decrease in production but a protective effect of Vit E was noted at the lower doses of AFB1.
Table 1. Egg production (%) of breeder hens kept on AFB1 contaminated feed with or without Vit E supplementation (Mean ± SD).
After 1 week of withdrawal of the AF from the feed, the mean weekly egg production levels in Groups D, E, F, G, J, K and L were significantly lower than the controls while the other groups showed no significant difference with the control. In week 5 (2 weeks after the withdrawal of AFB1 from the feed), the egg production of hens in Groups F, G, and L was significantly lower than the control while in the other groups there was no significant change when compared with the control.
Egg shell thickness
During week 1 a non-significant effect on the egg shell thickness was noted among all groups. During week 2, however, significantly lower values were observed in groups offered 5 and 10 mg kg−1 AFB1 alone and in combination with Vit E (i.e. Groups F, G, K and L). (Note: Statistical comparisons among all of the groups can be found in Supplemental Table S1.) During week 3, the shell thickness was significantly lower in the groups given higher doses of AFB1 alone (Groups F and G) when compared with the control group, while in Groups K and L (the same doses of AFB1 but with added Vit E) there was no significant change. After the withdrawal of the experimental diet, differences in shell thickness between the groups were not significant.
Aflatoxin B1 residues in eggs
The residues of AFB1 in the eggs from the hens kept on the experimental diet are presented in . No residues of AFB1 were detected in the eggs from the hens offered 0.1 and 0.5 mg kg−1 mycotoxin in their diet. The dietary concentration of AFB1 at 2.5 and above resulted in residues in the eggs, which were detected from week 1 of the experimental period. A dose-dependent and duration-dependent increasing trend was noted in the level of AFB1 residue in the eggs. In Group L (given 10 mg AFB1 along with Vit E), significantly higher levels of AFB1 residues were noted throughout the experimental period. After cessation of the mycotoxin feeding, the level of AFB1 residue showed a gradual decreasing trend, first in the 2.5 mg kg−1 group and then in the 5.0 mg kg−1 group. However, a detectable amount of AFB1 was observed in the highest dosed group until the end of the second week after the withdrawal of experimental feed.
Table 2. Aflatoxin B1 residues (ng/g) in the eggs obtained from the breeder hens kept on AFB1 contaminated feed with or without Vit E supplementation (Mean ± SD).
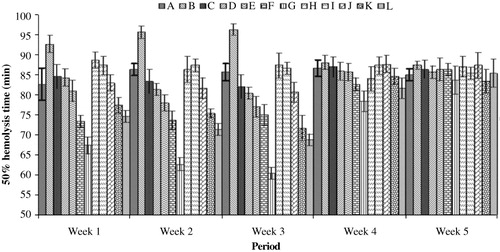
Resistance of red blood cells to oxidative stress
The resistance of RBCs to AAPH-induced oxidative damage in the different groups of breeder hens is shown in . During week 1 a significant decrease in the resistance to oxidative damage, as indicated by the haemolysis time, was noted in the groups of hens fed higher doses of AFB1 (5 and 10 mg kg−1 feed), either alone or in combination with Vit E. However, the dietary supplementation of Vit E had a significantly protective effect as indicated by the increased time for the RBC haemolysis. With the increasing duration of mycotoxin feeding (i.e. during weeks 2 and 3), the effect of low doses of the AFB1 feed (≥0.5 mg kg−1) became apparent with a significant increase in the oxidative damage, when compared with the control group. The protective effect of Vit E supplementation was noted in the groups given low doses (≤2.5 mg kg−1) of AFB1. After the withdrawal from the experimental diet, the hens in Group H (fed a diet with AFB1 at 10 mg kg−1) showed a significantly lower HT50, while all of the other groups showed non-significant difference values from the control group. At the end of the second week of the withdrawal of the mycotoxin-contaminated feed, no significant difference between the groups was noted.
Embryonic weights
The weights of the embryos were determined at day 11 of incubation and are presented in Supplemental Table S2. During the experimental period a significant difference was noted in the weight of the embryos, because the values were higher in the groups fed AFB1-free diets when compared with the mycotoxin-fed groups. After the withdrawal from the mycotoxin-contaminated feed, no significant difference between the groups was noted.
Embryonic mortalities
Early (day 1 to 10 of incubation) and late (day 11 to 21 of incubation) embryonic deaths in the different groups of layer breeder hens fed aflatoxin ± Vit E have been presented in Supplemental Figures S1 and S2 respectively. Embryonic mortalities showed a dose-related increasing trend and were highest (43% early [week 1]; 39% late [week 2]) in Group G (fed 10 mg AFB1 alone). Mortalities in Group B (fed Vit E alone) were even lower than that of the control group. Co-feeding of the two compounds (Vit E + AFB1) partially ameliorated the effect of AFB1, as seen by the lower mortalities in Groups H to L. After the withdrawal of the experimental diet, embryonic losses began to decrease in all of the groups, and during week 5 no significant difference among the groups was noted.
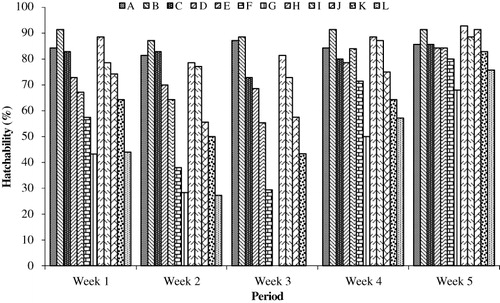
Hatchability of eggs
The hatchability in the different groups was calculated on a weekly basis and is presented in . A significant decrease in hatchability was noted in the groups fed higher doses of AFB1 alone or in combination with Vit E. The lowest values were noted in Group G during week 1 (44%) and week 2 (26%), given the highest dose of mycotoxin (10 mg kg−1). A non-significant ameliorative effect was noted by the addition of Vit E because almost similar values were observed in the two groups (AFB1 ± Vit E). After the withdrawal of the experimental feed, there was an improvement in the hatchability with the high-dose groups showing a slower improvement when compared with the low-dose groups. The effect of AFB1 feeding was noted until the end of the second week after the withdrawal of the mycotoxin-contaminated feed.
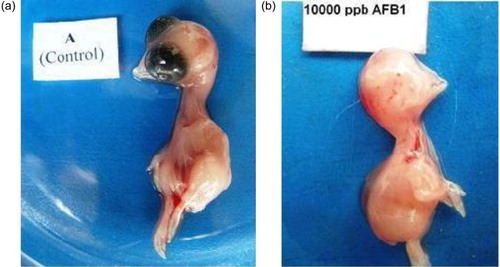
Embryonic deformities
The embryonic deformities and the percentage of defective embryos in the different groups at day 11 of incubation are presented in Supplemental Figures S3 and S4, respectively. No deformity was noted in the embryos from the eggs obtained during week 1 of the AFB1 feeding in the low-dose groups (i.e. Groups A, B, C, H, I and J). During weeks 2 and 3 the embryos from the low-dose groups such as Groups C and J also exhibited deformities in addition to the high-dose groups. One week after the withdrawal of AFB1 feed (week 4), the proportion of deformities decreased and during week 5 deformities were observed only in embryos from the high-dose groups (i.e. Groups F, G and L). The deformities observed were anophthalmia, maxillary retrognathism, mandibular hypoplasia (), and reduced body size. The addition of Vit E to the mycotoxin-contaminated feed provided only partial protection against AFB1-induced damage.
Discussion
The egg production of the hens showed a gradual decrease with increasing duration of AFB1 feeding. During week 1 a significant decrease in the egg production was noted only in Groups G and L, both being offered 10 mg kg−1 AFB1, alone and in combination with Vit E, respectively. During weeks 2 and 3 this decrease in egg production also occurred in the 0.5 mg kg−1 AFB1-fed group. The decrease in the egg production may be due to a decrease in feed intake, impaired metabolism due to hepatic damage (Khan et al., Citation2010; Manegar et al., Citation2010), decreased protein synthesis (Smith et al., Citation1971), impaired nutrient absorption, reduced pancreatic digestive enzyme production (Osborne & Hamilton, Citation1981) or reduced appetite (Sharline et al., Citation1980).
The effect of AFB1 feeding on the egg shell thickness was also noted during the second and third weeks of the experiment. No protective effects of Vit E supplementation were observed during week 2 of the experiment, but during week 3 a significant ameliorative effect was noted. After the cessation of mycotoxin feeding, the shell thickness values in all of the groups again returned to normal. The effect of mycotoxin feeding on the shell quality has previously been reported by several researchers and may be attributed to the impaired absorption of nutrients particularly calcium and phosphorous, and interference with vitamin D3 metabolism (Verma et al., Citation2003).
The introduction of AFB1 into the human food chain from animal-derived products such as meat and eggs is an important health issue. The Food and Agriculture Organization has estimated that up to 25% of the world's food crops and a higher percentage of animal feedstuffs are significantly contaminated by mycotoxins. Jacobson and Wiseman (Citation1974) reported that feeding AF at three levels (i.e. 0.1 parts/106 for 10 days, 0.2 parts/106 for 12 days and 0.4 parts/106 for 15 days) resulted in residues in egg white and yolk on average of 2.2 and 3.6 parts/106, respectively. The presence of AFB1 residue in eggs from hens fed mycotoxin-contaminated diets has been reported by several researchers (Qureshi et al., Citation1998; Pandey & Chauhan, Citation2007; Hassan et al., Citation2012b). In the present study, AFB1 residues were only detected in the eggs sampled from the hens fed contaminated feed at 2.5 mg or higher (with or without Vit E). The residue did appear in the eggs from the pooled samples of week 1 until the withdrawal from the contaminated feed. The deposition of aflatoxin at any stage of egg development and in any component (yolk/white) of the egg has been reported by several researchers (Bintvihok et al., Citation2002) and can be found in the egg the day after first exposure to the aflatoxin-contaminated feed (Trucksess et al., Citation1983). An interesting aspect of the present study was that relatively higher levels of AFB1 residue were observed in the eggs from the hens concurrently exposed to the two test compounds (i.e. Vit E and AFB1) when compared with the groups fed AFB1 alone. These results can possibly be attributed to the fact that feeding Vit E improves metabolism and feed intake of the hens, resulting in increased intake of the toxin. No toxic effects of Vit E feeding were observed in the group given Vit E at 0.1 mg kg−1; instead, an improvement in the production and hatchability was noted by its supplementation.
The oxidative damage induced by AFB1 in the different animal species and cell lines has been well reported by several researchers. Feeding AFB1-contaminated feed at 0.15, 0.3 and 0.6 mg kg−1 to broiler chicks resulted in a significant decrease in glutathione peroxidase, glutathione reductase, catalase activities, total glutathione and increased malondialdehyde concentration in the blood (Chen et al., Citation2013). The increased lipid peroxidation occurring with aflatoxicosis results in the cells being more vulnerable to damage. This was tested in the present study by exposing the RBCs to AAPH (a free radical generator) and monitoring their resistance to lysis. The cells from the hens given higher doses of AFB1 in their diet showed early lysis, as seen by the decreased HT50. The addition of Vit E to the diet resulted in partial but not significant protection. The dose-dependent trend noted in the study showed increased lipid peroxidation with higher doses of AFB1.
The in-ovo exposure of the developing embryo to AFB1 results in significant mortalities, embryonic deformities, and subsequent hatching of immune-suppressed chicks (Cilievici et al., Citation1980; Neldon-Ortiz & Qureshi, Citation1992; Qureshi et al., Citation1998). In the present study higher embryonic mortalities, depressed hatchability, and embryonic deformities were noted in the hatching eggs obtained from the AFB1-fed breeder hens. Mortalities such as these have been reported previously (Nishiyama & Kurebe, Citation1981; Iwaki et al., Citation1990) and were due to the cytotoxic effects induced by AFB1. These in turn can be linked to the depression of DNA, RNA and protein synthesis.
From the findings of the present study one can conclude that feeding AFB1 to breeder hens results in a decreased resistance to oxidative damage in the hen itself and in residues in the hatching eggs. The incubation of such contaminated eggs results in increased embryonic mortality/decreased hatchability and developmental defects in the chicks, and the addition of Vit E to mycotoxin-contaminated feed ensures only partial protection against AFB1-induced damage.
Supplemental data
Supplemental data for this article can be accessed here.
References
- AOAC (Association of Official Analytical Chemist). (2000). Official method of analysis. No. 990.33: Natural Toxins, 17th edn. Gaithersburg, MD: Association of Official Analytical Chemist, pp. 20–22.
- Agag, B.I. (2004). Mycotoxins in foods and feeds: aflatoxins. Association of Universal Bulletin in Environmental Research, 7, 173–205.
- Ahmad, M.F., Saleemi, M.K., Khan, M.Z., Muhammad, F., Hassan, Z.U., Khatoon, A., Bhatti, S.A., Abbas, R.Z., Rizvi, F. & Ahmed, I. (2012). Effects of Ochratoxin A feeding in White Leghorn cockerels on hematological and serum biochemical parameters and its amelioration with silymarin and vitamin E. Pakistan Veterinary Journal, 32, 520–524.
- Bertrand, S., Alonso-Alvarez, C., Devevey, G., Faivre, B., Prost, J. & Sorci, G. (2006). Carotenoids modulate the trade-off between egg production and resistance to oxidative stress in zebra finches. Oecologia, 147, 576–584. 10.1007/s00442-005-0317-8
- Binder, E.M., Tan, L.M., Chin, L.J., Handl, J. & Richard, J. (2007). Worldwide occurrence of mycotoxins in commodities, feeds and feed ingredients. Animal Feed Science and Technology, 137, 265–282. 10.1016/j.anifeedsci.2007.06.005
- Bintvihok, A., Thiengnin, S., Doi, K. & Kumagai, S., (2002). Residues of aflatoxins in the liver, muscle and eggs of domestic fowls. Journal of Veterinary Medical Science, 64, 1037–1039. 10.1292/jvms.64.1037
- Chen, J., Chen, K., Yuan, S., Peng, X., Fang, J., Wang, F., Cui, H., Chen, Z., Yuan, J. & Geng, Y. (2013). Effects of aflatoxin B1 on oxidative stress markers and apoptosis of spleens in broilers. Toxicology and Industrial Health.
- Choi, Y.K., Jung, K.K., Chae, K.Y., Jang, I., Lee, B.D. & Nahm, K.H. (1995). Effects of Vitamin E and selenium supplementation to diets containing aflatoxin B1 on the contents of liver lipids and various blood parameters in rats. Asian-Australian Journal of Animal Sciences, 83, 379–385.
- Cilievici, O., Gordos, I., Ghidus, E. & Moldovan, A. (1980). The toxic and teratogenic effect of aflatoxin B1 on the chick embryo development. Morphology and Embryology, 4, 309–314.
- Hassan, Z.U., Khan, M.Z., Khan, A. & Javed, I. (2012a). Ochratoxicosis in White Leghorn breeder hens: production and breeding performance. Pakistan Veterinary Journal, 32, 557–561.
- Hassan, Z.U., Khan, M.Z., Saleemi, M.K., Khan, A., Javed, I. & Noreen, M. (2012b). Immunological responses of male White Leghorn chicks kept on ochratoxin A (OTA)-contaminated feed. Journal of Immunotoxicology, 9, 56–63. 10.3109/1547691X.2011.627393
- Hussain, Z., Khan, M.Z. & Hassan, Z.U. 2008. Production of aflatoxins from Aspergillus flavus and acute aflatoxicosis in young broiler chicks. Pakistan Journal of Agricultural Sciences, 45, 95–102.
- Iwaki, M., Kitagawa, T., Akamatsu, Y. & Aibara, K. (1990). Cytotoxic effects of aflatoxin B1 and its association with cellular components in chicken embryo primary cultured cells. Biochimica et Biophysica Acta, 1035, 146–153. 10.1016/0304-4165(90)90109-A
- Jacobson, W.C. & Wiseman, H.C. (1974). The transmission of aflatoxin B1 in to eggs. Poultry Science, 53, 1743–1745. 10.3382/ps.0531743
- Khan, W.A., Khan, M.Z., Khan, A. & Hussain, I. (2010). Pathological effects of aflatoxin and their amelioration by Vitamin E in White Leghorn layers. Pakistan Veterinary Journal, 30, 155–162.
- Manegar, G.A., Shambulingappa, B.E. & Ananda, K.J. (2010). Studies on tolerance limit of aflatoxin in commercial broilers. Libyan Agriculture Research Center Journal International, 1, 177–181.
- Meluzzi, A., Sirri, F., Manfreda, G., Tallarico, N. & Franchini, A. (2000). Effects of dietary Vitamin E on the quality of table eggs enriched with n-3 long-chain fatty acids. Poultry Science, 79, 539–545. 10.1093/ps/79.4.539
- Micco, C., Miraglia, M., Benelli, L., Onori, R., Ioppolo, A. & Mantovani, A. (1988). Long term administration of low doses of mycotoxins in poultry. 2. Residues of ochratoxin A and aflatoxins in broilers and laying hens after combined administration of ochratoxin A and aflatoxin B1. Food Additives and Contaminants, 5, 309–314. 10.1080/02652038809373709
- Mori, A.V., Mendonca, Jr C.X., Almeida, C.R. & Pita, M.C. (2003). Supplementing hen diets with Vitamins A and E affects egg yolk retinol and α-tocopherol levels. The Journal of Applied Poultry Research, 12, 106–114. 10.1093/japr/12.2.106
- Mubarak, A., Rashid, A., Khan, I.A. & Hussain, A. (2009). Effect of Vitamin E and selenium as immunomodulators on induced aflatoxicosis in broiler birds. Pakistan Journal of Life and Social Sciences, 7, 31–34.
- Neldon-Ortiz, D.L. & Qureshi, M.A. (1992). Effects of AFB1 embryonic exposure on chicken mononuclear phagocytic cell functions. Developmental and Comparative Immunology, 16, 187–196. 10.1016/0145-305X(92)90018-8
- Nishiyama, S. & Kurebe, M. (1981). Prevention by estradiol of aflatoxin-induced cytotoxicity in cultured chick embryo liver cells. The Journal of Toxicological Sciences, 6, 159–168. 10.2131/jts.6.159
- Osborne, D.J. & Hamilton, P.B. (1981). Decreased pancreatic digestive enzymes during aflatoxicosis. Poultry Science, 60, 1818–1821. 10.3382/ps.0601818
- Pandey, I. & Chauhan, S.S. (2007). Studies on production performance and toxin residues in tissues and eggs of layer chickens fed on diets with various concentrations of aflatoxin AFB1. British Poultry Science, 48, 713–723. 10.1080/00071660701713534
- Potchinsky, M.B. & Bloom, S.E. (1993). Selective aflatoxin B1-induced sister chromatid exchanges and cytotoxicity in differentiating B and T lymphocytes in vivo. Environmental and Molecular Mutagenesis, 21, 87–94. 10.1002/em.2850210112
- Qureshi, M.A., Brake, J., Hamilton, P.B., Hagler, Jr., W.M. & Nesheim, S. (1998). Dietary exposure of broiler breeders to aflatoxin results in immune dysfunction in progeny chicks. Poultry Science, 77, 812–819. 10.1093/ps/77.6.812
- Rojas, W.R.U., Zeng, L., Madison, S.A., De Pinto, R.L. & Shay, B.J. (1998). Mechanistic studies on the decomposition of water soluble azo-radical-initiators. Journal of the Chemical Society Perkin Transactions, 2, 2009–2018.
- Saleemi, M.K., Khan, M.Z., Khan, A., Mehmood, M.A., Farooq, M., Hameed, S., Hassan, Z.U., Javed, M.R. & Javed, I. (2012). Molecular identification of black Aspergilli isolated from poultry feeds by sequencing their ITS regions. Pakistan Veterinary Journal, 32, 171–174.
- Sharline, K.S.B., Howarth, B.J. & Wyatt, R.D. (1980). Effect of dietary aflatoxin on reproductive chicks. Poultry Science, 72, 651–657.
- Shotwell, O.L., Hesseltine, C.W., Stubblefield, R.D. & Sorenson, W.G. (1966). Production of aflatoxin on rice. Applied Microbiology, 14, 425–428.
- Smith, J.W., Hill, C.H. & Hamilton, P.B. (1971). The effect of dietary modifications on aflatoxicosis in the broiler chicken. Poultry Science, 50, 768–774. 10.3382/ps.0500768
- Streit, E., Schatzmayr, G., Tassis, P., Tzika, E., Marin, D., Taranu, I., Tabuc, C., Nicolau, A., Aprodu, I., Puel, O. & Oswald, I.P. (2012). Current situation of mycotoxin contamination and co-occurrence in animal feed – focus on Europe. Toxins, 4, 788–809. 10.3390/toxins4100788
- Todd, L.A. & Bloom, S.E. (1980). Differential induction of sister chromatid exchanges by indirect-acting mutagen-carcinogens at early and late stages of embryonic development. Environmental Mutagenesis, 2, 435–445. 10.1002/em.2860020402
- Trucksess, M.W., Stoloff, L., Young, K., Wyatt, R.D. & Miller, B.L. (1983). Aflatoxicol and aflatoxins B1 and M1 in eggs and tissues of laying hens consuming aflatoxin-contaminated feed. Poultry Science, 62, 2176–2182. 10.3382/ps.0622176
- Verma, J., Johri, T.S. & Swain, B.K. (2003). Effect of varying levels of aflatoxin, ochratoxin and their combinations on the performance and egg quality characteristics in laying hens. Asian-Australasian Journal of Animal Sciences, 16, 1015–1019.
- Verma, R.J. & Nair, A. (2001). Ameliorative effect of Vitamin E on aflatoxin-induced lipid peroxidation in the testis of mice. Asian Journal of Andrology, 3, 217–221.
- Wogan, G.N. (1973). Aflatoxin carcinogens. In H. Bush (Ed.). Methods in Cancer Research (pp. 309–345). New York, NY: Academic Press.
- Zaki, M.M., El-Midany, S.A., Shaheen, H.M. & Rizzi, L. (2012). Mycotoxins in animals: occurrence, effects, prevention and management. Journal of Toxicology and Environmental Health Sciences, 4, 13–28. 10.5897/JTEHS11.072