Abstract
Tibial dyschondroplasia (TD) is an avian bone disorder of different aetiologies that may be associated with lameness. The disorder is characterized by focal disruption of endochondral bone formation, with a lack of matrix proteolysis and an accumulation of non-mineralized avascular cartilage. The aim of this study was to determine the expression of extracellular matrix metalloproteinase inducer (EMMPRIN/CD147) in normal, thiram-induced TD lesions and in the process of recovery from TD in broiler chickens. An extracellular matrix (ECM) degrading enzyme, matrix metalloproteinase-9 (MMP-9), was selected to investigate the effects of CD147 in the degradation of ECM. Gene expression was analysed by quantitative real-time polymerase chain reaction and protein levels by immunohistochemistry and western blotting. The birds were divided into three groups: thiram fed; recovery; and controls. Genes encoding CD147 and MMP-9 were down-regulated during the development of the disease, and were up-regulated during recovery. Western blotting also showed lower protein levels of CD147 in TD, which increased during the recovery phase associated with ECM degradation and growth plate repair. The findings of this study suggest that ECM has a crucial role in the occurrence of TD and that CD147 appears to play a pivotal role in matrix proteolysis in the chicken, similar to that in other species.
Introduction
Endochondral ossification occurs in growth plates located at the ends of long bones that contain chondrocytes at different stages. In mammals, the resting cartilage cells in the reserve region start proliferating slowly, and after accelerating their rate of proliferation they divide rapidly along the longitudinal axis of the bone. With characteristic changes in the gene expression profile, the most distal chondrocytes exit the cell cycle and differentiate into hypertrophic cartilage cells (Herzog et al., Citation2011). This pattern is less clear in the avian growth plate but the process of endochondral bone growth is considered to be functionally similar in both (Leach & Lilburn, Citation1992), converting the avascular tissue (cartilage) into the most vascularized area (bone) (Dan et al., Citation2009).
The chondrocytes are embedded in extracellular matrix (ECM), which supports them. Apart from functioning as a reservoir of various growth factors, it also contains ECM-remodelling enzymes and macromolecules. Matrix metalloproteinases (MMPs), a group of ECM-remodelling enzymes, have been reported to be involved in matrix proteolysis during avian endochondral ossification (Velada et al., Citation2011; Shahzad et al., Citation2014).
CD147, also known as ECM metalloproteinase inducer (EMMPRIN), is a highly glycosylated trans-membrane protein of approximately 40 to 60 kDa molecular weight. With multi-extracellular immunoglobulin domains, it is suggested that the protein may be functionally interactive with other proteins like cellular adhesion molecules in patho-physiological conditions, and is involved in cell–ECM interaction that induces the secretion of MMPs, mainly including MMP-1, MMP-2 and MMP-9 (Guo et al., Citation1997; Sun & Hemler, Citation2001; Xu et al., Citation2007). In addition to being an MMP inducer, CD147 has been reported to directly enhance angiogenesis via an MMP-independent mechanism (Xu et al., Citation2013).
Tibial dyschondroplasia (TD) is one of the most prevalent leg disorders in chickens and accounts for approximately 30% of such cases in broiler flocks (Pelicia et al., Citation2012). TD is characterized by the presence of a dull white avascular, non-mineralized cartilage mass that extends into the metaphysis of the proximal tibiotarsus (Leach & Nesheim, Citation1965). For the last few years, the role of MMPs in ECM has been studied extensively in TD occurrence (Orth, Citation1999; Simsa et al., Citation2007; Hasky-Negev et al., Citation2008; Dan et al., Citation2009). Among these, MMP-9 has been identified as one of the first enzymes involved in TD recovery (Dan et al., Citation2009). Because of its angiogenic properties (Mira et al., Citation2004) and its stimulation by CD147, we selected MMP-9 to investigate its role in TD occurrence and recovery, and our results strengthen the link between them. The aim of the present study was to examine the expression of ECM-regulating molecules.
Materials and Methods
Experimental design and induction of tibial dyschondroplasia
This experiment was planned taking into account all of the national legislations concerning the protection of animal welfare and following the strict guidelines and approval of the Institution Animal Care and Use Committee of Huazhong Agricultural University Wuhan, China.
Three hundred 1-day-old broiler chicks were obtained and maintained under the recommended temperature regime and standard hygienic conditions. The chicks were divided initially into two groups: a control group (n = 100), which received a standard normal diet rich in vitamin D; and a thiram group (n = 200), which was kept on the same diet as the control group but with the addition of 50 mg/kg tetramethylthiuram disulphide (thiram). After 7 days, 100 birds from the thiram group were separated, designated as the recovery group and fed with the non-thiram-containing diet. These three groups were raised for 14 days and the number of lame birds in each group was recorded daily.
Thirty birds from each group were euthanized on days 7, 10 and 14. TD was scored according to Pines et al. (Citation2005). From each group, some of the tibiotarsal bones were fixed in 4% paraformaldehyde and some growth plates were dissected out through the contour lines to avoid bone tissue, immediately frozen in liquid nitrogen and stored at −70°C for further analysis.
Immunohistochemistry for CD147
For immunohistochemistry analysis, the tibiotarsal bones were fixed overnight in 4% paraformaldehyde in phosphate-buffered saline at 4°C. The tissue was decalcified in 10% ethylenediamine tetraacetic acid, dehydrated in graded ethanol solutions, cleared in xylene and embedded in paraffin wax. Sections were cut at 4 to 5 µm thickness for preparation of slides. CD147 was detected and localized at the cellular level by immunohistochemistry with CD147 rabbit polyclonal antibodies (Abcam, Hong Kong, China) at a 1:500 dilution at 4oC overnight. After washing with phosphate-buffered saline, the sections were incubated with horseradish peroxidase-conjugated anti-rabbit or anti-goat secondary antibodies. Primary antibodies were omitted for the negative control.
RNA extraction and reverse transcription
Total RNA was extracted from individual cartilage growth plates and was reverse transcribed into cDNA with a reverse transcription kit (Transgen Biotech Company, Beijing, China) according to the manufacturer’s instructions using Oligo (dT)18 at reaction temperatures of 42°C for 30 min and 85°C for 5 min. The amplification of cDNA was performed by real-time polymerase chain reaction (PCR) and detection of the gene of interest was confirmed after visualization of the specific band in an agarose gel.
Quantitative real-time polymerase chain reaction
By using the specific primers based on published Gallus gallus sequences (), cDNA and fluorescent dye SYBR Green I (Takara, Dalian, China), quantitative real-time PCR was performed in quadruplex with the Step One-Plus™ Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) with the following cycling protocol: one cycle at 95°C for 30 sec, and 40 amplification cycles at 95°C for 8 sec, 60°C for 30 sec, and 72°C for 30 sec. Relative gene expression level was normalized according to the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene expression.
Table 1. Primers used for real-time PCR analysis.
Western blot analysis
Frozen growth plates were homogenized in ice-cold buffer (20 mM Tris, pH 8.0) and the homogenate was incubated at 4°C for 2 h. The supernatant was collected after the samples were centrifuged at 11,180 × g for 10 min. The protein concentration was determined using the BCA assay (Pierce, Rockford, Illinois, USA) and the samples were cryo-preserved at −70°C for subsequent use. For sodium dodecyl sulphate-polyacrylamide gel electrophoresis, the protein samples were separated on 12% polyacrylamide gel and transferred to the polyvinylidene fluoride membranes, which were subjected to 5% skimmed milk. After blocking, the membranes were incubated overnight at 4°C with primary anti-CD147 rabbit polyclonal antibodies (1:500 ab-70062; Abcam). Following extensive washing, the membranes were incubated with secondary antibodies (1:5000, horseradish peroxidase-labelled goat anti-rabbit or rabbit anti-goat secondary antibodies) for 1 h at room temperature. Western blotting detection was used to develop blots and the membranes were exposed to X-ray film for the observation of protein bands.
Statistical analysis
The quantitative real-time PCR analysis was performed in quadruplicate and the data are presented as means ± standard error of means. Comparison between mean values of control and treatment groups was carried out using one-way analysis of variance followed by Tukey’s honest test for continuous variables, and the differences were considered statistically significant if *P <0.05 and **P <0.01.
Results
Morphological analysis of TD-affected and recovered chickens and growth plates
The occurrence of TD is considered to be associated with lameness (Lynch et al., Citation1992); we therefore recorded the lameness daily to give an initial assessment of the probable occurrence of TD. No birds in the control or thiram groups were found lame up to day 3, but from day 4 the birds started showing signs of lameness in the thiram-fed group. On day 7, 70% of the birds were affected by lameness, which became severe by day 14 leading to the involvement of 100% of the chickens. In contrast, the removal of thiram from the diet on day 7 resulted in reduction of lameness to 60% by day 10, compared with the group that continued to consume thiram. This recovery continued to progress until day 14 when lameness was observed in 5% of birds compared with the 100% in the thiram-fed group. None of the birds showed lameness in the control group throughout the experiment.
From day 10, considerable differences were observed between the control and thiram groups. In the control group the tibiotarsal bone was ossified with a defined growth plate at the end, whereas the birds in the thiram-fed group had developed a lesion filled with cartilaginous material (). The differences had become more obvious by day 14. In the recovery group (from which the thiram was removed), the lesion started to disappear and the growth plate underwent a recovery process via modelling of the cartilage through resorption and its replacement by bone. On days 10 and 14 most of the area had become calcified and vascularized, leaving behind a small cartilaginous area, showing that the recovery could result in complete healing ().
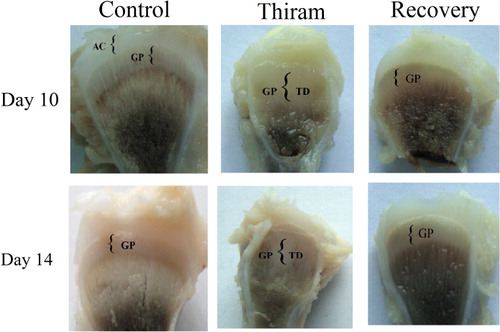
Gene expression and protein levels of CD147/EMMPRIN
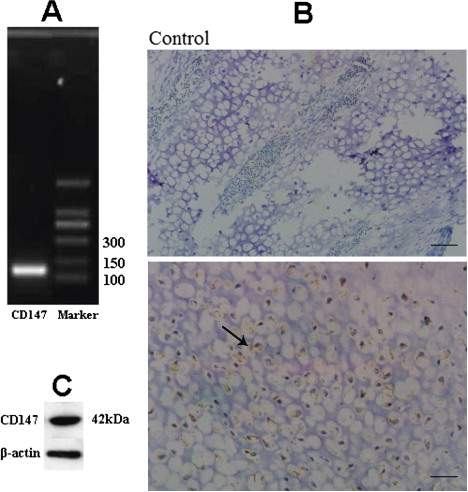
To determine CD147’s role in TD, we were first concerned to detect this gene using specific primers to amplify cDNA from the growth plate of chicken broiler tibiotarsus bone through PCR, taking into account its expected size. Western blotting using specific antibodies revealed the corresponding protein in the growth plate. These findings were later confirmed by immunohistochemistry ().
To find out the potential involvement of CD147 during the course of disease, a quantitative PCR analysis of mRNA level was carried out in the TD-affected birds and those in the recovery group. After 7 days of thiram administration to the broilers, the CD147 gene was found at lower levels compared with the control group and continued to be lower (P <0.01) in the TD-affected birds on day 10, while, on the contrary, the gene started to reappear in the recovery group (P <0.01) on the same day (i.e. 3 days after stopping thiram) compared with the TD-affected birds. Western blotting revealed the presence of the 42 kDa protein of CD147 in the recovery group while there was a reduction in this protein in the TD birds on day 10 ().
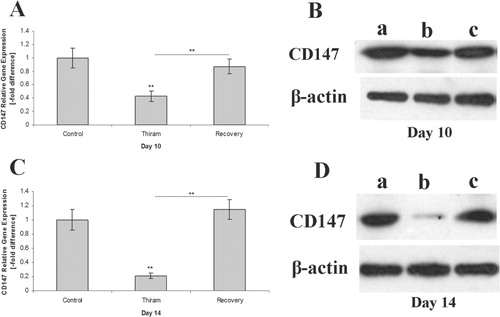
A significant (P <0.01) down-regulation (approximately four-fold) was observed in mRNA expression of CD147 gene in TD lesions compared with the control group on day 14, while it was markedly up-regulated (P <0.01) in recovered birds (approximately 6.5-fold) compared with the TD-affected ones. Western blotting further confirmed these expressions, depicting a significant reduction and increase in protein levels in TD lesions versus recovered tissues respectively ().
Expression of MMP-9, heat shock protein 90 and vascular endothelial growth factor genes
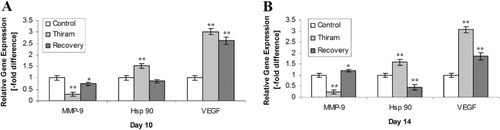
To explain the presence or absence of avascular cartilage in TD-affected and recovered tibiotarsal bones, the expression of the genes encoding vascular endothelial growth factor (VEGF) and heat shock protein 90 (Hsp90) were investigated. Both genes were found significantly up-regulated (P <0.01) in TD lesions and progressively down-regulated (P <0.01) during recovery from TD on days 10 and 14 compared with the control group. The status of ECM in the growth plate was assessed by determining expression of the MMP-9 gene responsible for ECM degradation through quantitative PCR. As compared with the control group, expression was found at a significantly lower level (P<0.01) during the course of disease but started showing expression (P <0.05) during the recovery period on days 10 and 14 ().
Discussion
Although the mechanism is still unclear, many aspects of TD occurrence have been well investigated since its discovery as a spontaneous disease by Leach & Nesheim (Citation1965). This prevalent skeletal abnormality is a leading cause of lameness in poultry raised for meat purposes with consequent large economic losses (Dan et al., Citation2009; Pelicia et al., Citation2012). The method of TD induction for experimental purposes has already been well established (Orth & Cook, Citation1994; Rath et al., Citation2005; Simsa et al., Citation2007) and a variety of protocols have been employed to induce TD, including manipulation of nutritional factors (Rennie et al., Citation1993), cysteine supplement (Bai & Cook, Citation1994) and thiram, a copper chelator compound (Rath et al., Citation2004; Gay et al., Citation2007; Tian et al., Citation2009). In this experiment, a vitamin D-rich diet was offered to the birds to stop the chances of rickets because a vitamin D-deficient diet only has been reported to cause this disease (Ben-Bassat et al., Citation1999; Nield et al., Citation2006).
In our study, TD was induced by thiram and lameness was first seen on the fourth day, progressing within 10 days to affect all of the birds. The natural process of recovery started immediately after thiram administration ceased and a healing process was observed within a week. Although complete recovery was not seen by the end of the experiment, a decreasing pattern in growth plate size and lesion could be observed.
EMMPRIN/CD147 is expressed on a wide variety of cell types and is best known as a potent inducer of MMPs directly in various biological processes (Damsker et al., Citation2009). The correlation of CD147 and MMP-9 has been elaborated in various reports explaining their ECM proteolysis and angiogenic properties (Yangqiong et al., Citation2011; Huang et al., Citation2013). Human articular chondrocytes have shown an extensive expression of CD147 in cartilage (Orazizadeh & Salter, Citation2008) and its importance in reducing arthritis has also been reported in mice (Damsker et al., Citation2009). Since its discovery in brain and kidney cells of the chicken (Seulberger et al., Citation1990), CD147 has not been widely studied in avian species. So, for the first time, we investigated the expression and localization of CD147 in tibiotarsal articular cartilage in the normal broiler chicken and showed its role in TD.
The importance of MMPs for endochondral bone growth is well known (Inada et al., Citation2004; Stickens et al., Citation2004); mice with a null mutation in the MMP-9 enzyme gene exhibited an abnormal pattern of growth plate vascularization and ossification (Vu et al., Citation1998) resembling a phenotype with TD lesions. Among the MMPs, the earliest to be involved in recovery is MMP-9—which fully occupies the disappearing lesion on day 10 (Dan et al., Citation2009), thus explaining its role in angiogenesis in the growth plate (Hasky-Negev et al., Citation2008). Velada et al. (Citation2011) found no evidence for the hypothesis that TD could occur due to increased synthesis of ECM and proved that it was due to decreased matrix proteolysis as a result of the down-regulation of ECM-remodelling enzymes (MMPs). Their study strengthened the link between the lack of MMP expression and abnormal endochondral bone formation. In our study, the samples collected from the TD lesion area showed less mRNA expression and protein activity of CD147 throughout the course of the disease even at the lowest level on day 14 with a possible defect in the release of regulatory molecules (MMPs) into the ECM. On the contrary, during the recovery phase, CD147 expression started elevating along with the increase in protein activity and resulted in the possible induction of MMP-9 that led to the degradation of ECM, propagation of the blood vessels, mineralization of the tissue and abrogation of the lameness. The reduced and elevated amounts of both CD147 and MMP-9 are in parallel in TD-affected and the recovery birds respectively and the mRNA expression levels of the latter are concomitant with previous studies (Simsa et al., Citation2007; Dan et al., Citation2009; Velada et al., Citation2011). This is the first study in which the expression of CD147 protein and mRNA has been evaluated in the occurrence and recovery of TD.
In the present study, the expression of VEGF and Hsp90 was also demonstrated in TD lesions to explain the presence or absence of avascular cartilage and the results are in accordance with previous reports (Orth & Cook, Citation1994; Rath et al., Citation2004, Citation2007; Velada et al., Citation2011). It is well known that hypoxia is one of the principal stimuli for the expression of VEGF and Hsp90. Shapiro et al. (Citation1997) have demonstrated that cartilage cells in chicken growth plate are not hypoxic due to the deep penetration of metaphyseal blood vessels into the growth plate, making it different from the mammalian growth plate (Leach & Gay Citation1987). However, Genin et al. (Citation2008) demonstrated the TD growth plate to be hypoxic, with higher levels of chaperones, and speculated that this might be due to the lack of vascularization. On the other hand, as mentioned earlier, Velada et al. (Citation2011) believed this hypoxic environment to be due to the decreased matrix proteolysis (not increased production of ECM) owing to the induction of VEGF. Our findings suggest that, during the recovery phase, when the stress was over, high expression levels of CD147 might lead to the matrix degradation indirectly, thus restoring the normoxic environment and resulting in angiogenesis. These findings emphasize the role of CD147 in conjunction with MMP-9 in the recovery process according to their recognized functions.
In conclusion, the present study has identified, for the first time, the expression and role of CD147 in tibiotarsal growth plate. This gene appears to play the same role in the avian growth plate as it does in mammalian growth plates. It is likely that a molecule with high and low expression in an experiment may have some important functions, and it appears that the previously described gene decreases in TD lesions and then reappears during the recovery process and shows its activity by orchestrating the growth plate and ultimately playing its role in mitigating the lameness. We demonstrate that, as in mammalian cells, CD147 has the same role in avian chondrocytes by stimulating the production and activation of MMP-9 (and possibly other MMPs) and promoting their involvement in tissue remodelling. These data strengthen the link between the CD147 expressions and their inducers, which lead to the pivotal findings for further research on bone growth.
Acknowledgements
The authors are very thankful to Professor Mark Pines, Institute of Animal Science, Agricultural Research Organization, The Volcani Center, Israel for his kind comments and valuable advice to improve this manuscript.
Additional information
Funding
References
- Bai, Y. & Cook, M.E. (1994). Histological study of tibial dyschondroplasia-like lesion from light-type chicks fed cysteine-supplemented diets. Avian Diseases, 38, 557–562. 10.2307/1592079
- Ben-Bassat, S., Genina, O., Lavelin, I., Leach, R.M. & Pines, M. (1999). Parathyroid receptor gene expression by epiphyseal growth plates in rickets and tibial dyschondroplasia. Molecular and Cellular Endocrinology, 149, 185–195. 10.1016/S0303-7207(98)00231-7
- Damsker, J.M., Okwumabua, I., Pushkarsky, T., Arora, K., Bukrinsky M.I. & Constant, S.L. (2009). Targeting the chemotactic function of CD147 reduces collagen induced arthritis. Journal of Immunology, 126, 55–62. 10.1111/j.1365-2567.2008.02877.x
- Dan, H., Simsa-Maziel, S., Hisdai, A., Sela-Donenfeld, D. & Ornan, M. (2009). Expression of matrix metalloproteinases during impairment and recovery of the avian growth plate. Journal of Animal Science, 87, 3544–3555. 10.2527/jas.2009-2068
- Gay, C.V., Gilman, V.R. & Leach, R.M. Jr. (2007). Immunolocalization of vascularization factors in normal, tibial dyschondroplasia and rachitic cartilage. Avian Pathology, 36, 445–451. 10.1080/03079450701591387
- Genin, O., Hasdai, A., Shinder, D. & Pines, M. (2008). Hypoxia, hypoxia-inducible factor-1 alpha (HIF-1a), and heat-shock proteins in tibial dyschondroplasia. Poultry Science, 87, 1556–1564. 10.3382/ps.2008-00124
- Guo, H., Zucker, S., Gordon, M.K., Toole, B.P. & Biswas, C. (1997). Stimulation of matrix metalloproteinase production by recombinant extracellular matrix metalloproteinase inducer from transfected Chinese hamster ovary cells. Journal of Biological Chemistry, 272, 24–27. 10.1074/jbc.272.1.24
- Hasky-Negev, M., Simsa, S., Tong, A., Genina, O. & Ornan, E.M. (2008). Expression of matrix metalloproteinases during vascularization and ossification of normal and impaired avian growth plate. Journal of Animal Science, 86, 1306–1315. 10.2527/jas.2007-0738
- Herzog, A., Genin, O., Hasdai, A., Shinder, D. & Pines, M. (2011). Hsp90 and angiogenesis in bone disorders--lessons from the avian growth plate. American Journal of Physiology-Regulatory Integrative and Comparative Physiology, 301, R140–R147. 10.1152/ajpregu.00134.2011
- Huang, H.J., Baozhao, X., Qiuxia, L., Xujing, X., Shangling, Z., Mingxia, W., Weixiang, P. & Jieruo, G. (2013). Infliximab reduces CD147, MMP-3, and MMP-9 expression in peripheral blood monocytes in patients with active rheumatoid arthritis. European Journal of Pharmacology, 698, 429–434.
- Inada, M., Wang, Y., Byrne, M.H., Rahman, M.U., Miyaura, C., Lopez-Otin, C. & Krane, S.M. (2004). Critical roles for collagenase-3 (MMP13) in development of growth plate cartilage and in endochondral ossification. Proceedings of the National Academy of Sciences of the USA, 101, 17192–17197. 10.1073/pnas.0407788101
- Leach, R.M. Jr. & Gay, C.V. (1987). Role of epiphyseal cartilage in endochondral bone formation. Journal of Nutrition, 117, 784–790.
- Leach, R.M. Jr. & Lilburn, M.S. (1992). Current knowledge on the etiology of tibial dyschondroplasia in the avian species. Poultry Science, 4, 57–65.
- Leach, R.M. Jr. & Nesheim, M.C. (1965). Nutritional, genetic and morphological studies of an abnormal cartilage formation in young chicks. Journal of Nutrition, 86, 236–244.
- Lynch, M., Thorp, B. & Whitehead, C. (1992). Avian tibial dyschondroplasia as a cause of bone deformity. Avian Pathology, 21, 275–285. 10.1080/03079459208418842
- Mira, E., Lacalle, R.A., Buesa, J.M., González de Buitrago, G., Jiménez-Baranda, S., Gómez-Moutón, C., Martínez-A, C., M.A. & Mañes, S. (2004). Secreted MMP9 promotes angiogenesis more efficiently than constitutive active MMP9 bound to the tumor cell surface. Journal of Cell Science, 117, 1847–1857.
- Nield, L.S., Mahajan, P., Joshi, A. & Kamat, D. (2006). Rickets: not a disease of the past. American Family Physician, 74, 619–626.
- Orazizadeh, M. & Salter, D.M. (2008). CD147 (extracellular matrix metalloproteinase inducer-EMMPRIN) expression by human articular chondrocytes. Iranian Biomedical Journal, 12, 153–158.
- Orth, M.W. (1999). The regulation of growth plate cartilage turnover. Journal of Animal Science, 77, 183–189.
- Orth, M.W. & Cook, M.E. (1994). Avian tibial dyschondroplasia: a morphological and biochemical review of the growth plate lesion and its causes. Veterinary Pathology, 31, 403–414. 10.1177/030098589403100401
- Pelicia, K., Aparecido, I.M. Jr., Garcia, E.A., Molino, A.B., Santos, G.C., Berto, D.A., Vieira-Filho, J.A., Murakami, E.S.M., Montenegro, A.T. & Silva, A.M. (2012). Evaluation of a radiographic method to detect tibial dyschondroplasia lesions in broilers. Brazilian Journal of Poultry Science, 14, 129–135.
- Pines, M., Hasdai, A. & Monsonego-Ornan, E. (2005). Tibial dyschondroplasia – tools, new insights and future prospects. World’s Poultry Science Journal, 61, 287–299.
- Rath, N.C., Huff, W.E., Balog, J.M. & Huff, G.R. (2004). Comparative efficacy of different dithiocarbamates to induce tibial dyschondroplasia in poultry. Poultry Science, 83, 266–274. 10.1093/ps/83.2.266
- Rath, N.C., Huff, W.E. & Huff, G.R. (2007). Thiram-induced changes in the expression of genes relating to vascularization and tibial dyschondroplasia. Poultry Science, 86, 2390–2395. 10.3382/ps.2007-00219
- Rath, N.C., Richards, M.P., Huff, W.E., Huff, G.R. & Balog, J.M. (2005). Changes in the tibial growth plates of chickens with thiram-induced dyschondroplasia. Journal of Comparative Pathology, 133, 41–52. 10.1016/j.jcpa.2005.01.005
- Rennie, J.S., Whitehead, C.C. & Thorp, B.H. (1993). The effect of dietary 1,25-dihydroxycholecalciferol in preventing tibial dyschondroplasia in broilers fed on diets imbalanced in calcium and phosphorus. British Journal of Nutrition, 69, 809–816. 10.1079/BJN19930081
- Seulberger, H., Lottspeich, F. & Risau, W. (1990). The inducible blood-brain barrier specific molecule HT7 is a novel immunoglobulin-like cell surface glycoprotein. EMBO Journal, 9, 2151–2158.
- Shahzad, M., Gao, J., Ping, Q., Liu, J., Wang, Z., Zhang, D. & Li, J. (2014). Expression of genes encoding matrilin-3 and cyclin-I during the impairment and recovery of chicken growth plate in tibial dyschondroplasia. Avian Diseases, 58, 468–47310.1637/10781-012614-ResNote.1.
- Shapiro, I.M., Mansfield, K.D., Evans, S.M., Lord, E.M. & Koch, C.J. (1997). Chondrocytes in the endochondral growth cartilage are not hypoxic. American Journal of Physiology, 272, C1134–C1143.
- Simsa, S., Hasdai, A., Dan, H. & Ornan, E.M. (2007). Differential regulation of MMPs and matrix assembly in chicken and turkey growth-plate chondrocytes. American Journal of Physiology-Regulatory Integrative and Comparative Physiology, 292, R2216–R2224. 10.1152/ajpregu.00864.2006
- Stickens, D., Behonick, D.J., Ortega, N., Heyer, B., Hartenstein, B., Yu, Y., Fosang, A.J., Schorpp-Kistner, M., Angel, P. & Werb, Z. (2004). Altered endochondral bone development in matrix metalloproteinase 13-deficient mice. Development, 131, 5883–5895.
- Sun, J. & Hemler, M.E. (2001). Regulation of MMP-1 and MMP-2 production through CD147/extracellular matrix metalloproteinase inducer interactions. Cancer Research, 61, 2276–2281.
- Tian, W.X., Zhang, W.P., Li, J.K., Bi, D.R., Guo, D.Z., Pan, S.Y., Zhang, Y.H. & Qin, P. (2009). Identification of differentially expressed genes in the growth plate of broiler chickens with thiram-induced tibial dyschondroplasia. Avian Pathology, 38, 161–166. 10.1080/03079450902737789
- Velada, I., Capela-Silva, F., Reis, F., Pires, E., Egas, C., Rodrigues-Santos, P. & Barros, M.T. (2011). Expression of genes encoding extracellular matrix macromolecules and metalloproteinases in avian tibial dyschondroplasia. Journal of Comparative Pathology, 145, 174–186. 10.1016/j.jcpa.2010.12.008
- Vu, T.H., Shipley, J.M., Bergers, G., Berger, J.E., Helms, J.A., Hanahan, D., Shapiro, S.D., Senior, R.M. & Werb, Z. (1998). MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell, 93, 411–422. 10.1016/S0092-8674(00)81169-1
- Xu, J., Xu, H.Y., Zhang, Q., Song, F., Jiang, J.L., Yang, X.M., Mi, L., Wen, N., Tian, R., Wang, L., Yao, H., Feng, Q., Zhang, Y., Xing, J.L., Zhu, P. & Chen, Z.N. (2007). HAb18G/CD147 functions in invasion and metastasis of hepatocellular carcinoma. Molecular Cancer Research, 5, 605–614. 10.1158/1541-7786.MCR-06-0286
- Xu, T., Guo, N., Xu, L., Gou, X. & Mi, M. (2013). CD147/EMMPRIN: an effective therapeutic target for hepatocellular carcinoma. Journal of Drug Targeting, 21, 224–231. 10.3109/1061186X.2012.702769
- Yangqiong, O., Weidong, L., Xuejun, L., Zhibin, L. & Min, L. (2011). Sinomenine reduces invasion and migration ability in fibroblast-like synoviocytes cells co-cultured with activated human monocytic THP-1 cells by inhibiting the expression of MMP-2, MMP-9. CD147. Rheumatology International, 31, 1479–1485.
