Abstract
The efficacy of a live Mycoplasma gallisepticum (MG) vaccine candidate (K-strain) was compared to commercially available vaccines in broiler-type chickens (Trial 1) and layer-type chickens (Trial 2). In Trial 1, three-week-old broiler-type chickens were vaccinated via aerosol with K-strain or an F-strain vaccine. The vaccinated chickens and 10 non-vaccinated controls were subsequently challenged with virulent R-strain via aerosol at six weeks post vaccination; both K-strain and F-strain vaccination resulted in significant protection from air sac and tracheal lesions, as well as R-strain colonization (P ≤ 0.05). In Trial 2, commercial layer-type chickens were vaccinated with ts-11 (via eye drop) or K-strain (via aerosol) at 12 weeks of age. At 25 weeks of age these birds were challenged with R-strain via aerosol. The ts-11 and K-strain vaccinated groups both had significantly lower air sac lesion scores and a lower prevalence of ovarian regression after challenge as compared to non-vaccinated chickens (P ≤ 0.05). K-strain vaccination also prevented significant tracheal lesions and R-strain colonization (P ≤ 0.05). K-strain shows great potential as a highly efficacious live MG vaccine in broiler and layer-type chickens for protection of the respiratory and reproductive systems as well as prevention of infection with field strains.
Introduction
Mycoplasma gallisepticum (MG) causes respiratory disease in chickens and turkeys, and reduces the performance of laying flocks worldwide (Carpenter et al., Citation1981; Mohammed et al., Citation1986; Ley, Citation2008). Although biosecurity and maintenance of MG-free replacement stock are ideal for the control of MG, large poultry populations in small geographic areas and multiple-age farms make eradication and control of MG by biosecurity alone difficult and MG vaccines have been used successfully in these situations (Whithear, Citation1996; Kleven, Citation1997, Citation2008a; Levisohn & Kleven, Citation2000). Vaccination has been shown to prevent clinical signs, reduce egg production losses and reduce egg transmission of MG (Carpenter et al., Citation1981; Hildebrand et al., Citation1983; Glisson & Kleven, Citation1984, Citation1985; Yoder & Hopkins, Citation1985; Whithear et al., Citation1990a; Evans et al., Citation1992, Citation2007).
The commercially available options for MG immunizing agents include oil-emulsion bacterins, live vaccines and a recombinant fowl pox vaccine expressing MG antigens. The characteristics of the commercially available MG immunizing agents have been investigated and compared (Glisson & Kleven, Citation1984, Citation1985; Abd-el-Motelib & Kleven, Citation1993; Whithear, Citation1996; Ley et al., Citation1997; Feberwee et al., Citation2006; Kleven, Citation2008a; Ferguson-Noel et al., Citation2012a). Inactivated MG vaccines have been widely used for control of the disease in several countries although the results have been variable (Hildebrand et al., Citation1983; Khan et al., Citation1986; Karaca & Lam, Citation1987); they appear to protect against loss of egg production in layers, but do not prevent infection or provide consistent protection against respiratory disease (Glisson & Kleven, Citation1984, Citation1985; Abd-el-Motelib & Kleven, Citation1993).
Live vaccines currently used worldwide to control MG include F-strain (Adler et al., Citation1960; Luginbuhl et al., Citation1967), 6/85 (Evans & Hafez, Citation1992) and ts-11 (Whithear et al., Citation1990a, b). F-strain vaccine has been used widely and is effective in displacing virulent (field) strains from poultry operations (CitationLevisohn & Kleven, 1981; Kleven et al., Citation1990, Citation1998). F-strain is very immunogenic but mildly virulent in chickens and virulent to turkeys (Rodriguez & Kleven, Citation1980; Lin & Kleven, Citation1982b; Branton et al., Citation1988; Abd-el-Motelib & Kleven, Citation1993); whereas ts-11 or 6/85 may induce a milder respiratory post-vaccination reaction and result in somewhat lower immunity than F-strain (Abd-el-Motelib & Kleven, Citation1993). The ts-11 vaccine has been reported to have minimal or no virulence for chickens and turkeys, and to induce good protection to MG in experimental and field situations (Abd-el-Motelib & Kleven, Citation1993; Whithear, Citation1996; Barbour et al., Citation2000).
Preliminary studies with K-strain have indicated its potential as a safe and efficacious live vaccine in chickens (Ferguson-Noel et al., Citation2012c). The objective of this study was to compare the efficacy of K-strain to commercially available MG vaccines. The efficacy of K-strain and F-strain was investigated in broiler-type chickens (Trial 1), and the protection elicited by K-strain and ts-11 in layer-type chickens was evaluated in Trial 2.
Materials and Methods
MG vaccines and strains
R-strain is a well-characterized virulent MG strain (Rodriguez & Kleven, Citation1980). The ts-11 (Merial Select, Gainesville, GA, USA) and F-strain (Pfizer Animal Health-Global Poultry, Durham, NC, USA) vaccines are commercially available and were transported, stored and administered by eye drop (ts-11) and aerosol (F-strain) according to the manufacturers’ recommendations. The K-strain vaccine (K5831) was developed at the Poultry Diagnostic and Research Center (University of Georgia, Athens, GA, USA). It is a naturally occurring avirulent MG strain; previous studies have indicated the safety and efficacy of this vaccine strain (Raviv et al., Citation2008; Ferguson-Noel et al., Citation2012c). Aerosol MG vaccinations and challenges (Kleven et al., Citation1972) were carried out using a commercial paint sprayer (Preval® Sprayer Division, Precision Valve Corporation, Yonkers, NY, USA). Approximately 1 ml of actively growing culture was sprayed per bird; the titre of each inoculum (in colour changing units (CCU)/ml) was determined as previously described (Rodwell & Whitcomb, Citation1983).
Serology
Sera were analysed for MG antibodies using the serum plate agglutination (SPA) test using commercial antigen (Charles River Laboratories, Wilmington, ME, USA). The haemagglutination-inhibition (HI) test was performed using antigen prepared from the A5969 strain and chicken erythrocytes. The SPA and HI tests were carried out according to procedures described by Kleven (Citation2008b). Commercial enzyme-linked immunosorbent assays (ELISAs) were also performed on the sera (IDEXX, Westbrook, ME). An SPA score ≥1 was considered positive. An HI titre of 1:20 was considered positive. A geometric mean sample/positive ratio of ≥0.5 on the ELISA test was considered positive.
Isolation and identification of mycoplasma
Cotton swabs from tracheas, choanal clefts and air sacs were inoculated in Frey's modified broth and agar and incubated at 37°C. Mycoplasma isolates were identified using direct immunofluorescence (Kleven, Citation2008b).
Random amplified polymorphic DNA (RAPD) analysis
Selected MG isolates were characterized by RAPD analysis. The procedure and primers used were described by Fan et al. (Citation1995). Two isolates per group were typed by this method.
DNA extraction and real-time PCR
The larynges of necropsied birds were collected in 4 ml sterile sterile phosphate buffered saline. Genomic DNA was extracted from 200 µl of the laryngeal wash using extraction was carried out using the MagMAX™-96 Viral RNA Isolation Kit (Applied Biosystems by Life Technologies, Carlsbad, CA, USA) on the MagMAX Express-96 Magnetic Particle Processors (Applied Biosystems by Life Technologies, Carlsbad, CA, USA) according to the manufacturers recommendations, using 50 µl of final elution buffer.
Real-time PCR (qPCR) using a Taqman® probe and primers targeted towards the MGA_0319 gene described by Callison et al. (Citation2006), was performed. Strain differentiating PCR (SD-qPCR) was also carried out after challenge to distinguish between the vaccines and challenge strain (R-strain) as previously described (Raviv et al., Citation2008). The assays are quantitative, using plasmids containing the genome targets as standard DNA controls (Raviv et al., Citation2008; Ferguson-Noel et al., Citation2012b).
Evaluation of lesions
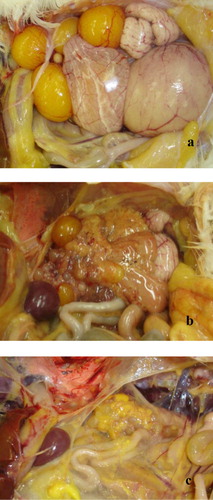
The lesions in chickens necropsied during the study were evaluated grossly by air sac lesions scoring on a scale from 0 to 4 (Kleven et al., Citation1972). Ovarian regression was evaluated by gross examination; ovaries were scored as immature (juvenile), normal (multiple follicles in various stages of development) or regressed (multiple atretic follicles that were flaccid and/or discoloured; see ; Ferguson-Noel et al., Citation2012a). The tracheal lesions were evaluated microscopically by measuring the width of the tracheal mucosa. A section was collected from the upper third of the trachea (approximately 2.5 cm distal from the larynx) and fixed in 10% neutral formalin. The tracheal mucosa thickness was measured at four equidistant points on histological slides of cross sections of tracheae (Whithear, Citation1996). The observer scoring all lesions was blind to the group being evaluated.
Chickens and experimental design
All animal procedures in these experiments were approved by the Institutional Animal Care and Use Committee of the University of Georgia, Athens, GA. In both experiments in this study the bird welfare was adequate and in accordance with the Institutional Animal Care and Use Committee. Chickens were euthanized by cervical dislocation and carbon dioxide and all birds in these studies were provided with feed and water ad libitum.
Trial 1 (Broilers)
Thirty-five 1-day-old commercial broiler-type chickens were acquired from a source known to be free of MG and Mycoplasma synoviae (MS). These chickens were divided into four groups and housed in four colony houses (3 × 3 m2) with pine shaving litter. At two weeks of age, 10 chickens were randomly selected from the houses and tested by serology (SPA, HI, and ELISA), qPCR and culture of choanal clefts to ensure that they were free of MG and MS. At three weeks of age, two of the groups of chickens (10 per group) were vaccinated with either F-strain (5.2 × 107 CCU/ml) or K-strain (5.6 × 107 CCU/ml) via aerosol. At six weeks post vaccination (WPV) the vaccinated chickens and 10 non-vaccinated controls were challenged via aerosol with R-strain (3.9 × 108 CCU/ml). Five chickens served as non-challenged negative controls. At 10 days post challenge (DPC) the chickens were euthanized and evaluated by air sac lesion scoring, measurement of the tracheal mucosa, serology, culture of air sacs and SD-qPCR.
Trial 2 (Layers)
One hundred and ten one-day-old female layer-type chickens (Hy-line, West Des Moines, IA) were acquired from a commercial source known to be free of MG and MS. They were housed in two floor pens (1.5 × 3 m2) with pine shaving litter. Ten birds were randomly selected and screened for Mycoplasma by serology (SPA and HI), tracheal culture and qPCR at 10 weeks of age. At 12 weeks of age, two groups of 30 chickens were moved to two different floor pens (1.5 × 3 m2) with pine shaving litter, and vaccinated with either ts-11 (4.7 × 103 CCU/ml via eye drop) or K-strain (2.4 × 107 CCU/ml via aerosol). Fifty birds were not vaccinated. At 24 weeks of age (12 WPV) twenty birds from each group were bled for serological testing. At 25 weeks of age the vaccinated groups and 30 non-vaccinated controls were challenged with virulent R-strain (1.7 × 109 CCU/ml) via aerosol. The remaining 20 non-challenged birds served as negative controls. The birds were euthanized and evaluated by air sac lesion scoring, ovarian regression, measurement of the tracheal mucosa, serology, culture of air sacs and SD-qPCR at 10 DPC.
Statistical analysis
The prevalence of ovarian regression, air sac lesion scores and MG isolations were analysed using the Kruskal–Wallis Rank Sums test. The mean tracheal mucosa thickness and mean (genome) copy numbers (MCNs) log10 were analysed using the Tukey-Kramer honest significant difference test. These analyses were performed using JMP® Statistics Made Visual (SAS Institute Inc., Cary, NC 27513). A P-value ≤0.05 was considered significant.
Results
Trial 1 (Broilers)
Pre-vaccination
All of the birds tested pre-vaccination in Trial 1 were negative for MG and MS by serological testing as well as culture and qPCR.
Serology
The serological results for this trial are presented in . The chickens (vaccinated and non-vaccinated) that were challenged with R-strain seroconverted on the SPA, HI and ELISA tests whereas the negative controls remained negative on the tests.
Table 1. (Trial 1) Serological response from broiler-type chickens at 10.4 weeks of age (7.4 WPV and 10 DPC with R-strain).
Air sac lesions
As presented in , the mean air sac lesion scores of the vaccinated groups (K-strain and F-strain) were significantly lower than the mean air sac lesion scores of the non-vaccinated challenged controls and not significantly different from the negative controls (P ≤ 0.05).
Table 2. (Trial 1) Air sac lesion scores, tracheal mucosa measurements, MG isolation and strain differentiating qPCR from vaccinated and non-vaccinated broiler-type chickens at 10.4 weeks of age (7.4 WPV and 10 DPC with R-strain).
Tracheal mucosa measurement
The groups vaccinated with K-strain and F-strain had significantly lower mean tracheal mucosa measurements than the non-vaccinated challenged controls. The mean measurements from the vaccinated groups were not significantly different from the negative controls (P ≤ 0.05). These results are shown in .
MG isolation and RAPD analysis
At 10 DPC with R-strain (10.4 weeks of age) MG was isolated from the air sacs of 100% (9/9), 80% (8/10) and 62.5% (5/8) of chickens in the non-vaccinated control, F-strain and K-strain groups, respectively. These results are summarized in . RAPD analysis was attempted to differentiate the isolates from the respective vaccines and R-strain. Two of the isolates from the F-strain group and one from the K-strain group were determined to match their respective vaccine strains. The remaining re-isolates matched R-strain.
Strain differentiating qPCR
At 10.4 weeks of age (7.4 WPV) F-strain was detected in 20% (2/10) of the birds in the F-strain vaccinated group, and K-strain was detected in 62.5% (5/8) of the birds vaccinated with K-strain. There was significantly more K-strain present in the tracheal samples from that group in comparison to F-strain in the F-strain vaccinated group when the MCN (genome) log10 of the vaccinated groups were analysed (P ≤ 0.05). The challenge strain (R-strain) was identified in 20% (2/10) of the birds in the F-strain group and 62.5% (5/8) of birds in the K-strain group. The MCNs log10 of R-strain in the vaccinated groups were significantly lower than the non-vaccinated challenged control (P ≤ 0.05). These results are presented in .
Trial 2 (Layers)
Pre-vaccination
All of the birds tested pre-vaccination were negative for MG and MS by serological testing as well as culture and qPCR.
Serology
At 24 weeks of age (12 WPV) the serological response of the birds vaccinated with K-strain was significantly stronger than that of the ts-11 vaccinated group by both SPA and HI testing (P ≤ 0.05). The chickens (vaccinated and non-vaccinated) that were challenged with R-strain significantly seroconverted on the SPA and HI tests as compared to the negative controls (P ≤ 0.05) at 10 DPC. The serological response in the ts-11 vaccinated group was significantly stronger than that of the K-strain group (P ≤ 0.05). These serological results are summarized in .
Table 3. (Trial 2) Serological response of layer chickens at 12 WPV with TS-11 or K-strain (24 weeks of age) and 10 DPC with R-strain (26.3 weeks of age).
Ovarian regression
A significantly lower prevalence of ovarian regression (follicle atresia) was observed in the groups vaccinated with ts-11 and K-strain compared to the non-vaccinated controls (P ≤ 0.05). The prevalence of ovarian regression in the K-strain vaccinated group was also significantly lower than the ts-11 group and not significantly different from the non-challenged controls (P ≤ 0.05). These results are presented in .
Table 4. Prevalence of ovarian regression (follicle atresia), air sac lesion scores, tracheal mucosa measurements, MG isolation and quantitative SD-qPCR from vaccinated and non-vaccinated layer chickens 10 DPC with R-strain at 25 weeks of age.
Air sac lesions
As presented in , the mean air sac lesion scores of the vaccinated groups (K-strain and ts-11) were significantly lower than the mean air sac lesion scores of the non-vaccinated controls (P ≤ 0.05). The mean score of the K-strain vaccinated group was also significantly lower than the ts-11 group and not significantly different from the non-challenged controls (P ≤ 0.05).
Tracheal mucosa measurement
As shown in , the group vaccinated with K-strain had a significantly lower mean tracheal mucosa measurement than the non-vaccinated controls and the ts-11 vaccinated group (P ≤ 0.05).
MG isolation
MG was isolated from the air sacs of 83% (19/23), 83% (25/30) and 69% (20/29) of the birds in the ts-11, K-strain and non-vaccinated challenged groups, respectively. There were no significant differences in the recovery of MG from the air sacs of the groups challenged with R-strain (P ≤ 0.05), as shown in .
Strain differentiating qPCR
As presented in , 26% (6/23) and 100% (23/23) of the ts-11 vaccinated group were positive by SD-qPCR for ts-11 and R-strain, respectively, at 10 DPC. Eighty-three percent (25/30) and 53% (16/30) of the birds vaccinated with K-strain were positive by SD-qPCR for K-strain and R-strain, respectively. The MCN log10 for ts-11 in the ts-11 group was significantly lower than the MCN log10 for K-strain in the K-strain group (1.2 ± 2.1 vs. 4.4 ± 2.1); also the MCN log10 for R-strain in the ts-11 group was significantly higher than the MCN log10 for R-strain in the K-strain group (7.0 ± 0.7 vs. 3.2 ± 3.1; P ≤ 0.05).
Discussion
MG vaccines are used to prevent or reduce respiratory disease and clinical signs in vaccinated birds and also to prevent egg production losses and egg transmission of MG. An ideal MG vaccine is avirulent, induces long-lived (lifelong) protection, poorly transmissible, affordable, easy to administer and stable. Although each of the currently available vaccines has its advantages, none of them attains the ideal in every respect (Whithear, Citation1996). There is a complex relationship between infectivity, pathogenicity and immunogenicity of MG strains (Levisohn, Citation1984); virulence, invasiveness and immunogenicity of MG strains are directly correlated (Lin & Kleven, Citation1982a). Therefore although the perfect vaccine is both avirulent and highly immunogenic, it may be difficult to balance both characteristics. In earlier studies we investigated the safety, efficacy, transmissibility, colonization and persistence, stability following back passage through chickens, vertical transmission, minimum infectious dose and protective dose of K-strain (Raviv et al., Citation2008; Ferguson-Noel et al., Citation2012c). In these studies, K-strain had a relatively low rate of transmission to direct contacts, and it was shown to colonize and persist in the upper respiratory tract of vaccinated chickens for at least five months. There was no increase in virulence following five back passages through chickens and no vertical transmission was detected. The preliminary studies also established that K-strain is efficacious, and in this study the efficacy of K-strain was directly compared to two well-characterized commercially available live MG vaccines (F-strain and ts-11) in two different types of commercial poultry (broiler and layers).
In both trials, MG vaccinations resulted in significant protection as evidenced by significantly lower air sac lesion scores, tracheal mucosa measurements and reduction of colonization with the challenge strain as compared to the non-vaccinated challenged controls (P ≤ 0.05). There were no significant differences in protection between F-strain and K-strain as measured by these parameters in Trial 1, although K-strain was significantly more protective than ts-11 in Trial 2 (P ≤ 0.05). It must be noted that the titre of the ts-11 vaccine was estimated to be only 4.7 × 103 CCU/ml, whereas the titre of the K-strain vaccine was 5.6 × 107 CCU/ml (Trial 1) and 2.4 × 107 CCU/ml (Trial 2); the titre of the F-strain vaccine in Trial 1 was 5.2 × 107 CCU/ml. The differences in titre may account for the difference in the results among the vaccines, as it has been shown that the titre of a live vaccine may be of paramount importance for efficacy (Whithear, Citation1996; Raviv et al., Citation2008). These titrations were conducted using standard methods, including incubation at 37°C; the ts-11 vaccine is temperature sensitive and grows poorly at this temperature; therefore it is likely that the actual dose of the vaccine was higher than estimated. Subsequent titrations of the same vaccine serial at the permissive temperature (33°C) yielded titres ranging from 3.6 × 106 to 1.4 × 107 CCU/ml. The titres of this lot of vaccine supplied by the manufacturer were 7.9 and 8.0 CCU/dose (approximately 108.3 and 108.4 CCU/ml). However, we cannot conclusively state that the vaccination with ts-11 was with a dose high enough to allow direct comparison to K-strain. The efficacy of ts-11 has been established in previous studies (Whithear et al., Citation1990a; Abd-el-Motelib & Kleven, Citation1993; Noormohammadi et al., Citation2002).
Both K-strain and ts-11 vaccination elicited a significantly lower prevalence of ovarian regression in laying chickens after challenge in Trial 2 (P < 0.05). As a substantial percentage of MG vaccinations worldwide are administered to long-lived poultry to protect against egg production drops as well as respiratory disease, the capacity of vaccines to protect the reproductive tract is an important characteristic for consideration. Although the pathogenesis of egg production drops in MG-infected layers has not been completely defined, it is likely due to more than one mechanism. Egg production typically declines with initial infection, then recovers and is maintained at a lowered level (Mohammed et al., Citation1987). Ovarian regression with follicular atresia has been described (Nunoya et al., Citation1997) and the prevalence of ovarian regression has been shown to be useful indicator of the ability of MG vaccines to protect against egg production drops (Ferguson-Noel et al., Citation2012a).
There were significantly lower levels of R-strain colonization in birds vaccinated with K-strain in both trials and F-strain in Trial 1. This can be regarded as a measure of the ability of these vaccines to prevent or reduce colonization with a field challenge and indicates their usefulness as part of a programme to displace and eradicate field strains in endemically infected complexes and regions (Levisohn & Kleven, Citation1981; Kleven et al., Citation1990, Citation1998).
It can be concluded from these studies that K-strain is a vaccine strain with, at minimum, equivalent efficacy to two commercially available live MG vaccines, and has the potential to protect vaccinated birds from respiratory and reproductive lesions, as well as colonization with field strains.
Acknowledgements
We gratefully acknowledge the assistance of Mrs Victoria Laibinis and Ms Ruth Spooner Wooten.
References
- Abd-el-Motelib, T.Y. & Kleven, S.H. (1993). A comparative study of Mycoplasma gallisepticum vaccines in young chickens. Avian Diseases, 37, 981–987. 10.2307/1591903
- Adler, H.E., McMartin, D. & Shifrine, M. (1960). Immunization against Mycoplasma infections of poultry. American Journal of Veterinary Research, 21, 482–485.
- Barbour, E.K., Hamadeh, S.K. & Eidt, A. (2000). Infection and immunity in broiler chicken breeders vaccinated with a temperature-sensitive mutant of Mycoplasma gallisepticum and impact on performance of offspring. Poultry Science, 79, 1730–1735. 10.1093/ps/79.12.1730
- Branton, S.L., Lott, B.D., Deaton, J.W., Hardin, J.M. & Maslin, W.R. (1988). F strain Mycoplasma gallisepticum vaccination of post-production-peak commercial leghorns and its effect on egg and eggshell quality. Avian Diseases, 32, 304–307. 10.2307/1590817
- Callison, S.A., Riblet, S.M., Sun, S., Ikuta, N., Hilt, D., Leiting, V., Kleven, S.H., Suarez, D.L. & García, M. (2006). Development and validation of a real-time Taqman polymerase chain reaction assay for the detection of Mycoplasma gallisepticum in naturally infected birds. Avian Diseases, 50, 537–544. 10.1637/7639-050106R.1
- Carpenter, T.E., Mallinson, E.T., Miller, K.F., Gentry, R.F. & Schwartz, L. D. (1981). Vaccination with F-strain Mycoplasma gallisepticum to reduce production losses in layer chickens. Avian Diseases, 25, 404–409. 10.2307/1589932
- Evans, J.D., Leigh, S.A., Branton, S.L., & Collier, S.D. (2007). Effects of increased dosages of the Mycoplasma gallisepticum vaccine MYCOVAC-L in layer chickens subsequently challenged with virulent M. gallisepticum: egg production and serologic response. Avian Diseases, 51, 912–917. 10.1637/7931-020807-REGR2.1
- Evans, R.D. & Hafez, Y.S. (1992). Evaluation of a Mycoplasma gallisepticum strain exhibiting reduced virulence for prevention and control of poultry mycoplasmosis. Avian Diseases, 36, 197–201. 10.2307/1591490
- Evans, R.D., Hafez, Y.S. & Orthel, F.W. (1992). Mycoplasma gallisepticum vaccination-challenge: an egg-production model. Avian Diseases, 36, 956–963. 10.2307/1591555
- Fan, H.H., Kleven, S.H. & Jackwood, M.W. (1995). Application of polymerase chain reaction with arbitrary primers to strain identification of Mycoplasma gallisepticum. Avian Diseases, 39, 729–735. 10.2307/1592409
- Feberwee, A., von Banniseht-Wysmuller, T., Vernooij, J.C., Gielkens, A.L. & Stegeman, J.A. (2006). The effect of vaccination with a bacterin on the horizontal transmission of Mycoplasma gallisepticum. Avian Pathology, 35, 35–37. 10.1080/03079450500465700
- Ferguson-Noel, N., Cookson, K., Laibinis, V.A. & Kleven, S.H. (2012a). The efficacy of three commercial Mycoplasma gallisepticum vaccines in laying hens. Avian Diseases, 56, 272–275. 10.1637/9952-092711-Reg.1
- Ferguson-Noel, N.M., Laibinis, V.A. & Farrar, M. (2012b). Influence of swab material on the detection of Mycoplasma gallisepticum and M. synoviae by real-time PCR. Avian Diseases, 56, 310–314. 10.1637/9972-102411-Reg.1
- Ferguson-Noel, N.M., Laibinis, V.A. & Kleven, S.H. (2012c). Evaluation of Mycoplasma gallisepticum K-strain as a live vaccine in chickens. Avian Diseases, 56, 44–50. 10.1637/9833-061411-Reg.1
- Glisson, J.R. & Kleven, S.H. (1984). Mycoplasma gallisepticum vaccination: effects on egg transmission and egg production. Avian Diseases, 28, 406–415. 10.2307/1590347
- Glisson, J.R. & Kleven, S.H. (1985). Mycoplasma gallisepticum vaccination: further studies on egg transmission and egg production. Avian Diseases, 29, 408–415. 10.2307/1590502
- Hildebrand, D.G., Page, D.E. & Berg, J.R. (1983). Mycoplasma gallisepticum (MG) – laboratory and field studies evaluating the safety and efficacy of an inactivated MG bacterin. Avian Diseases, 27, 792–802. 10.2307/1590323
- Karaca, K. & Lam, K.M. (1987). Efficacy of commercial Mycoplasma gallisepticum bacterin (MG-Bac) in preventing air-sac lesions in chickens. Avian Diseases, 31, 202–203. 10.2307/1590796
- Khan, M.I., McMartin, D.A., Yamamoto, R. & Ortmayer, H.B. (1986). Observations on commercial layers vaccinated with Mycoplasma gallisepticum bacterin on a multiple-age site endemically infected with Mycoplasma gallisepticum. Avian Diseases, 30, 309–312. 10.2307/1590533
- Kleven, S.H. (1997). Changing expectations in the control of Mycoplasma gallisepticum. Acta Veterinaria Hungarica, 45, 299–305.
- Kleven, S.H. (2008a). Control of avian mycoplasma infections in commercial poultry. Avian Diseases, 52, 367–374. 10.1637/8323-041808-Review.1
- Kleven, S.H. (2008b). Mycoplasmosis. In L. Dufour-Zavala, D.E. Swayne, J.R. Glisson, J.E. Pearson, W.M. Reed, M.W. Jackwood, & P.R. Woolcock (Eds.). A Laboratory Manual for the Isolation, Identification and Characterization of Avian Pathogens 5th edn (pp. 59–64). Jacksonville, FL: American Association of Avian Pathologists.
- Kleven, S.H., Fan, H.-H. & Turner, K.S. (1998). Pen trial studies on the use of live vaccines to displace virulent Mycoplasma gallisepticum in chickens. Avian Diseases, 42, 300–306. 10.2307/1592480
- Kleven, S.H., Khan, M.I. & Yamamoto, R. (1990). Fingerprinting of Mycoplasma gallisepticum strains isolated from multiple-age layers vaccinated with live F strain. Avian Diseases, 34, 984–990. 10.2307/1591393
- Kleven, S.H., King, D.D. & Anderson, D.P. (1972). Airsacculitis in broilers from Mycoplasma synoviae: effect on air-sac lesions of vaccinating with infectious bronchitis and Newcastle virus. Avian Diseases, 16, 915–924. 10.2307/1588772
- Levisohn, S. (1984). Early stages in the interaction between Mycoplasma gallisepticum and the chick trachea as related to pathogenicity and immunogenicity. Israel Journal of Medical Science, 20, 982–984.
- Levisohn, S. & Kleven, S.H. (1981). Vaccination of chickens with nonpathogenic Mycoplasma gallisepticum as a means for displacement of pathogenic strains. Israel Journal of Medical Science, 17, 669.
- Levisohn, S. & Kleven, S.H. (2000). Avian mycoplasmosis (Mycoplasma gallisepticum). Revue Scientifique et Technique [International Office of Epizootics], 19, 425–442.
- Ley, D.H. (2008). Mycoplasma gallisepticum infection. In Y.M. Saif, A.M. Fadly, J.R. Glisson, L.R. McDougald, L.K. Nolan, & D.E. Swayne (Eds.). Diseases of Poultry 12th edn (pp. 807–845). Ames: Blackwell Publishing Professional.
- Ley, D.H., McLaren, J.M., Miles, A.M., Barnes, H.J., Miller, S.H. & Franz, G. (1997). Transmissibility of live Mycoplasma gallisepticum vaccine strains ts-11 and 6/85 from vaccinated layer pullets to sentinel poultry. Avian Diseases, 41, 187–194. 10.2307/1592459
- Lin, M.Y. & Kleven, S.H. (1982a). Cross immunity and antigenic relationships among 5 strains of Mycoplasma gallisepticum in young leghorn chickens. Avian Diseases, 26, 496–507. 10.2307/1589895
- Lin, M.Y. & Kleven, S.H. (1982b). Pathogenicity of two strains of Mycoplasma gallisepticum in turkeys. Avian Diseases, 26, 360–364. 10.2307/1590106
- Luginbuhl, R.E., Tourtellotte, M.E. & Frazier, M.N. (1967). Mycoplasma gallisepticum – control by immunization. Annals of the New York Academy of Sciences, 143, 234–238. 10.1111/j.1749-6632.1967.tb27662.x
- Mohammed, H.O., Carpenter, T.E. & Yamamoto, R. (1987). Economic impact of Mycoplasma gallisepticum and Mycoplasma synoviae in commercial layer flocks. Avian Diseases, 31, 477–482. 10.2307/1590727
- Mohammed, H.O., Yamamoto, R., Carpenter, T.E. & Ortmayer, H.B. (1986). Comparison of egg yolk and serum for the detection of Mycoplasma gallisepticum and Mycoplasma synoviae antibodies by ELISA. Avian Diseases, 30, 398–408. 10.2307/1590547
- Noormohammadi, A.H., Jones, J.F., Underwood, G. & Whithear, K.G. (2002). Poor systemic antibody response after vaccination of commercial broiler breeders with Mycoplasma gallisepticum vaccine ts-11 not associated with susceptibility to challenge. Avian Diseases, 46, 623–628. 10.1637/0005-2086(2002)046[0623:PSARAV]2.0.CO;2
- Nunoya, T., Kanai, K., Yagihashi, T., Hoshi, S., Shibuya, K. & Tajima, M. (1997). Natural case of salpingitis apparently caused by Mycoplasma gallisepticum in chickens. Avian Pathology, 26, 391–398. 10.1080/03079459708419221
- Raviv, Z., Callison, S.A., Ferguson-Noel, N. & Kleven, S.H. (2008). Strain differentiating real-time PCR for Mycoplasma gallisepticum live vaccine evaluation studies. Veterinary Microbiology, 129, 179–187. 10.1016/j.vetmic.2007.11.017
- Rodriguez, R. & Kleven, S.H. (1980). Pathogenicity of two strains of Mycoplasma gallisepticum in broiler chickens. Avian Diseases, 24, 800–807. 10.2307/1589957
- Rodwell, A.W. & Whitcomb, R.F. (1983). Methods of direct and indirect measurement of mycoplasma growth. In S. Razin & J.G. Tully (Eds.). Methods in Mycoplasmology. Volume I, Mycoplasma Characterization Vol. I (pp. 85–196). New York, NY: Academic Press.
- Whithear, K.G. (1996). Control of avian mycoplasmoses by vaccination. Revue Scientifique et Technique, 15, 1527–1553.
- Whithear, K.G., Soeripto, Harrigan, K.E. & Ghiocas, E. (1990a). Immunogenicity of a temperature sensitive mutant Mycoplasma gaffisepticum vaccine. Australian Veterinary Journal, 67, 168–174. 10.1111/j.1751-0813.1990.tb07748.x
- Whithear, K.G., Soeripto, Harringan, K.E. & Ghiocas, E. (1990b). Safety of temperature sensitive mutant Mycoplasma gallisepticum vaccine. Australian Veterinary Journal, 67, 159–165. 10.1111/j.1751-0813.1990.tb07745.x
- Yoder, H.W., Jr. & Hopkins, S.R. (1985). Efficacy of experimental inactivated Mycoplasma gallisepticum oil-emulsion bacterin in egg-layer chickens. Avian Diseases, 29, 322–334. 10.2307/1590492