Abstract
The present report describes two outbreaks of serious enteritis in commercial laying hens where Clostridium perfringens and Clostridium colinum were simultaneously detected. At the age of 44 and 31 weeks, two laying hen flocks showed an increase of the mortality rate and a worsening of productive performance. Post-mortem examination revealed intestinal necrotic-haemorrhagic ulcerations and hepatic focal necrosis. The bacteriological examination yielded the isolation of C. colinum and C. perfringens toxin type A, NetB positive. In one outbreak, C. colinum was detected also by polymerase chain reaction in all the intestines of affected birds. In laying hens, C. colinum has never been isolated but only suspected as the causative agent of a slight enteric disease called duodenal focal necrosis. The present case report was characterized by severe enteritis presumably due to the synergistic effect of C. colinum and C. perfringens.
Introduction
Bacterial enteritis in laying hens (Gallus gallus) is primarily caused by anaerobic microorganisms belonging to the genera Clostridium and Brachyspira (Hampson & Swayne, Citation2008). The most frequent among these pathologies is necrotic enteritis (NE), caused by Clostridium perfringens, a Gram-positive sporogenic anaerobic bacillus that acts as a pathogen through the production of diverse types of toxins. C. perfringens is classified into five toxin types (A, B, C, D and E) in relation to the different combinations of major toxins produced. Historically, NE in poultry is associated with toxin types A and C; however, research conducted in several countries has demonstrated the implication of only toxin type A characterized by the production of α-toxin (Songer, Citation1996; Olkowski et al., Citation2008; Drigo et al., Citation2009). In the past, α-toxin produced by all the strains of C. perfringens was thought to be an essential virulence factor for the occurrence of the disease (Al-Shiekhly & Treuescott, Citation1977a,Citationb), but today it is acknowledged that its presence is not necessary for the development of the typical lesions (Keyburn et al., Citation2006). The toxin considered the most significant in the aetiopathogenesis of NE is a “pore-forming protein” named NetB (Keyburn et al., Citation2008). The gross lesions and the severity of the clinical signs can be more serious if the strain involved produces also a toxin belonging to the group “large clostridial cytotoxins” named TpeL (Coursodon et al., Citation2012). NE generally strikes broilers from two to six weeks of age, even though reports of full-blown disease exist in commercial pullets and laying hens from three to nine months of age (Kwatra & Chaudhury, Citation1976; Porter, Citation1998; Barnes et al., Citation2008).
Another important clostridial disease in poultry is ulcerative enteritis (UE), caused by Clostridium colinum, which primarily affects the Virginia quail (Colinus virginianus), in which the pathology often assumes an epidemic nature. C. colinum infection has also been reported in chickens but the disease has not been reproduced in orally inoculated birds (Berkhoff & Campbell, Citation1973; Berkhoff et al., Citation1973). Although C. colinum is considered a primary pathogen for poultry, the combination with other predisposing factors (e.g. coccidiosis, Gumboro disease, infectious anaemia and stress) may worsen the clinical picture (Davis, Citation1973). In laying hens, C. colinum has been suspected as the causative agent of so-called duodenal focal necrosis, although the presence of the pathogen in this disease has never been clearly demonstrated or lesions reproduced (Baltzely et al., Citation2008).
Materials and Methods
Case history
Over the course of 2013, in a Hyline commercial laying hen farm, two outbreaks of disease characterized by sudden increases of mortality were observed. The first episode occurred in June and involved a group of 44,000 birds of 47 weeks of age, while the second occurred in December in a group of 18,300 birds of 31 weeks of age.
Both groups of birds were cage-raised in different sheds of the same farm. The farmer stated that there was a sudden decrease in productive performance with reduction of the egg weight (from 65 to 60 g), decreased food consumption (from 106 to 93 g/head/day) and an average mortality rate of 0.5%. In the 31-week-old group, there were also birds with gastrointestinal signs localized in only one section of the shed. The principal sign was characterized by diarrhoea with the presence of incompletely digested food.
Thirteen bird carcasses (four from group 1 and nine from group 2) were sent to the veterinary diagnostic laboratory of Treviso for diagnostic purposes.
Bacteriological isolation and identification
Liver of one bird and intestinal content of two birds of the first outbreak with macroscopic lesions were sent for bacteriological examination. In addition, bacteriological examination was conducted from the intestinal ulcers of four birds of the second outbreak. The samples aseptically collected from liver and intestine were inoculated in Columbia agar (CA) with 5% of sheep blood (Becton Dickinson, NJ, USA), perfringens agar base (Oxoid, Basingstoke, UK) with 5% of sheep blood (PAB), cooked meat medium (Oxoid) and eosin methylene blue (Oxoid). The CA and eosin methylene blue plates were incubated for 24 hours at 37 ± 1°C in aerobiosis, while PAB, cooked meat medium and a second CA plate were incubated at the same temperature in anaerobiosis for 24–48 hours. The culture media were inspected after 24–48 hours. The identification of isolates was carried out through MALDI-TOF MS (Maldi Biotyper, Bruker Daltonics, MA, USA).
Histopathology and parasitology
Samples of liver with macroscopically evident lesions were subjected to virological and histopathological examination. The virological examination was conducted through electronic microscopy, isolation in embryonated-specific pathogen-free eggs and conventional cell lines (chick embryo hepatocytes). For the histological examination, portions of liver and intestine were fixed in 10% buffered formalin, included in paraffin and sectioned at 4 µm. The sections were then stained with haematoxylin and eosin stain and the preparations were observed under an optical microscope.
A parasitological examination was also conducted through direct microscopic observation (× 100) of intestinal scrapings made on the small intestine, caecum and at the level of the macroscopic lesions.
Polymerase chain reaction detection of C. colinum and toxin typing of C. perfringens
One colony of C. perfringens isolated from each tested sample (picked up from PAB or CA) was toxinotyped through multiplex-polymerase chain reaction (PCR) and the NetB toxin encoding gene was also investigated (Yoo et al., Citation1997; Keyburn et al., Citation2008). In birds sent in December, from the intestinal contents, the presence of C. colinum was also investigated by PCR in the intestinal tracts with evident lesions consistent with UE (Bano et al., Citation2008).
Results
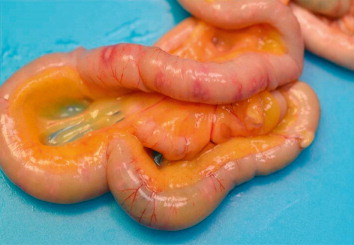
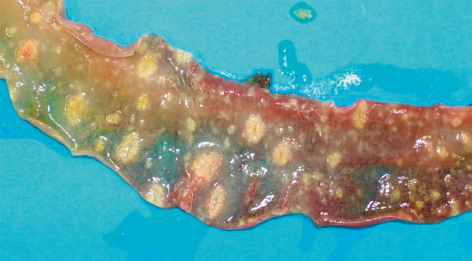
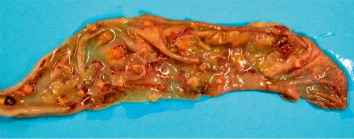
In all submitted carcasses, post-mortem examination revealed round-shaped intestinal lesions of variable diameter (0.5–3 mm), with necrotic-haemorrhagic centre, raised margins and occasionally coalescent. The ulcerations with the largest diameters were also visible through the serous membrane of the intestine (), while there were round necrotic areas in some tracts of the mucous membrane measuring 1–2 cm in diameter ( and ). The caecum lumens were enlarged by caseous material of haemorrhagic aspect composed by fibrin and necrotic debris, originating from ulcerations disseminated on the mucous membrane and visible after washing (). A focal necrotic hepatitis was observed in two birds from the first flock, while the spleen was generally non-reactive, pallid but with haemorrhagic suffusions on the surface.

At the histological level, NE was detected with various stages of erosion of the mucous surface, associated with the presence of bacterial aggregates and diffuse lymphoplasmacytic infiltration (). The liver was affected by multifocal heterophilic-necrotic hepatitis associated with bacterial aggregates and diffuse steatosis of the hepatocytes (). Results concerning PCR and bacteriological examination are summarized in . Bacteriological examination revealed high levels of toxin type A C. perfringens isolated from the intestine of birds belonging to both groups. Two strains of C. perfringens isolated over the course of the first outbreak were also positive for the NetB toxin encoding gene. None of the C. perfringens isolated from second group was positive for the NetB encoding gene. From the liver of one bird with necrotic lesions it was possible to isolate C. colinum in CA incubated in anaerobic conditions. C. colinum was identified at species level through MALDI-TOF MS (score 2.106) as well as through PCR performed directly from the isolate, while no direct PCR was conducted on the intestinal contents of birds from the first submission. In the intestines of all examined birds of the second outbreak, both C. perfringens and C. colinum were detected. In the birds belonging to the second group, C. colinum was not isolated but only detected by PCR from the intestinal contents. The virological examinations were negative, while the parasitological examination detected modest presence of coccidia oocysts only in three out of the 12 birds examined.
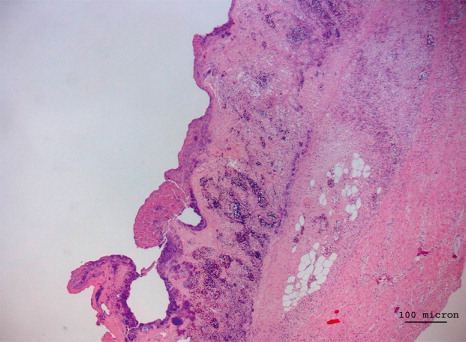
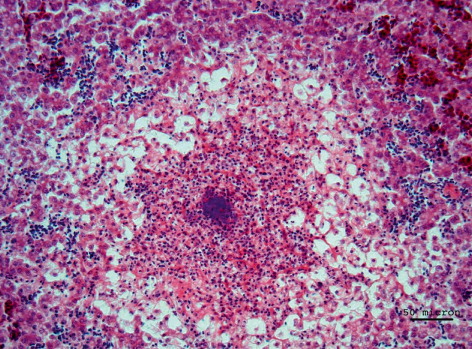
Table 1. Results of bacteriological and biomolecular examinations.
Discussion
NE is a typical disease of meat chickens that is becoming more and more common in laying hens. The different susceptibility to NE in the aforementioned production types (broiler and laying hen) may be explained by the possibility that predisposing factors (e.g. coccidiosis) contribute to the development of the pathology, rather than by a different sensitivity related to the age of the host. However, the lesions that are usually observed in NE outbreaks in laying hens do not reach the same serious level as described in the present report. In both the affected groups investigated in this study, the simultaneous presence of C. perfringens and C. colinum was detected. Some isolated strains in the first group harboured the NetB encoding gene, while the presence of C. colinum was not initially suspected and therefore not investigated directly from the intestinal lesions. The unexpected isolation of C. colinum from the liver permitted a retrospective diagnosis of a possible case of UE. In the second case, which occurred six months later in another group of laying hens, the presence of C. colinum was investigated directly through PCR, demonstrating the presence of C. colinum in all affected birds.
In the presented clinical case, it is impossible to establish with certainty which of the two Clostridium species has the predominant aetiological role, but the isolation of C. colinum in the liver presenting the classic necrotic lesions is nevertheless significant, as these are usually detected in UE in other host species. The only reports of UE in laying hens refer to a focal necrotic duodenitis in which C. colinum has never been isolated but only identified through molecular methods. A simultaneous C. colinum and C. perfringens co-infection in laying hens has never been reported. However, Beltran-Alcrudo et al. (Citation2008) reported the isolation of both bacteria in a case of UE in quails, considering C. colinum as primary agent of the pathology and attributing C. perfringens a secondary role.
Following the diagnosis of intestinal clostridiosis, the birds were treated for five days with tylosin in drinking water (50 g/100 L). It is well known that the strains of C. perfringens isolated from chickens are usually sensitive to tylosin, but the lack of selective growth media for C. colinum makes the isolation from particularly contaminated organs difficult, as well as the execution of tests to evaluate the susceptibility to antimicrobials (Martel et al., Citation2004; Gholamiandehkordi et al., Citation2009). The fact that after treatment there was a remission in clinical signs leads to the conclusion that C. colinum can also be susceptible to this molecule.
References
- Al-Shiekhly, F. & Truescott, R.B. (1977a). The pathology of necrotic enteritis of chickens following infusion of broth cultures of Clostridium perfringens into the duodenum. Avian Diseases, 21, 230–240. 10.2307/1589343
- Al-Shiekhly, F. & Truescott, R.B. (1977b). The pathology of necrotic enteritis of chickens following infusion of crude toxins of Clostridium perfringens into the duodenum. Avian Diseases, 21, 241–255. 10.2307/1589344
- Baltzely, T.A., Dunham, S.M., Lago, F., Rehberger, T.G. & Siragusa, G.R. (2008). Molecular pathogenesis markers of focal duodenal necrosis in layer hens. Proceedings of the 80th Northeastern Conference on Avian Diseases. State College, PA.
- Bano, L., Drigo, I., Macklin, K.S., Martin, R.S., Miller, R.S., Norton, R.A., Oyarzabal, O.A. & Bilgili, S.F. (2008). Development of a polymerase chain reaction assay for specific identification of Clostridium colinum. Avian Pathology, 37, 179–181. 10.1080/03079450801918662
- Barnes, H.J., Wages, D.P. & Opengart, K. (2008). Clostridial disease. In Y.M. Saif, A.M. Fadly, J.R. Glisson, L.R. McDougald, & L.K. Nolan (Eds.). Diseases of Poultry 12th edn (pp. 865–879). London: Blackwell Publishing.
- Beltran-Alcrudo, D., Cardona, C., McLellan, L., Reimers, N. & Charlton, B. (2008). A persistent outbreak of ulcerative enteritis in bobwhite quail (Colinus virginianus). Avian Diseases, 52, 531–536. 10.1637/8195-121307-Case
- Berkhoff, G.A. & Campbell, S.G. (1973). Etiology and pathogenesis of ulcerative enteritis (quail disease). The experimental disease. Avian Diseases, 18, 205–2012. 10.2307/1589127
- Berkhoff, G.A., Campbell, S.G. & Naylor, H.B. (1973). Etiology and pathogenesis of ulcerative enteritis (quail disease). Isolation of the causative anaerobe. Avian Diseases, 18, 186–194. 10.2307/1589125
- Coursodon, C.F., Glock, R.D., Moore, K.L., Cooper, K.K. & Songer, J.G. (2012). TpeL-producing strains of Clostridium perfringens type A are highly virulent for broiler chicks. Anaerobe, 18, 117–121. 10.1016/j.anaerobe.2011.10.001
- Davis, R.B. (1973). Ulcerative enteritis in chickens: coccidiosis and stress as predisposing factors. Poultry Science, 52, 1283–1287. 10.3382/ps.0521283
- Drigo, I., Agnoletti, F., Bacchin, C., Guolo, A., Cocchi, M., Bonci, M. & Bano, L. (2009). Diffusion of Clostridium perfringens NetB positive strain in healthy and diseased chicken. Italian Journal of Animal Science, 8, 761–764. 10.4081/ijas.2009.761
- Gholamiandehkordi, A., Eeckaut, V., Lanckriet, A., Timbermont, L., Bjerrum, L., Ducatelle, R., Haesebrouck, F. & Van Immerseel, F. (2009). Antimicrobial resistance in Clostridium perfringens isolates from broilers in Belgium. Veterinary Research Communications, 33, 1031–1037. 10.1007/s11259-009-9306-4
- Hampson, D.J. & Swayne, D.E. (2008). Avian intestinal Spirochetosis. In Y.M. Saif, A.M. Fadly, J.R. Glisson, L.R. McDougald, & L.K. Nolan. (Eds.). Diseases of Poultry 12th edn (pp. 922–940). London: Blackwell Publishing.
- Keyburn, A.L., Boyce, J.D., Vaz, P., Bannma, T.L., Ford, M.E., Parker, D., Di Rubbo, A., Rood, J.I. & Moore, R.J. (2008). NetB, a new toxin that is associated with avian necrotic enteritis caused by Clostridium perfringens. PLoS Pathogens, 4, 0001–0011. 0147-619X(1993)029[0233:CPCSVT]2.0.CO;2
- Keyburn, A.L., Sheedy, S.A., Ford, M.E., Williamson, M.M., Award, M.M., Rood, J.I. & Moore, R.J. (2006). Alpha-toxin of Clostridium perfringens is not an essential virulence factor in necrotic enteritis in chickens. Infection and Immunity, 74, 6496–6500. 10.1128/IAI.00806-06
- Kwatra, M.S. & Chaudhury, B. (1976). A presumptive diagnosis in the fowl (Gallus gallus domesticus) from Assam (India). Avian Diseases, 20, 401–406. 10.2307/1589280
- Martel, A., Devriese, L.A., Cauwerts, K., De Gussem, K., Decostere, A. & Haesebrouck, F. (2004) Susceptibility of Clostidium perfringens stains from broilers chickens to antibiotics and anticoccidials. Avian Pathology, 33, 3–7. 10.1080/0307945031000163291
- Olkowski, A.A., Wojnaroicz, C., Chirino-Trejo, M., Laarveld, B. & Sawicki, G. (2008). Sub-clinical necrotic enteritis in broiler chickens: novel etiological consideration based on ultra-structural and molecular changes in the intestinal tissue. Research in Veterinary Science, 85, 543–553. 10.1016/j.rvsc.2008.02.007
- Porter, R.E. Jr. (1998). Bacterial enteritides of poultry. Poultry Science, 77, 1159–1165. 10.1093/ps/77.8.1159
- Songer, J.G. (1996). Clostridial enteric disease of domestic animals. Clinical Microbiology Reviews, 9, 216–234.
- Yoo, H.S., Lee, S.U. & Park, Y.H. (1997). Molecular typing and epidemiological survey of prevalence of Clostridium perfringens types by multiplex PCR. Journal of Clinical Microbiology, 35, 228–232.