Abstract
A one-year-old male Himalayan monal (Lophophorus impejanus) was presented for veterinary attention with a history of chronic wasting, weakness and ataxia. The bird died, and post-mortem findings included mild non-suppurative encephalitis and degenerative encephalopathy, lymphoplasmacytic myenteric ganglioneuritis (particularly of the proventriculus), and Wallerian degeneration of the sciatic nerves. Avian bornavirus (ABV) was identified in the brain by immunohistochemistry and reverse-transcriptase polymerase chain reaction. Sequencing of the reverse-transcriptase polymerase chain reaction product indicated the presence of ABV genotype 4, which is generally associated with disease in psittacine birds. Subsequent to the death of the pheasant, ABV genotype 4 was identified at autopsy from a juvenile white-bellied caique (Pionites leucogaster) in the same collection. We hypothesize that the pheasant became infected through contact with psittacine birds with which it shared an aviary. We believe this to be the first reported case of natural ABV infection in a bird in the Order Galliformes.
Introduction
Avian bornavirus (ABV) was first identified in 2008 as an avian-specific member of the family Bornaviridae, and is accepted as the cause of proventricular dilatation disease (PDD) in psittacine birds (Honkavuori et al., Citation2008; Kistler et al., Citation2008; Payne et al., Citation2012). Classical clinical signs of PDD include ataxia, proprioceptive deficits, chronic wasting, regurgitation, and passing undigested seeds in faeces (Hoppes et al., Citation2010). These signs are typically accompanied by dilation of the proventriculus with undigested food material, as well as histologic lesions of lymphoplasmacytic inflammation of the central, peripheral and autonomic nerves and ganglia (Payne et al., Citation2011). Diagnosis of ABV infection at autopsy is generally based on the presence of characteristic histologic lesions and the use of immunohistochemistry and reverse-transcriptase polymerase chain reaction (RT-PCR) to identify viral RNA (Hoppes et al., Citation2010; Raghav et al., Citation2010). To date, 12 different ABV genotypes have been isolated from avian species including psittacine genotypes 1-7, canary genotypes ABV-C 1-3, estrildid finch genotype ABV-EF and ABV-CG, which has been identified in free-ranging waterfowl and other wild birds in North America (Delnatte et al., Citation2011; Payne et al., Citation2012; Rubbenstroth et al., Citation2013; Rubbenstroth et al., Citation2014). Histopathologic lesions consistent with PDD, without identification of concurrent ABV infection, have been described in additional avian families within the orders Passeriformes, Pelecaniformes, Falconiformes and Piciformes (Gancz et al., Citation2010). Infection within the Order Galliformes has not been reported to our knowledge.
The following case report describes the identification of ABV genotype 4 from a Himalayan monal (Lophophorus impejanus) that presented to the Ontario Veterinary College Avian and Exotics Service for neurological disease.
Materials and Methods
Case history
A one-year-old male Himalayan monal was presented to the Avian-Exotics Service of the Health Sciences Centre of the Ontario Veterinary College with a one week history of generalized weakness, inappetence, weight loss and ataxia. The bird was owned by an aviculturist and was housed in a large outdoor aviary with one additional Himalayan monal and eight parrots of various types (Amazona spp. and Cacatua spp.). On physical examination, the pheasant was emaciated (weighing 1.65 kg with a body condition score 2/5) and moderately dehydrated. It appeared ataxic, with proprioceptive deficits and intermittent head and wing droop. Whole body radiographs were performed under isoflurane anaesthesia and revealed multiple loops of intestines that were mildly dilated with air. Plasma biochemistry revealed elevations in creatinine kinase, consistent with the history of recumbency and trauma due to frequent falls [6,645 U/L, reference interval: 3,195–3,623 U/L in ring-necked pheasants (Phasianus colchicus)], and uric acid (824 μmol/L, reference interval: 136–302 μmol/L in ring-necked pheasants) and sodium (166 mmol/L, reference interval: 147–156 mmol/L in ring-necked pheasants), consistent with dehydration or kidney disease (Lloyd & Gibson, Citation2006; Kececi & Col, Citation2011). Stained and fresh smears made from a crop swab and of faeces were cytologically unremarkable. A faecal flotation was also negative for ova or parasites. Blood samples were submitted for assessment of lead and zinc levels, which were found to be 0.08 µg/ml and 1.6 µg/ml, respectively.
The pheasant was maintained in the hospital with supportive care including intravenous fluid therapy, enrofloxacin (15 mg/kg PO q12h), calcium EDTA (35 mg/kg IV once, discontinued after plasma heavy metal levels were obtained). While in the hospital the bird was also syringe fed periodically; however, crop emptying was delayed and syringe feeding was subsequently discontinued. The bird was discharged the following day at the owner's request and was sent home on enrofloxacin (15 mg/kg PO q12h), metronidazole (30 mg/kg PO q12h) and fenbendazole (50 mg/kg PO q12h for possible Baylisascaris sp. infestation). The bird was returned for a recheck 11 days later; at this time it presented with severe ataxia, disorientation and head and bilateral wing droop. The pheasant also had severe crop stasis and occasional ptyalism. Further in-hospital care and diagnostics were declined and the bird died at home the following day. The body of the pheasant was refrigerated and six days later was submitted to the Ontario Veterinary College Department of Pathobiology for post-mortem analysis.
Four months after the autopsy of the pheasant, a 13-week-old white-bellied caique (Pionites leucogaster) from the same avian collection was presented for post-mortem examination. The bird had been seen three weeks previously at the OVC-Avian and Exotics Service for crop stasis and melena that was successfully treated with amoxicillin/clavulanic acid (125 mg/kg PO q12h), nystatin (10,000 U/kg PO q12h), sucralfate (25 mg/kg PO q12h) and subcutaneous fluids.
Autopsy, histopathology and immunohistochemistry
Complete autopsies were performed on both dead birds and representative tissues were collected and placed in 10% formalin, paraffin embedded, sectioned at 4–6 µm, and stained with hematoxylin and eosin (H&E). Immunohistochemical staining of sections of the brain of the pheasant was performed using rabbit anti-ABV nucleocapsid protein antibody as described previously (Raghav et al., Citation2010).
PCR and sequencing
Nucleic acid extraction, real-time RT-PCR and sequencing were carried out as described previously (Delnatte et al., Citation2013). GenBank accession numbers for nucleotide sequences described in this report are KM508811 and KM508812.
Results
Autopsy, histopathology and immunohistochemistry
The carcass of the pheasant was thin and there were abundant lice within the feathers. Yellow viscous material was present within the oral cavity and at the commissures of the mouth. The eyes were bilaterally sunken in the orbits, the keel bone was prominent and pectoral muscles were pale. The crop was full and contained large pieces of fibrous plant material. The koilin layer of the gizzard was grey-green and markedly granular and fibrous in texture, and did not readily peel away from the epithelium. The caique was in poor body condition, was markedly autolyzed, and had widespread visceral gout. On the cranium, there were multiple petechial and ecchymotic haemorrhages.
Significant microscopic lesions in the pheasant were confined to the central, peripheral and autonomic nervous systems. In the cerebral parenchyma and meninges, there were increased numbers of lymphocytes and plasma cells expanding Virchow-Robin spaces and forming perivascular cuffs (). The tunica media of multiple vessels within the cerebral cortex contained aggregates of mineral. There were multifocal areas within the brainstem and mesencephalon that were diffusely vacuolated and contained scattered swollen, hypereosinophilic axons (spheroids), gemistocytic astrocytes with glassy eosinophilic cytoplasm and pyknotic debris (). The sciatic nerves, bilaterally, had multifocal, hypereosinophilic, swollen axons with scattered mineralization and foamy macrophages (gitter cells) within dilated myelin sheaths (Wallerian degeneration) (). Within the ganglia of the proventricular serosa there were low numbers of plasma cells and occasional macrophages. There was submucosal and periglandular oedema and protein exudation, accompanied by increased numbers of heterophils, haemorrhage and scattered pyknotic debris. The autonomic nerves of other coelomic organs (including the intestines) had mild Wallerian degeneration; low numbers of plasma cells, lymphocytes and macrophages were present within and surrounding nerve sheaths. The adrenal glands were histologically normal. Immunohistochemistry of sections of brain revealed subtle nuclear staining within neurons deep to the ependymal cells of the lateral ventricles ().
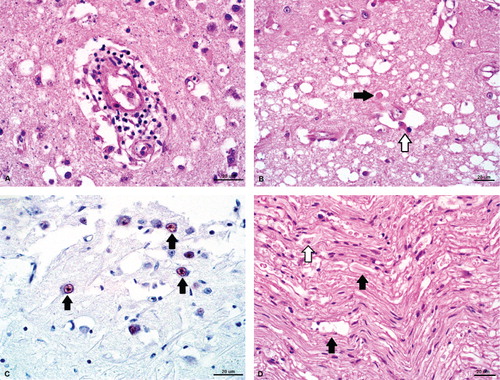
Microscopic lesions in the caique were consistent with a diagnosis of proventricular dilation disease. Wallerian degeneration and infiltration by low numbers of mononuclear cells and heterophils were present within nerves of the myenteric plexus of the crop. Within the ventriculus, there were multiple moderate lymphoplasmacytic infiltrates tracking through the tunica muscularis as well as forming cuffs surrounding the subserosal nerves and vessels. A lymphoplasmacytic infiltrate was present perivascularly within the cervical portion of the vagus nerve, sciatic nerve and brain; and within the interstitium of the adrenal glands. The presence of serosal urate deposits was confirmed, and there was marked atrophy of the Bursa of Fabricius as well as bursal granulomas with intra-lesional bacteria.
PCR and sequencing
For the pheasant, real-time RT-PCR for the ABV matrix (ABV-M) gene was first performed on frozen brain and was strongly positive with a crossing point value of 24 (Animal Health Laboratory, University of Guelph) (Delnatte et al., Citation2013). This result was replicated using a second sample of frozen brain as well as with scrolls of formalin-fixed paraffin embedded (FFPE) brain. The nucleotide sequence of the ABV matrix gene was 100% identical to an ABV genotype 4 sequence (Gen Bank FJ603679). RT-PCR carried out on frozen brain from the caique was also positive for the ABV-M gene; the resulting sequence had a single synonymous substitution and was 99.7% identical to the pheasant sequence ( and ). At the amino-acid level, sequences were 100% identical.
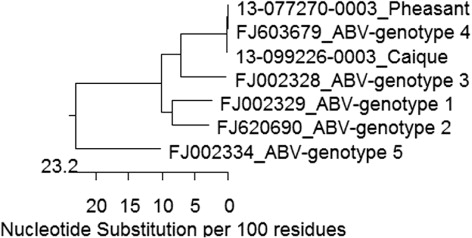
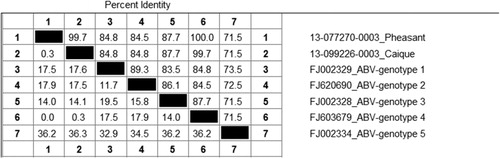
Scrolls of FFPE brain from the pheasant were also examined for the presence of avian paramyxovirus (APVM-1 Matrix rt-RT-PCR), avian influenza (Influenza A Matrix PCR) and West Nile virus (West Nile virus rt-RT-PCR); results for all three viruses were negative.
Discussion
We propose that the neurological disease in this Himalayan monal was caused by infection with ABV, specifically psittacine genotype 4. To our knowledge, this is the first case of natural disease caused by an avian bornavirus in a member of the Order Galliformes. The only previous description of bornaviral-associated infection or disease in a galliform bird was a report by Ludwig et al. in 1973, who infected day-old chicks via direct cerebral inoculation with Borna disease virus infected rabbit brain homogenates (Ludwig et al., Citation1973). These birds developed clinical signs of ataxia and hind limb paralysis 5 –8 weeks post-infection and had the intraneuronal, intranuclear, Joest-Degen inclusions that are classically seen with Borna disease virus infections in mammals. Further description of any histologic lesions was lacking. Persistent infection was demonstrated in two chickens, whose brains were infectious to rabbits 1.5 years after the birds were inoculated. Chickens could not be infected with ABV by experimental inoculation with psittacine (ABV-4) or Canada goose genotypes, or by direct exposure to cockatiels shedding ABV (Delnatte et al., Citation2013; Shivaprasad et al., Citation2010).
Specific testing for ABV in this pheasant was prompted by the character of the pathologic lesions identified, particularly the presence of non-suppurative autonomic ganglioneuritis, as well as the fact that the bird's owner also maintained a collection of psittacine birds within which ABV had previously been identified. The positive RT-PCR result was somewhat unexpected given the lack of previous identification of ABV in galliform birds. In order to ensure that laboratory contamination had not occurred, the results were replicated using a second sample of frozen brain and FFPE brain sections. As well, the records from the PCR laboratory and the autopsy suite were examined and it was determined that there had been no samples or birds with ABV-4 processed in either facility on the days in question. The combination of ABV antigen within the brain, in conjunction with histopathologic changes, and the positive RT-PCR results are strong evidence that this bird was truly infected with ABV.
The pathological changes in the nervous system of this pheasant explained the clinical signs shown by the bird. While the lymphoplasmacytic infiltrates noted are characteristic of ABV in psittacine birds, the presence of degenerative lesions, including Wallerian changes, in the brain and peripheral nerves are less so. Similar lesions were; however, described in parrots with PDD by Berhane et al. (Citation2001) and have been seen in birds with PDD and ABV infection by one author (DAS). Spinal cord was not, unfortunately, examined in the pheasant and so we are unable to determine whether a component of the peripheral nerve degeneration could have been secondary to spinal cord disease.
Consideration of other differential diagnoses was prompted by the presence of spongiosis and Wallerian degeneration. Lead toxicity, which can cause peripheral neuropathy, was ruled out on the basis of blood lead levels (Trampel et al., Citation2003). Aberrant migration of the raccoon roundworm, Baylisascaris procyonis, is a well-recognized cause of encephalomalacia in birds in Ontario; however, neither larvae nor larval tracks were observed in the neural parenchyma. Bacterial infection was considered unlikely based on the non-suppurative nature of the inflammatory infiltrates and negative culture of filter organs including lung, liver and kidney. Several other causes of viral encephalitis were ruled out by PCR testing.
We propose that the route of infection for this pheasant was faecal contamination of the environment that it shared with psittacine birds. In retrospect, the owner divulged that several of the parrots sharing the aviary with the pheasant were suspected to have PDD or had previously been exposed to other parrots with confirmed PDD. The presence of ABV in psittacines in the collection was confirmed at the autopsy of the juvenile caique several months later. Although experimental infection of galliform birds has not been successful, we concluded that the unique management features in this case: a ground-dwelling bird that was feeding and foraging on substrates contaminated the excrement of ABV-infected psittacine birds over a long period of time, provided a combination of sufficient viral inoculum over a sufficient duration of exposure to induce clinical ABV infection in this pheasant.
ABV-4 has previously been shown to cross the “Avian Order-Barrier”. Transmission of ABV genotype 4 from cockatiels to ducks, as evidenced by faecal shedding of viral RNA, was described under circumstances where mallards shared a cage with cockatiels, and under experimental conditions into Khaki Campbell ducks by inoculation (Hoppes et al., Citation2010; Payne et al., Citation2012). Disease was not identified in either scenario. ABV-CG has also been shown to affect a variety of avian species, not all of them anseriforms (Payne et al., Citation2012).
In summary, ABV genotype 4 was identified in the brain of a Himalayan monal with neurological disease consistent in clinical and pathological features with ABV infection of psittacine birds, and this pheasant was from an aviary where the same genotype of ABV was present in psittacine birds. We thus conclude that natural transmission of ABV genotype 4 from a psittacine bird reservoir to this Himalayan monal resulted in the development of clinical signs and death. Psittacine ABV spillover to other galliforms, such as chickens and turkeys, may also be possible under favourable circumstances or high infectious pressure.
Disclaimer
Taxonomic designation used in this manuscript does not reflect recent updates to the taxonomy of the Bornaviridae as per Kuhn, J.H., Dürrwald, R., Bào, Y., Briese, T., Carbone, K., Clawson, A. N., deRisi, J.L., Garten, W., Jahrling, P.B., Kolodziejek, J., Rubbenstroth, D., Schwemmle, M., Stenglein, M., Tomonaga, K., Weissenböck, H., & Nowotny, N. (2014). Taxonomic reorganization of the family Bornaviridae. Archives of Virology, 160, 621–632.
Acknowledgements
The authors gratefully acknowledge Dr Josepha DeLay for assistance with interpretation of the immunohistochemistry.
References
- Berhane, Y., Smith, D.A., Newman, S., Taylor, M., Nagy, É., Binnington, B. & Hunter B. (2001). Peripheral neuritis in psittacine birds with proventricular dilatation disease. Avian Pathology, 30, 563–570.
- Delnatte, P., Berkvens, C., Kummrow, M., Smith, D.A., Campbell, D., Crawshaw, G., Ojkic, D. & DeLay, J. (2011). New genotype of avian bornavirus in wild geese and trumpeter swans in Canada. Veterinary Record, 169, 108–108.
- Delnatte, P., Ojkic, D., DeLay, J., Campbell, D., Crawshaw, G. & Smith, D.A. (2013). Pathology and diagnosis of avian bornavirus infection in wild Canada geese (Branta canadensis), trumpeter swans (Cygnus buccinator) and mute swans (Cygnus olor) in Canada: a retrospective study. Avian Pathology, 42, 114–128.
- Gancz, A.Y., Clubb, S. & Shivaprasad, H.L. (2010). Advanced diagnostic approaches and current management of proventricular dilatation disease. The Veterinary Clinics of North America. Exotic Animal Practice, 13, 471–494.
- Honkavuori, K.S., Shivaprasad, H.L., Williams, B.L., Quan, P.-L., Hornig, M., Street, C., Palacios, G., Hutchison, S.K., Franca, M., Egholm, M., Briese, T. & Lipkin, W.I. (2008). Novel borna virus in psittacine birds with proventricular dilatation disease. Emerging Infectious Diseases, 14, 1883–1886.
- Hoppes, S., Gray, P.L., Payne, S., Shivaprasad, H.L. & Tizard, I. (2010). The isolation, pathogenesis, diagnosis, transmission, and control of avian bornavirus and proventricular dilatation disease. The Veterinary Clinics of North America. Exotic Animal Practice, 13, 495–508.
- Kececi, T. & Col, R. (2011). Haematological and biochemical values of the blood of pheasants (Phasianus colchicus) of different age. Turkish Journal of Veterinary and Animal Sciences, 35, 149–156.
- Kistler, A.L., Gancz, A., Clubb, S., Skewes-Cox, P., Fischer, K., Sorber, K., Chiu, C.Y., Lublin, A., Mechani, S., Farnoushi, Y., Greninger, A., Wen, C.C., Karlene, S.B., Ganem, D. & Derisi, J.L. (2008). Recovery of divergent avian bornaviruses from cases of proventricular dilatation disease: identification of a candidate etiologic agent. Virology Journal, 5, 88–102.
- Lloyd, S. & Gibson, J.S. (2006). Haematology and biochemistry in healthy young pheasants and red-legged partridges and effects of spironucleosis on these parameters. Avian Pathology, 35, 335–340.
- Ludwig, H., Becht, H. & Groh, L. (1973). Borna disease (BD), a slow virus infection biological properties of the virus. Medical Microbiology and Immunology, 158, 275–289.
- Payne, S., Covaleda, L., Jianhua, G., Swafford, S., Baroch, J., Ferro, P.J., Lupiani, B., Heatley, J. & Tizard, I. (2011). Detection and characterization of a distinct bornavirus lineage from healthy Canada geese (Branta canadensis). Journal of Virology, 85, 12053– 12056.
- Payne, S.L., Delnatte, P., Guo, J., Heatley, J.J., Tizard, I. & Smith, D.A. (2012). Birds and bornaviruses. Animal Health Research Reviews / Conference of Research Workers in Animal Diseases, 13, 145–156.
- Raghav, R., Taylor, M., Delay, J., Ojkic, D., Pearl, D.L., Kistler, A.L., Derisi, J.L., Ganem, D. & Smith, D.A. (2010). Avian bornavirus is present in many tissues of psittacine birds with histopathologic evidence of proventricular dilatation disease. Journal of Veterinary Diagnostic Investigation, 22, 495–508.
- Rubbenstroth, D., Rinder, M., Stein, M., Höper, D., Kaspers, B., Brosinski, K., Horie, M., Schmidt, V., Legler, M., Korbel, R. & Staeheli, P. (2013). Avian bornaviruses are widely distributed in canary birds (Serinus canaria f. domestica). Veterinary Microbiology, 165, 287–295.
- Rubbenstroth, D., Schmidt, V., Rinder, M., Legler, M., Corman, V.M. & Staeheli, P. (2014). Discovery of a new avian bornavirus genotype in estrildid finches (Estrildidae) in Germany. Veterinary Microbiology, 168, 318–323.
- Shivaprasad, H.L., Weissenböck, H., Hoppes, S., Gray, P.L., Payne, S. & Tizard, I. (2010). Pathology of experimental avian bornavirus infection in psittacines and chickens. In Proceedings of the American Association of Veterinary Laboratory Diagnosticians (p. 97). Minneapolis, MN, USA,
- Trampel, D.W., Imerman, P.M., Carson, T.L., Kinker, J.A. & Ensley, S.M. (2003). Lead contamination of chicken eggs and tissues from a small farm flock. Journal of Veterinary Diagnostic Investigation, 15, 418–422.
