Abstract
Genotyping of seven infectious bronchitis virus (IBV) strains isolated in Brazil showed that all belonged to the common Brazilian genotype and that these strains were closest to the subcluster of strain IBV/Brazil/2007/USP-19. Pathotyping of four selected Brazilian strains showed that they all caused a considerable level of ciliostasis in the trachea but at a somewhat lower level than did M41 and Brazilian strains 50/96, 57/96, 62/96 and 64/96 representing four different serotypes that had been reported earlier. In contrast to the M41 challenge strain, all Brazilian isolates replicated in kidney tissue in a high percentage of non-vaccinated challenged birds, clearly showing that they are nephropathogenic. As for the tracheal protection, the results using Massachusetts (Mass) vaccination against the recent strains seemed to show protection higher on average than for the strains reported earlier. A single or twofold vaccination with a Mass vaccine resulted in a mean tracheal protection level against the four challenge strains of 92% and 90%, respectively, whereas a single and twofold vaccination with a Mass vaccine halved the percentage of infected kidneys (14% and 13%, respectively, P < .05) compared to that of the unvaccinated birds (27%). The combination of the Mass and the 793B vaccine provided on average a tracheal protection of 99% and a reduction of the percentage of infected kidneys to a mean of 2%. This was a significantly (P < .05) higher protection than that achieved by a single or twofold Mass vaccination, showing the added value of the 793B vaccination following priming with a vaccine of the Mass type.
Introduction
Infectious bronchitis (IB), caused by infectious bronchitis virus (IBV), is one of the most economically important viral diseases of poultry and can be involved in respiratory disease, drops in egg production and hatchability, nephritis and enteric problems. IBV, a Gammacoronavirus (Nidovirales: Coronaviridae: Coronavirinae: Gammacoronavirus: Avian coronavirus) occurs as many different serotypes and genotypes, defined based on analysis of the genome or antigenicity of the spike envelope glycoprotein S1 (Jackwood & De Wit, Citation2013).
In Brazil, the incidence of IBV-positive flocks ranges from 50% to 100% in the areas where most of the poultry population is found (Brandão et al., Citation2009) and has been reported to cause serious problems for the Brazilian poultry industry (Assayag et al., Citation2012a, b, c). Experiments reported by Cook et al. (Citation1999) and Di Fabio et al. (Citation2000) showed that 14 out of 15 field isolates obtained in 1995 were of the non-Massachusetts (Mass) serotype and belonged to at least four antigenic groups that were different from the ones described in other countries. Genotyping was not performed for those strains but vaccination with a Mass vaccine (Nobilis IB MA5) at the day of hatch was able to induce heterologous cross-protection in the respiratory tract at 5 weeks post challenge, varying from 37% to 88% following challenge with strains representing the four different serotypes. A single vaccination at 14 days of age with the heterologous 4/91 vaccine (Nobilis IB 4/91 of the 793B serotype) induced between 76% and 84% cross-protection against these strains, whereas the combination of both vaccines induced 87% and 88% protection against the two strains that were tested. More recent papers have reported the widespread existence of a unique Brazilian lineage which is predominant over other established types such as Mass, 4/91 and Connecticut IBV types (Brandão et al., Citation2009; Felippe et al., Citation2010; Villarreal et al., Citation2010; Chacon et al., Citation2011) with 63.4–79.3% nucleotide identity in the S gene compared to the Mass strain (Abreu et al., Citation2006; Villarreal et al., Citation2010; Chacon et al., Citation2011). Although these recent papers give us a valuable insight into the genetic epidemiology of IBV in Brazil, they lack data concerning serotyping, pathotyping and protection provided by vaccination against the recent Brazilian IBV strains. It has been shown in several papers reporting the level of homology of the S1 gene (or a part of it) and the level of cross-protection that there is on average a higher chance of a good level of cross-protection between strains with a high level of homology than between strains with low homology (Cavanagh et al., Citation1997; Cook et al., Citation2001; Meir et al., Citation2004; Gelb et al., Citation2005; Ladman et al., Citation2006). However, all data together show that this relationship is rather weak (De Wit et al., Citation2011a). Some strains which differ by only a few per cent in the sequenced part of the genome showed a significant drop in cross-protection (Meir et al., Citation2004; Abdel-Moneim et al., Citation2006; De Wit et al., Citation2012), whereas there was a high level of cross-protection against other strains with a much lower homology (Meir et al., Citation2004).
To update the biologically relevant knowledge of recent Brazilian IBV strains, four recent isolates from broilers with respiratory distress were tested for their pathogenicity for the respiratory tract and kidney as well for the level of cross-protection induced by three different vaccination programmes. These programmes included: (a) a single vaccination with a Mass vaccine at day of hatch, (b) a double vaccination with the same Mass vaccine at day of hatch and day 14, or (c) a Mass vaccination at day of hatch and a second vaccination with a 4/91-793B-type vaccine at 14 days of age.
Materials and Methods
Genotyping and selection of virus strains.
Eleven IBV strains that had originally been isolated at the University of Sao Paulo were sent to GD Animal Health (Deventer, the Netherlands) for further testing. Information about the flocks and the organs from which the isolates were obtained, including coding and Genbank accession number, is listed in . The strains that could be re-isolated (n = 7, ) were subsequently tested by RT-PCR and sequenced using primers XCE1+ and XCE3- (Cavanagh et al., Citation1999) targeted to the S1 subunit of the S gene (nucleotides encoding for amino-acids 234–362 regarding the spike protein of the M41 strain, Genbank accession number AY851295.1). A maximum likelihood tree based on the JTT matrix-based model and 100 bootstrap replicates was built with the putative amino-acids sequences using Mega 6 (Hall, Citation2013). Based on the results of the genotyping, the origin of the different flocks and the tissue of origin of the strains, four strains were selected for testing by pathotyping and use in the vaccination/challenge experiment.
Table 1. Origin and identification of the seven Brazilian genotype IBV strains used in this study.
Vaccines and vaccination
Two vaccines of the Mass serotype were used: Nobilis IB MA5 (MSD Animal Health, indicated as vaccine Mass A) and Nobilis IB H120 (MSD Animal Health, further indicated as vaccine Mass B) and a vaccine of the 4/91–793B serotype (Nobilis IB 4/91, MSD Animal Health, further indicated here as vaccine 793B). All vaccines were applied by eye-drop (1 droplet in each eye) with one commercial dose of vaccine per bird. After inoculation, vaccines were back-titrated in 8-day-old embryonated SPF eggs. The titres for vaccines Mass A, Mass B and 793B were 105.2, 104.6 and 104.3 median embryo infectious doses (EID50) per 0.1 mL, respectively.
Pathotyping in 1-day-old chicks
All experiments were conducted with the formal approval of the local animal welfare committee and registered according to the Dutch legislation. Four groups of 20 and two groups of 10 1-day-old SPF layer chickens were placed in isolators and challenged by eye-drop at day of hatch with 104 EID50 of the selected strains: Brazil Be, Brazil Ce, Brazil Ar, Brazil Bk, M41 or mock infected. The pathogenicity of the challenge viruses for the respiratory tract and kidneys of 1-day-old chickens was determined at 5 and 8 days post challenge (d.p.c.) using the ciliostasis test and immunohistochemistry.
Protectotyping and vaccination/challenge experiment
In total 19 groups of 1-day-old SPF layer chickens were housed in separate isolators. For each of the four selected IBV strains Brazil Be, Brazil Ce, Brazil Ar, Brazil Bk, four groups of 20 birds were used, of which one group was not vaccinated, a second group was vaccinated only at day of hatch with a Mass vaccine (Mass A or Mass B), a third group was vaccinated at day of hatch and at 14 days with the same Mass vaccine (Mass A or Mass B) and a fourth group was vaccinated at day of hatch with a Mass vaccine (Mass A or Mass B) and at 14 days with a 793B vaccine. Vaccine Mass A was used for strains Brazil Ar and Brazil Bk, whereas vaccine Mass B was used for strains Brazil Be and Brazil Ce. The choice to use vaccine A or B with a specific field strain was made randomly. The Mass, M41 strain was also used for challenge and two challenge control groups with 20 birds were included. One group was not vaccinated, a second group was vaccinated only at the day of hatch with a Mass vaccine (Mass A). Group 19 was not vaccinated or challenged. The challenge strains were applied by eye-drop (one droplet in each eye) with 104 EID50 per bird at 32 days of age. Water and feed were supplied ad libitum, lighting was according to the advice of the breeding company.
Ciliostasis
The level of protection of the trachea was determined using the ciliostasis test on five tracheal rings per chicken at 5 d.p.c. (De Wit et al., Citation2013). The level of beating of the cilia in each ring (TOC score) was expressed as 4 ( < 25% beating of cilia), 3 (25–50% beating), 2 (50–75% beating), 1 (75–99% beating) or 0 (all beating). One chicken could score between 0 and 20 (five rings from each trachea; maximum score 4). An individual chicken was recorded as protected against challenge if the ciliostasis score was less than 10 (modified from Cook et al., Citation1999). For each group, a ciliostasis protection score (0–100%) was calculated by the formula:
![]()
Immunohistochemistry
Immunohistochemistry (IHC) was used for the detection of IBV antigen in the kidney tissue using monoclonal antibody 48.4 directed against the nucleoprotein (De Wit et al., Citation2011b). The following scoring system was used: 1 when a bird had IBV-positive cells in the kidney, 0.5 when the staining results were suspect, 0.25 when the ureter was IBV-positive, but the kidney was negative, and 0 when the staining of both kidney and ureter were negative for that bird.
Statistical analysis
Statistical analyses was performed by logistic regression using Stata.
Results
Genotyping
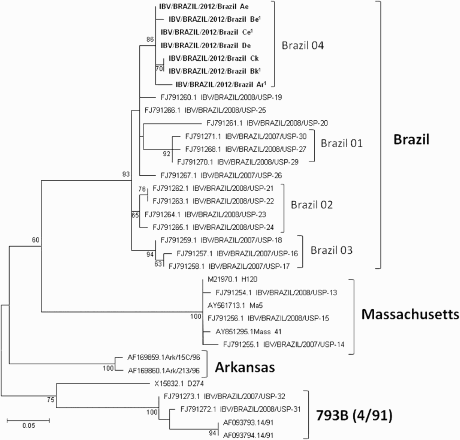
The results of the genotyping of the seven strains that could be re-isolated are shown in . The three Brazilian subclusters (Brazil 01, 02 and 03) described by Villarreal et al (Citation2010) are evident, while the seven strains from this study resulted in a fourth (Brazil 04) subcluster, all of them being close to the Brazilian strain IBV/Brazil/2007/USP-19 (FJ791260). In strains Brazil Ck and Brazil Bk, both isolated from kidneys, grouped in a separate node when compared to those isolated from the enteric contents (Brazil Ae, Be, Ce and De) and the respiratory tract (Brazil Ar), with a 100% amino acid identity between them; a I332R amino acid mutation was found amongst the kidney and the enteric/respiratory strains, while V287G and Q291E differentiate the kidney/enteric and the respiratory strains (positions are given regarding the spike protein of the M41 strain Genbank accession number AY851295.1). Based on the result of the infectivity studies, genotyping and origin of the strains, including organ of isolation, strains IBV/BRAZIL/2012/Brazil Be (Brazil Be), IBV/BRAZIL/2012/Brazil Ce (Brazil Ce), IBV/BRAZIL/2012/Brazil Ar (Brazil Ar) and IBV/BRAZIL/2012/Brazil Bk (Brazil Bk) were selected for pathotyping and for a vaccination/challenge experiment. They showed a mean amino-acids identity of 98.2% with each other, while the mean amino-acids identities of these four strains with the Mass A, Mass B vaccines used and the M41 challenge strain varied between 79.1% and 80%. Similar identities were seen with the 793B vaccine.
Figure 1. Amino-acids maximum likelihood tree (JTT matrix-based model, 100 bootstrap replicates) for the partial S1 subunit of the IBV spike protein (amino acid 231–346), showing the strains used in the present study (in bold) and the IBV D274, Massachusetts, 793B (4/91) and Arkansas types. The Brazilian strains used for the pathotyping and a vaccination/challenge experiment are identified with1. Numbers at each node are bootstrap values. The bar represents the number of amino-acids substitutions per site.
Pathotyping in 1-day-old chicks
The results of the pathotyping of strains M41, Brazil Be, Brazil Ce, Brazil Ar and Brazil Bk in 1-day-old SPF layers are shown in . All IBV strains caused severe damage to the trachea. The damage to the cilia caused by Brazil Ar in the 1-day-old SPF chickens was comparable to that caused by the M41 strain (complete damage at 5.d.p.c.), whereas the damage (loss of cilia) caused by the other three Brazilian strains was around 80% at 5 d.p.c. At 8 d.p.c., the trachea showed partial recovery for all strains. In contrast to M41, all Brazilian strains replicated in a high percentage of the kidneys with accompanying interstitial nephritis, which identified them as nephropathogenic strains. Strains Brazil Be and Brazil Ce showed percentages of kidney positivity of 53% and 40%, respectively, at 8 d.p.c. The highest level of positive kidneys was seen following inoculation of strain Brazil Ar (73% at 5 d.p.c. and 53% at 8 d.p.c.). For strain Brazil Bk, around 40% of the birds showed IBV replication in the kidney at both 5 and 8 d.p.c.
Table 2. Pathogenicity for 1-day-old SPF chickens of the Brazilian IBV strains compared to M41
Protectotyping and vaccination/challenge experiment
The results of the protectotyping and vaccination/challenge experiment are shown in . All strains caused damage in the trachea of the unvaccinated chickens after challenge at day 32. The M41 strain and the Brazil Ce strain caused 100% and 97% damage to the cilia, respectively. Strains Brazil Be (79% damage), Brazil Ar (53% damage) and Brazil Bk (69% damage) did not cause a very high level of damage to the cilia of the unvaccinated birds. All four Brazilian strains replicated in a high percentage of the kidneys, with accompanying interstitial nephritis, which identified them as nephropathogenic IBV strains.
Table 3. Overview of the results of the vaccination/challenge experiment in which the four Brazilian IBV strains and M41 were tested using Mass and 793B vaccines.
Vaccine Mass A was used as the vaccine for strains Brazil Ar and Brazil Bk, whereas vaccine Mass B was used with strains Brazil Be and Brazil Ce. A single vaccination with vaccine Mass A or Mass B showed 85%, 88%, 94% and 100% protection of the trachea against ciliostasis following challenge with strains Brazil Ar, Brazil Be, Brazil Bk and Brazil Ce, respectively (an average level of protection of 92%, ). The detectable level of renal replication of the challenge viruses in the groups given a single vaccination with a Mass vaccine was the highest at 8 d.p.c., being 3%, 13%, 0% and 40%, post challenge with strains Brazil Ar, Brazil Be, Brazil Bk and Brazil Ce, respectively (average of 14%).
A twofold vaccination with Mass vaccine A or B provided 94%, 85%, 91% and 90% protection of the trachea for against ciliostasis following challenge with strains Brazil Ar, Brazil Be, Brazil Bk and Brazil Ce, respectively (an average level of protection of 90%). The detectable level of renal replication of the challenge viruses in the groups with a twofold vaccination with Mass vaccines A or B was again the highest at 8.d.p.c. being 10%, 20%, 1% and 20% post challenge with strains Brazil Ar, Brazil Be, Brazil Bk and Brazil Ce, respectively (average of 13%).
The birds that had been vaccinated with Mass A or B and subsequently with the 793B vaccine showed a level of protection of 99%, 100%, 100% and 98% against tracheal damage by challenge strains Brazil Ar, Brazil Be, Brazil Bk and Brazil Ce, respectively (average of 99%). The detectable level of renal replication of the challenge viruses in the groups vaccinated with a Mass and a 793B vaccine was again the highest at 8 d.p.c. and was 0%, 3%, 0% and 3% post challenge with strains Brazil Ar, Brazil Be, Brazil Bk and Brazil Ce, respectively (average of 2%).
Statistical analyses showed that a single or double vaccination with a Mass vaccine reduced the percentage of IBV-positive kidneys significantly (P < .05) compared to the non-vaccinated birds. The results for the birds that had been vaccinated once or twice with Mass did not differ significantly from each other. The birds that were vaccinated with a Mass and subsequently with a 793B vaccine had the lowest level of IBV-positive kidneys; significantly less than in the birds that had been vaccinated once with Mass (P = .03) or twice with Mass (P = .02).
Discussion
Genotyping of the seven IBV strains used in this study showed that all belonged to the general Brazilian genotype and that these strains were most close to the subcluster of strain IBV/Brazil/2007/USP-19 (Villarreal et al., Citation2010) (). Interestingly, all S1-genes of the isolates that were detected in the different organs of the same chickens were not identical. Although an I332R amino-acid mutation was found to differentiate the kidney and the enteric/respiratory strains in this study, leading to the co-segregation of strains isolated from the kidneys in , this mutation cannot be regarded as a marker, due to the short segment of S1 sequenced and the small number of samples from diverse tissues analyzed herein.
Pathotyping of the four selected Brazilian strains showed that they all caused a considerable level of ciliostasis in the trachea but at a somewhat lower level than M41 and the four Brazilian strains 50/96, 57/96, 62/96, and 64/96 of four different serotypes that were reported by Cook et al. (Citation1999) and Di Fabio et al. (Citation2000). In this last paper these strains were coded differently, being 1, 8, 13 and 15, respectively. At 5 d.p.c., the average damage in the trachea after challenge of the 1-day-old SPF birds and 32-day-old SPF layer chickens was 85% and 74%, respectively ( and ), compared to 99% and 98% for the M41 reference strain and between 90% and 100% for strains 50/96, 57/96, 62/96, and 64/96 isolated in 1995. In contrast to the M41 challenge strain, all Brazilian strains replicated in the kidney tissue with accompanying interstitial nephritis in a high percentage of the non-vaccinated challenged birds of both ages showing clearly that the strains are nephropathogenic. The average percentage of IBV-positive kidneys was higher at 8 d.p.c. than at 5 d.p.c., as can be expected for nephropathogenic strains (Lambrechts et al., Citation1993; De Wit & Cook, Citation2014). Like the tracheal damage, the percentage of the non-vaccinated 32-day-old chickens that showed IHC-positive kidneys at 5 and 8 d.p.c. was on average about half that for the 1-day-old chickens. This lower percentage is most likely influenced by the difference in age since young chickens become more resistant to the nephropathogenic effects of IBV as age increases (Chong & Apostolov, Citation1982; Albassam et al., Citation1986; Butcher et al., Citation1990; Animas et al., Citation1994). The previous studies with the four Brazilian strains did not examine the kidney, which makes it impossible to compare the nephropathogenicity of the recent strains with the older strains.
As for the tracheal protection, the results of the Mass vaccinations followed by challenge with the recent isolates seemed to show on average higher protection than for the earlier strains (being 37%, 88%, 82% and 64%, Cook et al., Citation1999). A single or twofold vaccination with a Mass vaccine resulted in an average tracheal protection level against the four challenge strains of 92% and 90%, respectively, whereas a single and twofold vaccination with a Mass vaccine halved the percentage of infected kidneys (14% and 13%, respectively, P < .05) compared to that of the unvaccinated birds (27%). The combination of the Mass and the 793B vaccine provided an average tracheal protection of 99% and a reduction in the percentage of infected kidneys to an average of 2%. This was a significantly (P < .05) higher protection than that was achieved by a single or twofold Mass vaccination, showing the added value of the 793B vaccination following a Mass priming. These findings illustrate the importance of studying both tracheal and renal protection when nephropathogenic strains are used within a vaccination and challenge experiment, preferably using a test for the renal samples that can distinguish between local replication (such as IHC) and tests that might be positive due to viraemia or contamination from airsac at sampling of the kidney (De Wit & Cook, Citation2014).
References
- Abdel-Moneim, A.S., El-Kady, M.F., Ladman, B.S. & Gelb, J. (2006). S1 gene sequence analysis of a nephropathogenic strain of avian infectious bronchitis virus in Egypt. Virology Journal, 3, 78.
- Abreu, J.T., Resende, J.S., Flatschart, R.B., Folgueras-Flatschart, A.V., Mendes, A.C., Martins, N.R., Silva, C.B., Ferreira, B.M. & Resende, M. (2006). Molecular analysis of Brazilian infectious bronchitis field isolates by reverse transcription-polymerase chain reaction, restriction fragment length polymorphism, and partial sequencing of the N gene. Avian Diseases, 50, 494–501.
- Albassam, M.A., Winterfield, R.W. & Thacker, H.L. (1986). Comparison of the nephropathogenicity of four strains of infectious bronchitis virus. Avian Diseases, 30, 468–476.
- Animas, S.B., Otsuki, K., Tsubokura, M. & Cook, J.K.A. (1994). Comparison of the susceptibility of chicks of different ages to infection with nephrosis/nephritis-causing strain of infectious bronchitis virus. Journal of Veterinary Medical Science, 56, 449–453.
- Assayag, M.S.J., Chacon, J.L.V. & Ferreira, A.P. (2012a). Economic Impact of IB in a Brazilian Poultry Integration System. In M. Lierz, U. Heffels-Redmann, E.F. Kaleta & J. Heckmann (Eds.). Proceedings of the VII. International Symposium on Corona- and Pneumoviruses and Complicating Pathogens (pp. 80–83). Rauischholzhausen: Druckerel Schroder, Wetter, Germany.
- Assayag, M.S.J., Rocha, P.T. & Kuana, S. (2012b). Performance of Vaccines Containing Different Massachusetts Strains of IBV in the Slaughterhouse Condemnation. In M. Lierz, U. Heffels-Redmann, E.F. Kaleta & J. Heckmann (Eds.). Proceedings of the VII. International Symposium on Corona- and Pneumoviruses and Complicating Pathogens (pp 189–193). Rauischholzhausen: Druckerel Schroder, Wetter, German.
- Assayag, M.S.J., Rocha, P.T., Pedroso, A.C. & Pereda, A. (2012c). Epidemiology of IB and the Impact in the Condemnations in the Slaughterhouse. In M. Lierz, U. Heffels-Redmann, E.F. Kaleta & J. Heckmann (Eds.). Proceedings of the VII. International Symposium on Corona- and Pneumoviruses and Complicating Pathogens (pp. 74–79). Rauischholzhausen: Druckerel Schroder, Wetter, German.
- Brandão, P.E., Sandri, T.L., Souza, S.P., Kuana, S.L., Richtzenhain, L.J. & Villarreal, L.Y.B. (2009). Recombination, Point Mutations and Positive Selection on the Basis of the Molecular Diversity of Brazilian Strains of Avian Infectious Bronchitis Virus. In U. Heffels-Redmann & E.F. Kaleta (Eds.). The VI International Symposium on Avian Corona- and Pneumoviruses and Complicating Pathogens (pp. 47–58). Rauischholzhausen: Druckerel Schroder, Wetter, German.
- Butcher, G.D., Winterfield, R.W. & Shapiro, D.P. (1990). Pathogenesis of H13 nephropathogenic infectious bronchitis virus. Avian Diseases, 34, 916–921.
- Cavanagh, D., Ellis, M.M. & Cook, J.K.A. (1997). Relationship between sequence variation in the S1 spike protein of infectious bronchitis virus and the extent of cross-protection in vivo. Avian Pathology, 26, 63–74.
- Cavanagh, D., Mawditt, K., Britton, P. & Naylor, C.J. (1999). Longitudinal field studies of infectious bronchitis virus and avian pneumovirus in broilers using type-specific polymerase chain reactions. Avian Pathology, 28, 593–605.
- Chacon, J.L., Rodrigues, J.N., Assayag Jr., M.S., Peloso, C., Pedroso, A.C. & Ferreira, A.J. (2011). Epidemiological survey and molecular characterization of avian infectious bronchitis virus in Brazil between 2003 and 2009. Avian Pathology, 40, 153–162.
- Chong, K.T. & Apostolov, K. (1982). The pathogenesis of nephritis in chickens induced by infectious bronchitis virus. Journal of Comparative Pathology, 92, 199–211.
- Cook, J.K.A., Chesher, J., Baxendale, W., Greenwood, N., Huggins, M.B. & Orbell, S.J. (2001). Protection of chickens against renal damage caused by a nephropathogenic infectious bronchitis virus. Avian Pathology, 30, 423–426.
- Cook, J.K.A., Orbell, S.J., Woods, M.A. & Huggins, M.B. (1999). Breadth of protection of the respiratory tract provided by different live-attenuated infectious bronchitis vaccines against challenge with infectious bronchitis viruses of heterologous serotypes. Avian Pathology, 28, 477–485.
- De Wit, J.J., Boelm, G.J., van Gerwe, T.J.W.M. & Swart, W.A.J.M. (2013). The required sample size in vaccination-challenge experiments with infectious bronchitis virus, a meta-analysis. Avian Pathology, 42, 9–16.
- De Wit, J.J. & Cook, J.K.A. (2014). Factors influencing the outcome of infectious bronchitis vaccination and challenge experiments. Avian Pathology, 43, 485–497.
- De Wit, J.J., Cook, J.K.A. & van der Heijden, H.M. (2011a). Infectious bronchitis virus variants: a review of the history, current situation and control measures. Avian Pathology, 40, 223–235.
- De Wit, J.J., Guerrero, P., Calvo, J. & Hidalgo, H. (2012). Report of the Genotyping, Pathotyping and Protectotyping of Recent Strains from Chile. In M. Lierz, U. Heffels-Redmann, E.F. Kaleta & J. Heckmann (Eds.). Proceedings of the VII. International Symposium on Corona- and Pneumoviruses and Complicating Pathogens (pp 230–241). Rauischholzhausen: Druckerel Schroder, Wetter, German.
- De Wit, J.J., Nieuwenhuisen-van Wilgen, J., Hoogkamer, A., Van de Sande, H., Zuidam, G.J. & Fabri, T.H.F. (2011b). Induction of cystic oviducts and protection against early challenge with infectious bronchitis virus serotype D388 (genotype QX) by maternally derived antibodies and by early vaccination. Avian Pathology, 40, 463–471.
- Di Fabio, J., Rossini, L.I., Orbell, S.J., Paul, G., Huggins, M.B., Malo, A., Silva, B.G. & Cook, J.K.A. (2000). Characterization of infectious bronchitis viruses isolated from outbreaks of disease in commercial flocks in Brazil. Avian Diseases, 44, 582–589.
- Felippe, P.A., da Silva, L.H., Santos, M.M., Spilki, F.R. & Arns, C.W. (2010). Genetic diversity of avian infectious bronchitis virus isolated from domestic chicken flocks and coronaviruses from feral pigeons in Brazil between 2003 and 2009. Avian Diseases, 54, 1191–1196.
- Gelb, J., Weisman, Y., Ladman, B.S. & Meir, R. (2005). S1 gene characteristics and efficacy of vaccination against infectious bronchitis virus field isolates from the United States and Israel (1996 to 2000). Avian Pathology, 34, 194–203.
- Hall, B.G. (2013). Building phylogenetic trees from molecular data with MEGA. Molecular Biology and Evolution, 30, 1229–1235.
- Jackwood, M. & de Wit, J.J. (2013). Infectious Bronchitis. In G. Swayne, L.R. McDougald, L.K. Nolan, D.L. Suarez, V.L. Nair (Eds.). Diseases of Poultry 13th edn (pp. 139–159). Ames, IA: Blackwell Publishing Professional.
- Ladman, B.S., Loupos, A.B. & Gelb, J. (2006). Infectious bronchitis virus S1 gene sequence comparison is a better predictor of challenge of immunity in chickens than serotyping by virus neutralization. Avian Pathology, 35, 127–133.
- Lambrechts, C., Pensaert, M. & Ducatelle, R. (1993). Challenge experiments to evaluate cross-protection induced at the trachea and kidney level by vaccine strains and Belgian nephropathogenic isolates of avian infectious bronchitis virus. Avian Pathology, 22, 577–590.
- Meir, R., Rosenblut, E., Perl, S., Kass, N., Ayali, G., Perk, S. & Hemsani, E. (2004). Identification of a novel nephropathogenic infectious bronchitis virus in Israel. Avian Diseases, 48, 635–641.
- Villarreal, L.Y., Sandri, T.L., Souza, S.P., Richtzenhain, L.J., de Wit, J.J. & Brandao, P.E. (2010). Molecular epidemiology of avian infectious bronchitis in Brazil from 2007 to 2008 in breeders, broilers, and layers. Avian Diseases, 54, 894–898.
