ABSTRACT
Chicken astrovirus (CAstV) was recently indicated as the factor of the “white chicks” condition associated not only with increased embryo/chick mortality but also with weakness and white plumage of hatched chicks. In February 2014, organ samples (livers and kidneys) from dead-in-shell embryos, as well as 1-day-old whitish and normal chicks, were delivered from one hatchery in Poland for disease diagnosis. The samples originated from the same 30-week-old breeder flock in which the only observed abnormal signs were 4–5% decrease in the number of hatched chickens and the presence (about 1%) of weaker chicks with characteristic whitish plumage among normal ones. CAstV was detected in submitted samples and was then isolated in 10-day-old embryonated specific pathogen free (SPF) chicken eggs. We also reproduced an infection model for the “white chicks” condition in SPF layer chickens using the isolated PL/G059/2014 strain as the infectious agent. Results of experimental reproduction of the “white chicks” condition were somewhat more serious than field observation. The administration of the CAstV material into the yolk sac of 8-day-old SPF chicken eggs caused delay and prolongation of hatching, as well as death of embryos/chicks, and also a change of plumage pigmentation. Only two chicks of a total of 10 inoculated SPF eggs survived and were observed for 2 months. A gradual elimination of the CAstV genome was noted in this period. Moreover, a few contact-naive SPF chicks, which had been placed in the same cage, were infected with CAstV. Molecular characterization of detected CAstV was performed by nucleotide sequencing of the full ORF2 region encoding the capsid precursor protein gene. Phylogenetic studies showed that the PL/G059/2014 isolate clustered in the subgroup Aiii of CAstV. In the light of the new classification rules, the Polish PL/G059/2014 CAstV isolate could be assigned to a new species of the Avastrovirus genus.
Introduction
Poultry farming is a very important sector of food production of many industrialized and developing countries. Maintaining good health status when raising poultry is an imperative for financial stability and production success. Despite applied immunoprophylaxis and biosecurity an unexpected “failure” may occur. The cause of some health problems has remained unknown but more sophisticated molecular techniques for the determination of their aetiological factors may allow the study of viral factors of enteritis, including astroviruses.
Astroviruses are small, round, nonenveloped viruses with a star-like morphology and a diameter of 25–35 nm. Their genome constitutes linear, positive-sense ssRNA with about 7 kb of size (Carter & Willcocks, Citation1996; Denison et al., Citation1998). Historically, astroviruses are known to cause enteritis not only in humans but also in various animal species such as chickens, turkeys, sheep, cattle, swine, dogs, cats, mice and others (Schultz-Cherry, Citation2013). In poultry they have caused enteritis combined with growth depression and higher mortality (McNulty et al., Citation1984; Yu et al., Citation2000; Jindal et al., Citation2010) but their presence was also described in healthy flocks of chickens and turkeys (Day et al., Citation2007; Domanska-Blicharz et al., Citation2011, Citation2014). In addition to their role in enteritis, astroviruses can also cause other disease conditions of poultry. Duck astrovirus (DAstV) infections trigger hepatitis with high morbidity and mortality of ducklings (Gough, Citation1984). Infections of chickens with avian nephritis virus (ANV) cause diarrhoea, growth retardation, kidney damage and gout resulting in increased mortality (Shirai et al., Citation1992; Bulbule et al., Citation2013). Astroviruses were also detected in domestic geese, guinea fowl, pigeons and different species of wild aquatic birds (Cattoli et al., Citation2007; Zhao et al., Citation2011; Bidin et al., Citation2012a, b; Chu et al., Citation2012). Chicken astroviruses (CAstV) are distributed worldwide and mostly associated with above-mentioned enteritis and recently also with gout, but sometimes their presence is not connected with disease signs (Pantin-Jackwood et al., Citation2006; Domanska-Blicharz et al., Citation2011; Citation2014; Jindal et al., Citation2011; Roussan et al., Citation2012; Smyth et al., Citation2012, Bulbule et al., Citation2013; Moura-Alvarez et al., Citation2013).
Astroviruses detected in various species of birds belong to the Astroviridae family, the Avastrovirus genus. At first, they were further divided into separate species depending on the host from which they originated. According to these criteria, six different astroviruses were identified in avian species, in turkeys: the turkey astrovirus type 1 (TAstV-1) and type 2 (TAstV-2), in chickens: ANV and the chicken astrovirus, and in ducks: DAstV type 1 (DAstV-1) and type 2 (DAstV-2). However, because that astroviruses could be transmitted between different species, the rule of their classification was changed to a system based on the amino acid structure of the viral capsid protein (http://ictvonline.org/proposals/2010.017a-cV.A.v3.Avastrovirus.pdf). Currently, astroviruses detected in avian species belong to three official avastrovirus species: 1, 2, and 3 (http://ictvonline.org/virustaxonomy.asp).
During the XVIIIth Congress of the World Veterinary Poultry Association (WVPA) in Nantes a new disease syndrome, named the “white chicks” condition was reported by Smyth et al. (Citation2013a). This condition was observed in various European and non-European countries where in some hatcheries increased embryo mortality was noted and hatched chicks were weak with white tinged feathers. In affected embryos/chicks subcutaneous oedema was observed and the most severe lesions were located in livers, which were greenish or mottled. In hatcheries, among hatched offspring a percentage of chicks with white plumage next to the “normal” ones were observed. Molecular studies have indicated CAstV as the aetiological factor of the observed “white chicks” condition (Smyth et al., Citation2013b). For easier identification of CAstV causing the “white chicks” condition, the abbreviation WC-CAstV will be used in the text in contrast to CAstV in general.
In this paper we describe the “white chicks” condition identified in a hatchery in western Poland. An attempt at reproducing this condition in SPF chicks as well as the molecular characteristics of the detected astrovirus will be also outlined.
Materials and methods
Case description
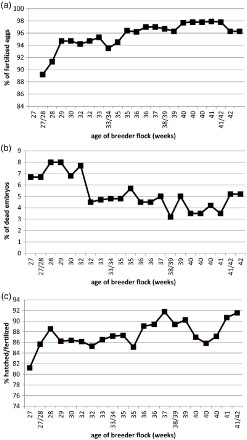
In February 2014, liver and kidney samples of 1-day-old chicks originating from one 30-week-old parent flock were delivered to the Poultry Diseases Department of National Veterinary Research Institute (NVRI) in Pulawy. Reduced hatchability and differences in appearance (white plumage) and condition (weaker) among hatched birds were observed (). The received samples were segregated into three groups: the organs of “normal” hatched chicks (N), chicks with white plumage colour (W) and the organs of dead embryos (D). The analysis of the breeder flock's performance parameters, monitored for a period of 15 months did not indicate any serious disturbances ((a)–(c)). The percentage of fertilized eggs from 28-week-old hens increased from approximately 92% to more than 98% 15 months later and was close to expected values. Similarly, the number of dead embryos occurring in the first week of development originating from a 27-week-old breeder flock was about 7% and 15 months later about 4% and such values were typical for this age of flock. The only slightly changed parameter was the 4–5% decrease in the ratio of hatched chicks to fertilized eggs, which had been set in the incubator during a 4 week period beginning from the 30th week of age, in comparison to previous flocks. Ten randomly selected layers from this breeder flock were swabbed only once when they were 44 weeks old. The presence of chicks with changed, white plumage among normal ones was observed in the hatchery for approximately 4 weeks. However, their number was not high and accounted for 1% at the peak of their appearance.
Figure 1. One-day-old chicks originating from the flock of breeder chickens at the age of 30 weeks. Numerous chicks with whitish plumage appear next to the normal ones.

Figure 2. Selected parameters of breeder flock performance recorded for a 15 weeks period: (a) the percentage of fertilized eggs, (b) the percentage of embryos which died in the first week of development, and (c) the ratio of hatched chicks to fertilized eggs set in the incubator.
In the hatchery careful selection of chicks was applied to eliminate weak ones with white plumage. The other ones, which looked normal, were devoted to rearing. Performance parameters of three flocks with normal broilers originating from this selection were monitored. These chicken broiler flocks came from the same breeders at the age of 30, 32 and 34 weeks. The health status and performance parameters of these flocks were within the norm according to the veterinarians taking care of them. The presence of individuals with lameness and fibrinous inflammation of the serous membranes and a period of mild diarrhoea were observed for a short time. Huddling of chickens in groups close to the henhouse walls was also noted. Various samples (cloacal swabs or internal organs) from these three broiler flocks at different ages (8–40 days) were additionally provided. Each flock was sampled twice during the production cycle. All samples were stored at −20°C until used for studies.
Sample processing
The received organ samples were homogenized in phosphate-buffered saline (PBS) (10% wt/vol) with antibiotics (2000 U/ml of penicillin and 2 mg/ml of streptomycin). Cloacal swabs from the flock were pooled in two samples (10 swabs into two pools of five each) also in PBS but with a higher concentration of antibiotics. The swabs and homogenates were clarified by centrifugation at 4°C for 15 min. at 1500 g. For the RNA isolation 200 µl of the supernatant was collected. Viral RNA was extracted using commercial GeneMATRIX Universal RNA Purification Kit (EURx Ltd., Gdańsk, Poland) according to the manufacturer's instructions.
Molecular methods for detection of CAstV and other avian pathogens
Detection of CAstV genome was performed by real-time RT-PCR (rRT-PCR) according to Smyth et al. (Citation2010) with primers and probe previously described as useful for detection of all CAstV types (universal CAstV). Primers spanned the 70 bp section of the intragenomic region between ORF1b/ORF2 (from 4920 to 4989 nt of the entire GA2011 strain genome sequence, GenBank Accession No. JF414802). The rRT-PCR was performed using with the QuantiTect Probe RT-PCR Kit (QIAGEN, Hilden, Germany), using the ABI 7300 device (Applied Biosystems Inc., Foster City, CA, USA). The submitted samples were also tested for the presence of other avian pathogens: fowl adenovirus, infectious bronchitis virus, infectious bursal disease virus, avian reovirus, rotavirus and parvovirus (Zierenberg et al., Citation2001; Callison et al., Citation2006; Day et al., Citation2007; Wang et al., Citation2009; Zsak et al., Citation2009; Gunes et al., Citation2012).
Virus isolation in SPF embryos
For virus isolation, six 10-day-old SPF chicken embryos (VALO BioMEDIA, Osterholz-Scharmbeck, Germany) were inoculated onto the chorioallantoic membrane (CAM) with organ sample material of dead embryos (D) (0.2 ml/egg) prepared as described above and filtered through a 0.45-μm syringe filter. The inoculated eggs were incubated at 37°C and candled daily for 5 days. Embryonic death occurring within one day of inoculation was considered to be non-specific. After cooling, the embryos were subjected to a thorough assessment and CAM, allantoic fluids and altered organs were harvested and stored at −70°C for further testing. The isolated virus was designated PL/G059/2014.
Molecular and phylogenetic analysis of detected CAstV
The rRT-PCR positive samples were then tested in one-step RT-PCRs in an attempt to characterize detected virus by sequence analysis and to distinguish the astrovirus suspected to be responsible for “white chicks” condition from other CAstV. The sequences of self-designed primers, applied annealing temperatures and sizes of obtained products are listed in . The RT-PCRs were conducted using the Qiagen One Step RT-PCR Kit (QIAGEN) and the mixture contained 1 μl of the enzyme mix, 5 μl of the 5× One Step RT-PCR buffer, 0.5 mM of each dNTP, 0.5–0.75 mM of each primer (depending on protocol), 0.2 M of betaine solution, 2.5 μl of RNA sample and water to the total volume of 25 μl. The RT-PCR conditions involved a RT step of 48°C for 30 min, an initial denaturing step of 95°C for 15 min, followed by 35 cycles each comprising denaturation at 94°C for 30 s, annealing at different temperatures depending on the primers used () for 30 s and extension at 72°C for 1 min with the step of final extension of 7 min. The resulting products were loaded onto a 2% agarose gel with the addition of ethidium bromide (1 mg/ml) and electrophoresed for approximately 1 h in 130–150 V and then visualized in UV light using a system for gel visualization and analysis (MiniBIS PRO, DNR Bio-Imaging Systems Ltd., Jerusalem, Israel).
Table 1. Primers, products sizes and annealing temperatures used in the study.
The product from RT-PCR reactions was sequenced in both directions by the commercial service Genomed (Warsaw, Poland). The obtained forward and reverse nucleotide sequences were edited and aligned in the final consensus sequence using the SeqMan (DNASTAR, Madison, WI, USA). Multiple alignments of the novel amino acid sequence and several isolates available in the GenBank database (www.ncbi.nlm.nih.gov) were constructed with the use of the Clustal W method using MEGA6 software. A phylogenetic tree was constructed by the neighbour-joining method.
Preliminary experimental reproduction of the condition, clinical signs and cloacal swab samples
Ten 8-day-old SPF chicken embryos were inoculated via the yolk sac with PL/G059/2014 material (allantoic fluid) diluted in PBS (50% vol/vol). Six embryos were inoculated with only PBS and served as the controls. After being sealed with paraffin wax, the eggs were incubated at 37°C and 55% relative humidity with rotation up to the 17th day of embryonic development. During this period the eggs were inspected daily for dead embryos. Live embryos were moved to the hatcher with an appropriately decreased temperature and humidity. The hatching progress, that is, the start of ‘pipping', the number of hatched chicks, their condition and appearance, length of hatching process and embryonic deaths were carefully observed for the period of 8 days from the 18th day of embryo development to the end of hatch. The hatched chicks were moved to an open cage and six days after the hatch, two SPF chickens (contact) of the same age were introduced to the group and kept for 45 days. The hatcher as well as the open cage were in the same isolation room of a BSL-3 biosafety level animal facility where negative air pressure was maintained. Cloacal swabs were collected from infected chicks on the first day of life (meconium) and from contact birds on the 6th day of life just before their introduction to infected ones and then every 3–5 days to check for virus presence. Dead embryos were necropsied and their internal organs (liver, kidney, pancreas, digestive and respiratory tracts) were removed for virus detection. These experiments were approved by the II Local Ethical Committee of the University of Life Sciences in Lublin, Poland (permission no. 19/2015).
Accession number
The gene sequences were submitted to the GenBank database and were assigned the following accession number: KR052479.
Results
CAstV detection in the field samples and virus isolation
CAstV genome was identified with the universal CAstV-rRT-PCR in RNA extracted from supernatants of kidney and liver samples of the 1-day-old “white chicks” (W) and dead embryos (D) collected in the field but not from internal organ samples of “normal” chickens (N). Three chicken broiler flocks, originating from the same breeder flock, were sampled twice during the production cycle. All cloacal swabs from the first sampling and only one from the second sampling were CAstV-positive. Swabs from 40- and 17-day-old broilers, which originated from the breeder flock at the age of 30- and 34-weeks, respectively, were negative.
The results of conventional RT-PCR test with self-designed primers, targeting specific regions of the “white chicks” CAstV genome, confirmed its presence in chicks with white plumage colour (W) and the organs of dead embryos (D) as well as in samples from inoculated SPF embryos. However, RT-PCR/WC-CAstV was negative for most of the pools of swabs from field broiler flocks, except for two of them from 1- and 8-day-old broilers originating from the breeder flock at the age of 30- and 37-weeks, respectively ().
Table 2. Data on examined broiler flocks originating from the same breeder flock.
Infected SPF embryos sacrificed on the fifth day after inoculation showed slight inhibition in development, and were congested with visible subcutaneous oedema. Significant changes were observed in the liver, which was enlarged and congested. There was also a greenish exudate in the body cavity. Samples collected from the infected embryos, that is, CAM, allantoic fluid and tissues/organs were positive by rRT-PCR/CAstV and RT-PCR/WC-CAstV. Moreover, molecular results revealed the highest quantity of the virus genome in embryonic parenchymal organs (liver, pancreas, spleen with a Ct value of 16) and digestive system (Ct value of 20) compared to the respiratory system (Ct value of 26).
The RNA/DNA eluted from field samples, as well as from infected embryos, was negative for infectious bronchitis virus, infectious bursal disease virus, avian reovirus, rotavirus, adenovirus and parvovirus by RT-PCR or PCR.
Reproduction of “white chicks” condition
Inoculation of material isolated from “white chicks” into the yolk sac of 8-day-old embryonated SPF chicken eggs generally resulted in problems with hatchability (embryo/chick mortality) and weakness of hatched chicks. Out of 10 inoculated chicken embryos, one died within the first day of inoculation, which was considered to be non-specific. The remaining nine live embryos were moved from the incubator to the hatcher on the 17th day of embryo development. The hatching period was delayed and prolonged as the first chicks pipped on the 22nd day and the last ones on the 25th day of embryonic development. Three live chicks hatched on the 22nd day and the next one on the 23rd day of development, but the latter was very weak and died the same day. Out of the remaining five embryos, three began the hatching process as eggs shells were partially cracked, however they were too weak to emerge and two died on the 23rd day and the third on the 25th day of development. Two embryos were found dead inside the shells on the 25th day of incubation. Out of three chicks hatched on the 22nd day, two were visually normal and in good condition, but the third clearly differed in appearance (smaller and with whitish plumage) and health status (weak) from the others. This white chick died on the 6th day of life.
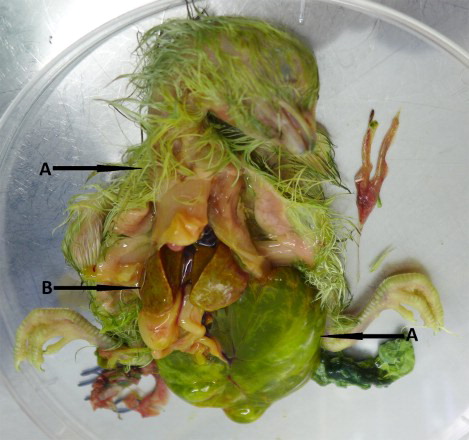
In three embryos which died during the hatching process the most visible changes were the presence of green colour in their plumage, allantoic fluids and remains of yolk sacs (). Urate deposits were also found on the body surface. Oedema, mainly gelatinous, was seen in subcutaneous tissues around the embryos’ necks and heads. Most lesions were observed in livers which were enlarged with a pale colour, brittle consistency and numerous pinpoint haemorrhages on the surface (). Kidneys, spleens and pancreas were enlarged and very congested. In one embryo greenish content of the stomach and bloodshot intestines were also found. Two embryos died inside the shells, in one abdominal and brain hernia were additionally observed, and the other one was clearly undeveloped with strongly congested organs. Unfortunately, two hatched live chicks died at 1- and 6-days post hatch during the night and were under the heat source until morning, making it impossible to examine the dried carcasses for lesions. The embryo inoculation method seemed to be safe as the hatchability and the health status of the hatched chicks were within the norm. From six control eggs five chicks hatched on the 21st day of embryonic development and the one which died revealed no lesions. High quantities of CAstV RNA were detected in allantoic fluids and organs of dead embryos with a Ct value around 17–24. The contents of the dried carcasses of the hatched chicks which died on the 1st and 6th day of their life gave weak CAstV-positive results with a Ct value around 34.
Figure 3. Gross lesions in dead embryos found during experimental reproduction of “white chicks” condition. (a) Green colour of plumage, allantoic fluid and remains of yolk sac of WC-CAstV-infected embryo and (b) enlarged, pale liver with numerous pinpoint haemorrhages on the surface.
Two live infected chickens and two additional SPF chickens of the same age, introduced to the infected ones, were carefully observed for the period of 35 days and no clinical signs were noted. CAstV-specific RNA was not detected in the meconium of the hatched specimens, but beginning from the 3rd day after hatching, all the collected cloacal swabs were positive. Detectable CAstV RNA decreased with the age of the chicken with the youngest chicks yielding the most and with no virus detection in 35-day-old birds. The 6-day-old contact chickens were CAstV-negative at the day of their introduction to the infected group, but were detected positive from the 3rd to 24th day after contact by rRT-PCR/CAstV. No viral RNA was found in swabs of 25-day-old infected chickens and 35-day-old contact birds (29 days after contact) ().
Table 3. Cloacal shedding of WC-CAstV in chickens hatched from eggs experimentally infected via the yolk sac at 8 day of embryonic development and in contact birds.
Molecular characterization
Successful amplification of a 2500 nt fragment of the WC-CAstV genome spanning about 300 nt from the it should be the 3' end of ORF1b end of ORF1b and all ORF2 was achieved with samples originating from liver and kidney homogenates of 1-day-old “white chicks” (W), originally delivered for disease recognition, as well as from organs of two dead 23-day-old embryos from the experimental infection. All the obtained sequences were identical to the PL/G059/2014 isolate. The capsid gene the of PL/G059/2014 strain consists of 2159 nt encoding a protein of 719 amino acids. Phylogenetic analysis of capsid protein using the parameters recommended by the Astroviridae Study Group of the International Committee on Taxonomy of Viruses with the MEGA 6 software (Neighbour-Joining method, evolutionary distances: p-dist method corresponding to amino acid substitutions per site, pairwise deletion option) showed that PL/G059/2014 belonged to the group previously described as Aiii (). In this group the capsid protein ranged from 717 to 721 amino acids with 721 aa-long capsid protein of the British VF08-18/7 and VF08-48 strains and a 717 aa-long capsid of the South-African 612 strain. The pairwise comparison of nucleotides and amino acid identities among AvianAstVs groups formed by the neighbour-joining tree are shown in . The nucleotide and amino acid identities of strains in group A were 76.2–99.3% and 78.2–99.2%, respectively. PL/G059/2014 clustered in subgroup Aiii together with VF08-65 strain with 98.3% nt and aa homology. Nucleotide and aa identities with CAstVs of groups Ai and Aii were 79.8–80.4% and 79.0–80.1% and 82.4% and 82.1%, respectively. Comparison of nt and aa structure of group A to strains from group B revealed low similarity. They shared nt and aa similarity only at the level of 48.4–53.5% and 47.6–48.3% and 39.5–40.1% and 40.3–40.8% with Bi UK and Indian strains, respectively. Nucleotide and aa identity with strains of Bii subgroup were from 47.2% to 54.4% and from 35.6% to 39.3%, respectively. However, the capsid protein length in group B was more diverged, from 664 aa of CAstV 4175 strain to 743 aa of GA2011 strain. Comparisons of PL/G059/2014 with other species of avian astroviruses showed that it shared nt and aa similarities of, respectively 45.5% and 38.6% with Avastrovirus 1 (TAstV-1). Nucleotide identity with representatives of Avastrovirus 2, namely ANV-1 and ANV-2, were 43.2% and 44.0%, respectively, and aa identity 26.7% and 27.0%, respectively. In turn, nt and aa homology with Avastrovirus 3 representative were higher and were 54.3% and 42.7% with TAstV-2 and 57.1% and 43.7% with DAstV, respectively.
Figure 4. Phylogenetic tree of the complete amino acid sequences of the AvianAstV ORF2 available in GenBank constructed with the Clustal W method.
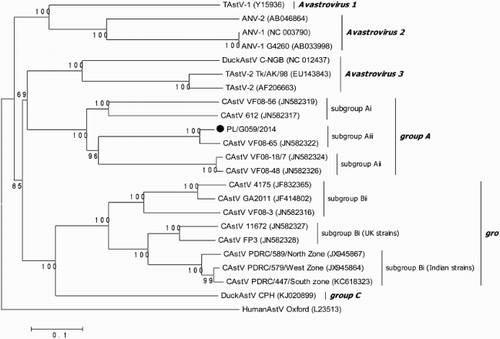
Table 4. Pairwise comparison of nucleotide and amino acid (in brackets) identities among AvianAstVs groups formed by the neighbour-joining tree.
The presence of conserved and variable regions in N- and C-terminal sequences of the capsid protein of CAstVs was also demonstrated. The conserved regions were displayed in N-termini between residues 1 and 415 (similarity of 99.5% with VF08-65 strain and 83.4–88.1% with the rest of group A strains) and C-termini between residues 654 and 719 (similarity of 100% with the VF08-65 strain and 92.6–95.8% with rest of group A strains). There was a variable region span between 416 and 653 aa with identity of 95.7% to VF08-65 strain and 68.5–69.5% with the rest of A group strains. The percentage of dissimilarity was increased when capsid proteins between PL/G015/2014 and strains of B group were compared.
Discussion
In this paper, we described the isolation, identification and molecular characterization of an astrovirus strain from livers and kidneys of dead-in-shell embryos as well as from dead and living one-day-old chickens with a disease condition characterized by white colour of plumage identified in one hatchery in Poland. We also reproduced an infection model for the “white chicks” condition in SPF layer chickens using the strain PL/G059/2014 as the infectious agent. In the initial study, embryo mortality as well as whitish plumage of feathers similar to that observed in the hatchery were seen in chickens hatched from eggs inoculated into the yolk sac of 8-day-old embryonated SPF chicken eggs.
The first report of the “white chicks” condition caused by the CAstV infection was presented at the XVIIIth Congress of WVPA in Nantes (Smyth et al., Citation2013a). This condition was observed in different countries, including Finland, Norway, Sweden and Canada where increased embryo mortality was observed in some hatcheries and hatched chicks were weaker with white plumage and rarely survived longer than 24 hours. In Finland, the first case occurred in 2006 and cases have been observed on many farms every year since then. In the parent flocks, 30–40 weeks of age, a marked, approximately 3–10% decline in egg production, lasting for up to two weeks was observed, although in flocks of a poorer hygienic status recovery may take longer. The hatchability from eggs of some flocks was reduced by 29% for a two week period, although in one case the reduction reached up to 68% (Smyth et al., Citation2013b). In the Polish hatchery these problems were not as serious and were identified only because of a careful observation of the hatch parameters by the hatchery manager. Accurate data of this hatchery performance were gathered for months and allowed us to gain the standard baseline. We noticed a slight 4–5% decrease of eggs hatchability and the presence of 1% of whitish chicks among normal ones for the period of 4 weeks in case of eggs which had been delivered from only one breeder flock of 30 weeks of age, similar to the ones previously observed in Nordic countries and Canada. No decline in eggs production or disease signs were observed in the affected breeder flock in Poland. Such a wide range of observed disease signs and deviation of hatchability parameters may result from different zootechnical housing conditions of birds on the farm and/or health status of layers which could be influenced by any other parasitic, bacterial or viral infections.
From 10 embryonated SPF chicken eggs infected into the yolk sac only two chickens survived for a longer time, so it seems that the results of preliminary experimental reproduction of the “white chicks” condition were somewhat more serious than field observations. The administration of CAstV material into SPF chicken eggs caused delay and prolongation of hatch as well as death of embryos/chicks and also change of plumage pigmentation. Half of the inoculated embryos died either during development (two embryos) or during emergence from eggshells (three embryos) and among four hatched chicks, one hatched with yellow plumage and died at one-day-old, and another one hatched with white plumage died at 6-days-old. The observed problems with hatchability were specifically caused by virus activity and not associated with the yolk-sac administration technique since hatchability and chick quality from the eggs inoculated with PBS were good. The role of astrovirus as a possible factor in chick hatchability problems was indicated previously. The British FP3 and 11672 viruses recently assigned as CAstVs were isolated, respectively, from the meconium of dead-in-shell chick embryos and weak chicks during the investigation of hatchability problems (Spackman et al., Citation1984; Todd et al., Citation2009a; Todd et al., Citation2009b). The possibility of astrovirus-induced mortality of duck and goose embryos was also recently described in Croatia. The astroviruses detected in dead-in-shell goslings and ducklings were identified as ANV and TAstV-1, respectively (Bidin et al., Citation2011, Citation2012b). It is not known if these previously reported astrovirus infections of poultry embryos were horizontally or vertically transmitted. An infectious agent could reach eggs from a parent during fertilization by semen, during egg development in the oviduct of the hen or immediately after oviposition. Some infectious organisms can pass through the eggshell because of contact with contaminated faeces, urates or bedding. In the case reported here, the fact that hatchability problems were associated with eggs originating from only one breeder flock, the periodicity of the occurrence of the “white chicks” condition and especially preliminary experimental reproduction of this health problem may be an indication of vertical astrovirus transmission, but further studies are required to elucidate this. Cloacal swabs from 44-weeks-old layers, the only investigated samples of the suspected breeder flock, were CAstV-negative but it seems that this flock was the most probable source of astrovirus infections of embryos. In our experiment the PL/G059/2014 virus shedding via the cloacal route of chicks infected during embryonic development and contact birds lasted 3 and 4 weeks, respectively. This is consistent with the results of Toffan et al. (Citation2012) revealing a long period of viral excretion of astrovirus lasting up to 25 dpi. Problematic birds with the “white chicks” condition originated from 30- to 34-week-old breeder layers, so at the age of 44 weeks they were already clearly free of the virus. Such a period of virus shedding could also explain the results which we obtained through testing samples from three chicken broiler flocks originating from layers of the same breeder flock but of different age. Younger broilers at the age of 1 and 8 days were positive for WC-CAstV but older ones, 17-, 25-, 38- and 40-day-old ones were negative.
Of interest is the appearance of chickens with white plumage colour as a result of astrovirus infection. The pigment of plumage in bird embryos, that is, melanin, is produced by melanocytes that arise from precursor cells in the neural crests and migrate during early embryonic development to their ultimate sites of residence. Ocular melanocytes of the retinal and iridial pigment epithelium and the pectin arise from the outer layer of the embryonic optic cup (Smyth Citation1990). The albino birds have a defect in melanin synthesis that results in partial or complete absence of this pigment in the skin, feather and eyes (Chang et al., Citation2006). Chickens from the same breeder flock, after the rejection of weaker ones and those with white plumage, were intended for breeding but they behaved differently, in that they huddled in groups close to the henhouse walls. However, the huddling did not seem to be caused by impaired thermoregulation as increased temperatures typically applied in such a situation did not give expected results. Interestingly, the birds spread throughout the house following a decrease in the intensity of light as much as 50%, so the huddling may be caused more by disturbances in the sensitivity to light (photophobia) than in the thermoregulation. Disturbances in light sensitivity observed in “normal” broiler chicks, which originated from the infected breeder flock, might be the result of only “partially” impaired melanin levels. In the chicken, there are different degrees of depigmentation, and consequently several types of albinism. The lack or reduced melanin levels during embryogenesis could result in impairing impaired visual receptors and brain (Smyth Citation1990). Recently, impaired sight in chickens with the albino mutation was described (Jorge & Cunha, Citation2008). The mechanism and cause of pigmentation change in the “white chicks” condition is not known.
Molecular characterization of detected CAstV was performed by nucleotide sequencing of the full ORF2 region encoding the capsid precursor protein. The phylogenetic analysis based on ORF2 showed that the PL/G059/2014 isolate clustered in subgroup Aiii. Such an affiliation is in contrast to results of Smyth et al. (Citation2013a) and Smyth et al. (Citation2013) in which the CAstVs detected from “white chicks” syndrome were assigned to the group B CAstVs. Unfortunately, their sequence is unavailable, so the estimation of their molecular relationship was not possible. However, it should be mentioned that the field material delivered to the laboratory could contain different populations of CAstV and the isolation technique would have allowed for the isolation of only one of them. It is also possible that the sequencing technique sequenced one strain only and did not detect any other CAstV populations. CAstV of different group or subgroup could be present in the same broiler flock (Smyth et al., Citation2012) Similarly, antibodies elicited by various CAstV groups were detected in broiler and parent flocks (Todd et al., Citation2009a; Todd et al., Citation2009b). Also in our studies we detected CAstVs different from PL/G059/2014 in broilers originating from the suspected breeder flock and these infections were probably acquired during the broilers’ growth on the farm, in spite of the fact that these flocks were in good health. Differences in CAstV-induced signs/disorders could be determined not only by varying virus pathogenicity but also different route of inoculation, age and strain of birds, and the level of antibodies, maternally derived or acquired during their life. Our knowledge about biology of astroviruses is poor and only a few full sequences have been described. The availability of these few sequences suggests that avian astroviruses may employ a different replication strategy which in turn could differently affect their biology, including the pathogenicity of the virus (Kang et al., Citation2012). Further work is needed to determine the full genome sequence of the detected astrovirus and also the prevalence of these viruses in the poultry population.
It should be also mentioned that in the light of the new classification rules, the Polish PL/G059/2014 CAstV studied here could be assigned to a new species of the Avastrovirus genus. Probably within this genus more than three official species exist as in the Mamastrovirus genus which is further divided into 19 species. Thus there is a need for new species arrangement within the Avastrovirus genus, as recently reported by Smyth et al. (Citation2012).
In conclusion, our paper described the occurrence of the “white chicks” condition in Poland. This condition was characterized by hatchability problems (increased embryo mortality and the presence of white plumage among hatched chicks) observed in one hatchery and affecting only eggs from one breeder flock. The virus belonged to the subgroup Aiii of the Avastrovirus genus. It was isolated from livers and kidneys of dead-in-shell embryos as well as of dead and living one-day-old chicks. We also reproduced an infection model for the “white chicks” condition in SPF layer chickens using the strain PL/G059/2014 as the infectious agent.
Acknowledgements
We would like to thank Monika Olszewska and Michal Jozwiak from the Department of Poultry Diseases, National Veterinary Research Institute in Pulawy for their excellent veterinary works.
References
- Bidin, M., Bidin, Z., Majnaric, D., Tisljar, M. & Lojkic, I. (2012a). Circulation and phylogenetic relationship of chicken and turkey-origin astroviruses detected in domestic ducks (Anas plathyrhynchos domesticus). Avian Pathology, 41, 555–562. doi: 10.1080/03079457.2012.733340
- Bidin, M., Lojkic, I., Bidin, Z., Tisljar, M., Majnaric, D. & Mikec, M. (2011). Detection and characterization of avian nephritis virus in ducklings. Avian Pathology, 40, 173–177. doi: 10.1080/03079457.2011.551873
- Bidin, M., Lojkic, I., Tisljar, M., Bidin, Z. & Majnaric, D. (2012b). Astroviruses associated with stunting and pre-hatching mortality in duck and goose embryos. Avian Pathology, 41, 91–97. doi: 10.1080/03079457.2011.642796
- Bulbule, N.R., Mandakhalikar, K.D., Kapgate, S.S., Deshmukh, V.V., Schat, K.A. & Chawak, M.M. (2013). Role of chicken astrovirus as a causative agent of gout in commercial broilers in India. Avian Pathology, 42, 464–473. doi: 10.1080/03079457.2013.828194
- Callison, S.A., Hilt, D.A., Boynton, T.O., Sample, B.F., Robison, R., Swayne, D.E. & Jackwood, M.W. (2006). Development and evaluation of a real-time Taqman RT-PCR assay for the detection of infectious bronchitis virus from infected chickens. Journal of Virological Methods, 138, 60–65. doi: 10.1016/j.jviromet.2006.07.018
- Carter, M.J. & Willcocks, M.M. (1996). The molecular biology of astroviruses. Archives of Virology. Supplementa, 12, 277–285.
- Cattoli, G., De Battisti, C., Toffan, A., Salviato, A., Lavazza, A., Cerioli, M. & Capua, I. (2007). Co-circulation of distinct genetic lineages of astroviruses in turkeys and guinea fowl. Archives of Virology, 152, 595–602. doi: 10.1007/s00705-006-0862-4
- Chang, C.M., Coville, J.L., Coquerelle, G., Gourichon, D., Oulmouden, A. & Tixier-Boichard, M. (2006). Complete association between a retroviral insertion in the tyrosinase gene and the recessive white mutation in chickens. BMC Genomics, 7, Article no. 19. doi: 10.1186/1471-2164-7-19
- Chu, D.K., Leung, C.Y., Perera, H.K., Ng, E.M., Gilbert, M., Joyner, P.H., Grioni, A., Ades, G., Guan, Y., Peiris, J.S. & Poon, L.L. (2012). A novel group of avian astroviruses in wild aquatic birds. Journal of Virology, 86, 13772–13778. doi: 10.1128/JVI.02105-12
- Day, J.M., Spackman, E. & Pantin-Jackwood, M. (2007). A multiplex RT-PCR test for the differential identification of turkey astrovirus type 1, turkey astrovirus type 2, chicken astrovirus, avian nephritis virus, and avian rotavirus. Avian Diseases, 51, 681–684. doi: 10.1637/0005-2086(2007)51[681:AMRTFT]2.0.CO;2
- Denison, M.R., Sims, A.C., Gibson, C.A. & Lu, X.T. (1998). Processing of the MHV-A59 gene 1 polyprotein by the 3C-like proteinase. Advances in Experimental Medicine and Biology, 730, 121–127. doi: 10.1007/978-1-4615-5331-1_16
- Domanska-Blicharz, K., Seroka, A. & Minta, Z. (2011). One-year molecular survey of astrovirus infection in turkeys in Poland. Archives of Virology, 156, 1065–1072. doi: 10.1007/s00705-011-0958-3
- Domanska-Blicharz, K., Jacukowicz, A., Bocian, L. & Minta, Z. (2014). Astroviruses in Polish commercial turkey farms in 2009–2012. Avian Diseases, 58, 158–164. doi: 10.1637/10611-070813-ResNote.1
- Gough, R.E. (1984). Laboratory confirmed outbreaks of duck virus enteritis (duck plague) in the United Kingdom from 1977 to 1982. Veterinary Record, 114, 262–265. doi: 10.1136/vr.114.11.262
- Gunes, A., Marek, A., Grafl, B., Berger, E. & Hess, M. (2012). Real-time PCR assay for universal detection and quantitation of all five species of fowl adenoviruses (FAdV-A to FAdV-E). Journal of Virological Methods, 183, 147–153. doi: 10.1016/j.jviromet.2012.04.005
- Jindal, N., Patnayak, D.P., Chander, Y., Ziegler, A.F. & Goyal, S.M. (2010). Detection and molecular characterization of enteric viruses in breeder turkeys. Avian Pathology, 39, 53–61. doi: 10.1080/03079450903490289
- Jindal, N., Patnayak, D.P., Chander, Y., Ziegler, A.F. & Goyal, S.M. (2011). Comparison of capsid gene sequences of turkey astrovirus-2 from poult-enteritis-syndrome-affected and apparently healthy turkeys. Archives of Virology, 156, 969–977. doi: 10.1007/s00705-011-0931-1
- Jorge, W. & Cunha, L.M. (2008). Inheritance of a new albino mutation in Brazilian free-range black chickens. Brazilian Journal of Poultry Science, 10, 153–156.
- Kang, K.I., Icard, A.H., Linnemann, E., Sellers, H.S. & Mundt, E. (2012). Determination of the full length sequence of a chicken astrovirus suggests a different replication mechanism. Virus Genes, 44, 45–50. doi: 10.1007/s11262-011-0663-z
- McNulty, M.S., Allan, G.M., Connor, T.J., McFerran, J.B. & McCracken, R.M. (1984). An entero-like virus associated with the runting syndrome in broiler chickens. Avian Pathology, 13, 429–439. doi: 10.1080/03079458408418545
- Moura-Alvarez, J., Chacon, J.V., Scanavini, L.S., Nunez, L.F., Astolfi-Ferreira, C.S., Jones, R.C. & Piantino Ferreira, A.J. (2013). Enteric viruses in Brazilian turkey flocks: single and multiple virus infection frequency according to age and clinical signs of intestinal disease. Poultry Science, 92, 945–955. doi: 10.3382/ps.2012-02849
- Pantin-Jackwood, M.J., Spackman, E. & Woolcock, P.R. (2006). Molecular characterization and typing of chicken and turkey astroviruses circulating in the United States: implications for diagnostics. Avian Diseases, 50, 397–404. doi: 10.1637/7512-020606R.1
- Roussan, D.A., Shaheen, I.A., Khawaldeh, G.Y., Totanji, W.S. & Al-Rifai, R.H. (2012). Simultaneous detection of astrovirus, rotavirus, reovirus and adenovirus type I in broiler chicken flocks. Polish Journal of Veterinary Sciences, 15, 337–344. doi: 10.2478/v10181-012-0052-0
- Schultz-Cherry, S.L. (2013). Astrovirus infections. In D.E. Swayne, J.R. Glisson, L.R. McDougald, L.K. Nolan, D.L. Suarez & V.L. Nair (Eds.), Diseases of Poultry (13th ed., pp. 384–396). Ames: Iowa State Press.
- Shirai, J., Tanimura, N., Uramoto, K., Narita, M., Nakamura, K. & Kawamura, H. (1992). Pathologically and serologically different avian nephritis virus isolates implicated in etiology of baby chick nephropathy. Avian Diseases, 36, 369–377. doi: 10.2307/1591515
- Smyth, J.R. Jr. (1990). Genetics of plumage, skin and eye pigmentation in chickens. In R.D. Crawford (Ed.), Poultry Breeding and Genetics (pp. 109–168). Amsterdam: Elsevier.
- Smyth, V., Kaukonen, E., Trudgett, J., Welsh, M. & Todd, D. (2013a). Chicken astrovirus detected in hatchability problems associated with “white chicks”. In Abstracts of the XVIII Conference of World Veterinary Poultry Association (p. 537). Nantes.
- Smyth, V., Trudgett, J., Wylie, M., Jewhurst, H., Conway, B., Welsh, M., Kaukonen, E. & Perko-Makela, P. (2013b). Chicken astrovirus detected in hatchability problems associated with ‘white chicks’. Veterinary Record, 173, 403–404. doi: 10.1136/vr.f6393
- Smyth, V.J., Jewhurst, H.L., Wilkinson, D.S., Adair, B.M., Gordon, A.W. & Todd, D. (2010). Development and evaluation of real-time TaqMan(R) RT-PCR assays for the detection of avian nephritis virus and chicken astrovirus in chickens. Avian Pathology, 39, 467–474. doi: 10.1080/03079457.2010.516387
- Smyth, V.J., Todd, D., Trudgett, J., Lee, A. & Welsh, M.D. (2012). Capsid protein sequence diversity of chicken astrovirus. Avian Pathology, 41, 151–159. doi: 10.1080/03079457.2011.652938
- Spackman, D., Gough, R.E., Collins, M.S. & Lanning, D. (1984). Isolation of an enterovirus-like agent from the meconium of dead-in-shell chicken embryos. Veterinary Record, 114, 216–218. doi: 10.1136/vr.114.9.216-a
- Todd, D., Wilkinson, D.S., Jewhurst, H.L., Wylie, M., Gordon, A.W. & Adair, B.M. (2009a). A seroprevalence investigation of chicken astrovirus infections. Avian Pathology, 38, 301–309. doi: 10.1080/03079450903055421
- Todd, D., Smyth, V.J., Ball, N.W., Donnelly, B.M., Wylie, M., Knowles, N.J. & Adair, B.M. (2009b). Identification of chicken enterovirus-like viruses, duck hepatitis virus type 2 and duck hepatitis virus type 3 as astroviruses. Avian Pathology, 38, 21–29. doi: 10.1080/03079450802632056
- Toffan, A., Catania, S., Salviato, A., De Battisti, C., Vascellari, M., Toson, M., Capua, I. & Cattoli, G. (2012). Experimental infection of poults and guinea fowl with genetically distinct avian astroviruses. Avian Pathology, 41, 429–435. doi: 10.1080/03079457.2012.704980
- Wang, Y., Qi, X., Gao, H., Gao, Y., Lin, H., Song, X., Pei, L. & Wang, X. (2009). Comparative study of the replication of infectious bursal disease virus in DF-1 cell line and chicken embryo fibroblasts evaluated by a new real-time RT-PCR. Journal of Virological Methods, 157, 205–210. doi: 10.1016/j.jviromet.2009.01.001
- Yu, M., Tang, Y., Guo, M., Zhang, Q. & Saif, Y.M. (2000). Characterization of a small round virus associated with the poult enteritis and mortality syndrome. Avian Diseases, 44, 600–610. doi: 10.2307/1593099
- Zhao, W., Zhu, A.L., Yuan, C.L., Yu, Y., Zhu, C.X., Lan, D.L., Yang, Z.B., Cui, L. & Hua, X.G. (2011). Detection of astrovirus infection in pigeons (Columbia livia) during an outbreak of diarrhoea. Avian Pathology, 40, 361–365. doi: 10.1080/03079457.2011.587792
- Zierenberg, K., Raue, R. & Muller, H. (2001). Rapid identification of "very virulent" strains of infectious bursal disease virus by reverse transcription-polymerase chain reaction combined with restriction enzyme analysis. Avian Pathology, 30, 55–62. doi: 10.1080/03079450020023203
- Zsak, L., Strother, K.O. & Day, J.M. (2009). Development of a polymerase chain reaction procedure for detection of chicken and turkey parvoviruses. Avian Diseases, 53, 83–88. doi: 10.1637/8464-090308-Reg.1
