ABSTRACT
Live Eimeria vaccines against coccidiosis in poultry initiate immunity using a vaccine dose containing few oocysts; protection is enhanced through subsequent faecal–oral transmission (“cycling”) of parasites in the poultry house. Spray-administered Eimeria vaccines can permit wide variations in doses ingested by individual chicks; some chicks may receive no primary vaccination at all. Consequently, protective immunity for the entire flock depends on successful environmental cycling of vaccine progeny. Pullets missing primary vaccination at day of age can become protected from coccidial challenge through cycling of vaccine progeny oocysts from vaccinated (V) cage mates. This study tested whether 40% cage floor coverage (CFC) with a durable material could improve protection against challenge in these “contact-vaccinated” (CV) or successfully V pullets. The six treatment groups tested were CV, V or sham-vaccinated pullets cage-reared on either 0% or 40% CFC. Oocyst output was measured separately for each group for 30 days following vaccine administration. Lesion scores, body weights and total oocyst outputs were measured to quantify protection at 30 days of age against single or mixed Eimeria species challenge infections. Use of 40% CFC to promote low-level oocyst cycling impacted the flock in two ways: (1) more uniform flock immunity was achieved in the 40% CFC (CV similar to V pullets) compared with 0% CFC and (2) protection was enhanced in the 40% CFC compared with the 0% CFC. The use of CFC is an easily adopted means of improving live Eimeria vaccination of caged pullets.
Introduction
Coccidiosis of commercial chickens, caused by highly host-specific apicomplexan parasites in the genus Eimeria, is one of the major parasitic diseases affecting the global chicken industry (Dalloul & Lillehoj, Citation2005; Bera et al., Citation2010; Zhang et al., Citation2013). After ingestion of sporulated (infective) oocysts initiation of the life cycle begins with an endogenous asexual followed by the sexual phase (Rose, Citation1987). At the end of the sexual phase, birds can shed large numbers of unsporulated oocysts (Parry et al., Citation1992; Johnston et al., Citation2001). Successful exogenous sporulation of these oocysts occurs within 22 and 77 hours, depending on the species, in an environment with suitable temperature and moisture levels, and available oxygen (Norton & Chard, Citation1983; Williams, Citation1998; Al-Badri & Barta, Citation2012).
Coccidiosis, sometimes with concurrent or subsequent necrotic enteritis (caused by Clostridium perfringens), has been observed as an issue of layer birds reared on wire mesh floors (Gingerich, Citation2012). Coccidiosis in layers is of greatest economic importance following movement to a new production facility (e.g. the egg production barn) (McDougald et al., Citation1990). This recognized risk of coccidiosis following movement of pullets to an egg production facility suggests that relatively large numbers of oocysts can be ingested by birds housed in a conventional cage environment if no protective immunity was developed during pullet rearing (Price et al., Citation2013).
Live Eimeria vaccines depend on delivery of a small dose of vaccine oocysts to birds to initiate primary infections and start to elicit a protective immune response. All these live vaccines require repeated infections of vaccinates via low-dose faecal–oral transmission (“cycling”) of the oocysts at the flock level in the poultry house environment. Strong immunological protection against a mixed Eimeria species infection depends on this environmental cycling of the vaccine organisms (Williams, Citation1998; Price et al., Citation2014).
Delivery of live Eimeria vaccines is usually accomplished via spray application after which chicks ingest vaccine oocysts via preening themselves and each other immediately after delivery (Price et al., Citation2014). Spray delivery of live Eimeria vaccines invariably leads to non-uniform ingestion with some chicks ingesting more or fewer oocysts than others (Price et al., Citation2014). Some chicks that are administered a live Eimeria vaccine by spray delivery may receive no primary vaccination at all (Price et al., Citation2014) and therefore can only get exposed to vaccinal oocysts via environmental cycling. Protective immunity for the entire flock thus depends on successful cycling of vaccine progeny among the majority of the flock.
Although environmental cycling has been demonstrated for floor-reared broilers (Velkers et al., Citation2012), providing conditions that promote successful cycling of vaccine progeny in conventionally reared (caged) pullets can be challenging. Adding degradable material to the cage floors for the period immediately following spray vaccination to retain faecal material and promote oocyst cycling has been proposed (Soares et al., Citation2004). The success of such cage modifications has been determined experimentally for both fibre trays and a double layer of chick paper (e.g. Price et al., Citation2013), at least for uniformly inoculated birds.
An initial study evaluated the utility of adding degradable cage floor coverage (CFC) beneath uniformly vaccinated (V) (oral-gavaged) pullets to determine the optimal percentage CFC (Price et al., Citation2013). Subsequent studies demonstrated that chick paper is efficacious as a CFC material to enhance cycling (Price, Citation2015). The present study was conducted to test explicitly if the addition of double-layered chick paper acting as CFC can address non-uniform vaccine delivery and promote protective immunity in caged-reared pullets that were completely missed during live Eimeria vaccine administration.
Materials and Methods
Experimental birds
White Lohmann-LSL chicks were obtained from Archers Hatchery (Brantford, ON, Canada); all pullets were vaccinated at the hatchery against Marek's disease virus and infectious bursal disease virus per the standard protocol prior to transportation to the Poultry Unit of the Arkell Research Station at the University of Guelph (Arkell, Ontario, Canada). Stocking densities met Canadian Agri-Food Research Council (Anonymous, Citation2003) and institutional Animal Care Committee recommendations in accordance with Canadian Council on Animal Care guidelines (Tennessen et al., Citation2009) throughout the study.
Pullets were provided water and non-medicated ration ad libitum for the duration of the trial. Room temperature, light intensity and relative humidity were monitored as described by Price et al. (Citation2014).
Parasites
Coccidia used in the vaccine and challenge doses were single-oocyst-derived Guelph strains of Eimeria acervulina, Eimeria brunetti, Eimeria maxima, Eimeria necatrix and Eimeria tenella. All vaccine and challenge doses except for E. necatrix were mixed from a single stock of oocysts for each species. Vaccine inoculum and mixed species low-dose challenge were mixed from an initial stock of E. necatrix oocysts but there were insufficient oocysts in that stock for the E. necatrix single species and high-dose mixed species challenges; for that reason a second, freshly passaged stock of E. necatrix oocysts was used to formulate the two challenge doses.
Vaccination and challenge study
This study consisted of day of age vaccination followed by virulent challenge infections 30 days later (). The vaccination phase encompassed the period from 0 to 30 days of age during which immunity was expected to develop against the Eimeria species in the live Eimeria vaccine within cages with 0% or 40% CFC. During this vaccination phase (see Vaccination phase, below), V chicks were commingled with an equal number of sham-vaccinated cage mates (so-called contact-vaccinated; CV) in each cage replicate. Additional sham-vaccinated (SV) chicks were housed at the same density under the same rearing conditions, but not commingled, to provide immunologically naïve birds for the challenge phase.
Figure 1. Experimental time line describing the phases, pullets per cage, cage replicates and timing of measurements for two CFC modifications (0% or 40%). During the vaccination phase (days 0–30), V and CV pullets were commingled (housed together) during the brooding and growing periods. At 30 days of age, pullets were either high-dose challenged or low-dose challenged. There were seven distinct high-dose challenges with 24 pullets per challenge: E. acervulina alone; E. brunetti alone; E. maxima alone; E. necatrix alone; E. tenella alone; high-dose mix of all five Eimeria species and a sham-challenge (seven challenges × 24 pullets/challenge × three vaccine treatment groups × two CFC modifications = 1008 high-dose challenged pullets; in addition to the two cages (28 pullets per cage) of the SV, uninfected control birds; n = 1064 total). The remaining V, CV and SV pullets were all administered a low mixed dose of the five Eimeria species and housed at six birds/cage (1 challenge × 24 pullets/challenge × 3 vaccine treatment groups × 2 CFC modifications = 144 pullets). Total oocyst output was measured daily from each cage of low-dose challenged birds.
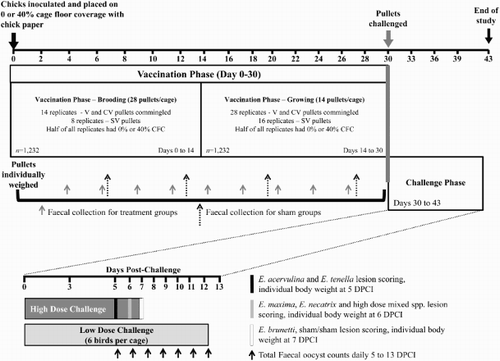
The challenge infection phase occurred from 30 to 43 days of age during which protective immunity was assessed functionally by measuring pre- and post-challenge body weights (BW), total oocyst output and parasite-induced intestinal lesions following virulent challenge with individual or mixed Eimeria species (see Challenge phase, below).
Vaccination inocula
During the vaccination phase, the V birds were inoculated by oral-gavage at day of age with 320 total oocysts of mixed Eimeria species (E. acervulina – 100 oocysts per chick; E. brunetti – 75 oocysts per chick; E. maxima – 50 oocysts per chick; E. necatrix – 75 oocysts per chick and E. tenella – 20 oocysts per chick) suspended in 0.5 ml of 0.9% saline. The CV and SV birds were sham inoculated using 0.5 ml of 0.9% saline by oral-gavage.
Housing during the vaccination phase
To test the effect of CFC, chicks from these three vaccine inoculations were distributed into two CFC modifications: (1) 0% CFC and (2) 40% CFC with chick paper. Pullets reared with 0% CFC were reared on wire mesh floor with no covering material.
The 40% CFC was a double-layered, commercially sourced (Lykir Limited, Mount Brydges, ON, Canada) corrugated chick paper measured to cover 40% (∼1240 cm2) of the floor area (∼3100 cm2) of conventional 20" x 24" (51cm x 61cm) cages (Ford Dickison Inc., Mitchell, ON, Canada). The chick paper has been shown to last approximately 5 weeks before degrading (Price, Citation2015). Cage assignments were randomized and half of the cages had 0% CFC and half had 40% CFC.
All chicks were placed into cleaned rearing cages at a stocking density of 110 cm2 per chick (28 birds per cage) from 0 to 14 days of age. For welfare reasons, brown waxed paper (Uline, Brampton, ON, Canada) was placed on the cage floor (beneath the CFC if present) from 0 to 10 days of age and replaced every 24 hours as described by Price et al. (Citation2015). At 14 days of age, pullets from each cage were split into two cages to provide a stocking density of 221 cm2 per bird (14 birds per cage) from 14 to 30 days of age. In cages with mixed V and CV birds, equal numbers of V and CV birds remained in both cages after halving the stocking density.
Vaccination phase
A total of 1232 day of age chicks were neck-tagged (Ketchum Manufacturing Inc., Brockville, ON, Canada), weighed individually, and assigned randomly to the V (392 birds), CV (392 birds) or SV (448 birds) treatment groups.
Following vaccination, birds were assigned randomly to 42 cages of 28 birds each; 14 of these cages contained 28 SV birds and the remaining 28 cages contained a commingled population of 14 V and 14 CV birds. Two additional cages each containing 28 SV birds acted as uninfected controls. Half of all cages had 0% CFC and half had 40% CFC. Stocking density was halved at 14 days of age as described above.
Challenge infection phase
At 30 days of age any remaining CFC was removed from the cages and pullets were administered challenge doses to assess the success of the vaccination phase. Pullets were randomly selected to be challenged either with a single Eimeria species, a high dose of mixed Eimeria species, a low dose of mixed Eimeria species or saline only (“sham-challenge”) (, ). Challenge doses were established using a breed- and age-matched dose titration trial run in parallel to this experiment (data not shown).
Table 1. A summary of the challenge species, the oocyst dose per pullet and the use of each Eimeria species dose in the challenge phase.
Twenty-four birds from each combination of vaccination and CFC coverage (i.e. V – 0%, CV – 0%, SV – 0%, V – 40%, CV – 40% and SV – 40%) were challenged with one of seven different challenges () comprising a single Eimeria species, a high dose of mixed Eimeria species or sham-challenge. These virulent challenges were used to assess lesion scores and post-challenge BW (controlling for pre-challenge BW). The numbers of oocysts used in each of the high-dose challenges () were selected so that naïve birds would demonstrate clinical coccidiosis without causing mortalities.
Similarly, for each combination of vaccine inoculum (V, CV, SV) and CFC (0% or 40%) group (six combinations in total), 24 pullets were randomly selected and challenged with a low dose of mixed Eimeria species () and housed at six birds per cage. Total oocyst output during challenge was the measurement of interest from pullets administered the low-dose mixed Eimeria species challenge (see Oocyst output below); the low challenge dose was selected to minimize the crowding effect (Williams, Citation2001).
Oocyst output
Flotation of faecal material in saturated salt (Long et al., Citation1976) from each cage containing SV birds was conducted on a weekly basis during the vaccination phase to confirm that no inadvertent infections of the SV had occurred.
Oocyst shedding by V and CV birds was enumerated every 3 days starting at 3 days post-inoculation (DPI) during the vaccination phase. To collect samples, comingled V and CV pullets within a single cage were separated into two cages lined with waxed paper for one hour to obtain separate faecal samples from the V and CV birds. At the end of each collection, pullets were returned to their original cages.
During the challenge phase, total oocyst output was obtained from V, CV and SV pullets infected with a low-dose mixed Eimeria species challenge (n = 144). Total faecal droppings from these low-dose challenged birds were collected by placing brown wax paper below cages for 24-hour periods from 5 to 13 days post-challenge infection (DPCI). Total oocyst output was measured as described by Price et al. (Citation2013).
Polymerase chain reaction for oocyst output samples
Faecal samples obtained during the vaccination phase that contained large numbers of oocysts (i.e. 9, 15, 18, 27 DPI) were pooled. These pooled samples were used to assess the presence or absence of specific Eimeria species in the samples. All faecal samples from the low-dose mixed Eimeria species challenged pullets were pooled quantitatively (e.g. equivalent amounts of faeces from each 24-hour faecal collection from 5 to 13 DPCI were pooled to provide a single sample from the 0% V low-dose challenged birds). Pooled faecal samples were diluted 10-fold (v/v) in 2.5% (w/v, aqueous) potassium dichromate (K2Cr2O7) and homogenized; 15 ml of faecal suspension was centrifuged (10 minutes at 1500 × g) to collect the oocysts and other particulates. Oocysts were purified from faecal debris by salt flotation using standard methods as described by Ryley et al. (Citation1976). DNA from the oocysts was extracted as described by El-Sherry et al. (Citation2013). Yield and purity were determined spectrophotometrically using a NanoDrop 2000 instrument (NanoDrop, Wilmington, DE, USA). Extracted DNA was passed through column purification (QiaQuick® Gel Extraction Kit, Qiagen, Germantown, MD, USA) prior to a nested polymerase chain reaction (PCR) procedure designed to detect all Eimeria species found in the vaccine.
Initially, coccidia-specific PCR amplification of a 803 bp portion of the mitochondrial cytochrome c oxidase subunit I (COI) gene was accomplished using coccidia-specific COI primers 400F and 1202R () with 100 ng of template DNA as previously described (El-Sherry et al., Citation2013). PCR products were electrophoresed on a 1.5% agarose submarine gel in Tris–Acetate–EDTA buffer (40 mM Tris, 20 mM acetic acid and 1 mM EDTA) at ∼100 V for approximately 45 min. The resulting gel was stained with ethidium bromide (C21H20BrN3) and the size of products estimated by comparison with a 100 bp–10 kb DNA ladder (Bio Basic Inc., Mississauga, ON, Canada) visualized using UV trans-illumination. Positive bands were excised and DNA purified using a QiaQuick® Gel Extraction Kit (Qiagen) for use in a species-specific PCR. Approximately, 50 pg of the gel-extracted DNA was used as template for the nested PCR with newly designed, Eimeria species-specific COI primers located within the region previously amplified using the 400F and 1202R primers (). Reactions contained approximately 50 pg of the gel-extracted DNA, 50 mM MgCl2, 1 mM dNTPs, 1×PCR buffer and 0.4U Platinum® Taq (Life Technologies Inc., Carlsbad, CA, USA). The PCR thermal profile was as follows: initial heat activation of polymerase at 96°C for 10 min; 35 cycles of denaturation at 94°C for 30 s annealing at 50–60°C for 30 s (see for anneal temperature and primer combinations) and extension at 72°C for 60 s and a final extension at 72°C for 10 min. The PCR products were visualized and sized as described previously. Both positive and negative control samples were run concurrently; the presence or absence of each Eimeria species was noted for each sample.
Table 2. Summary of the primer combinations, size and anneal temperatures for the polymerase chain reaction for oocyst output samples.
Lesion scores and BW
Pullets were killed humanely (n = 1064) by cervical dislocation (Charbonneau et al., Citation2010) for lesion scoring. Depending on the Eimeria species, challenge lesion scores and BW were assessed at 5 DPCI (E. acervulina and E. tenella), 6 DPCI (E. maxima, E. necatrix and high-dose mixed Eimeria species challenge) or 7 DPCI (E. brunetti and sham-challenged). Lesion scoring of the intestinal tracts was made by a single researcher who was blinded with regard to the identity of the pullets (i.e. the lesion scorer received only the isolated intestinal tract and the associated neck-tag number; neck-tag numbers were randomized among all groups). Intestinal lesion scores induced by coccidia were ranked from 0 to 4 in the upper, middle and lower intestinal regions as well as the caeca and rectum using the Johnson and Reid (Citation1970) scoring system.
Individual BW were measured at the day of challenge (“pre-challenge BW”) and at the day of lesion score (“post-challenge BW”) for only those pullets used for lesion scoring.
Statistical analyses
The statistical software SAS 9.2 (Cary, NC, USA) was used for all analyses. For all analyses, a PROC MIXED, ANOVA programme was used. Random effects were used to control for potential clustering (cage and replication) for all analyses. For all comparison tests, P-values of ≤0.05 were deemed significant.
Vaccination phase statistical analyses
Analyses were conducted for the oocysts per gram of faeces (OPG) for each DPI during the vaccination phase. Residual analyses were performed to determine the necessity of a natural log transformation to account for large variances; the lack of normal distribution in the raw data indicated that natural log transformation was required and this was applied before all statistical tests. A Toeplitz covariance structure was used to account for repeated cage measures. Tests of significance were reported between 0% and 40% CFC modifications at a single DPI using a t-statistic test for pairwise comparisons.
Challenge phase statistical analyses
For all BW analyses, post-challenge BW was controlled for pre-challenge BW. All reports of statistical significance below refer to post-challenge BW that have been controlled with respect to pre-challenge BW whether explicitly stated or not. For post-challenge BW, controlling for pre-challenge BW, a t-statistic test was used for comparisons between vaccine inoculation and CFC groups. The t-statistic test was also used to account for interaction terms and contrast statements.
Mean lesion scores were presented as a cumulative lesion score (maximum cumulative score of 20) combining scores of 0–4 from five regions of the intestinal tract (upper intestine, middle intestine, lower intestine, caeca and rectum). The inclusion criteria to test for statistical significance were that, within a comparison of two CFC groups given the same challenge infection for the same region of the intestine, a mean lesion score of 1.0 or higher was reported for the SV group. For all lesion scores, a difference between a score of 0 and 1 was assumed to be the same as a difference between a score of 1 and 2 and so on. For mean lesion scores, tests of statistical significance between vaccine inoculation and CFC groups, and to account for interaction terms, t-statistic tests for pairwise comparisons were used.
From a single vaccine inoculation and CFC group combination (e.g. CV – 0% CFC), mean total oocyst output numbers were pooled from 5 to 13 DPCI. A residual analysis was performed to determine the necessity of a natural log transformation to account for large variances; the lack of normal distribution in the raw data indicated that natural log transformation was required and this was applied before all statistical tests. For tests of statistical significance between vaccine inoculation and CFC groups, and to account for interaction terms, t-statistic tests for pairwise comparisons were used.
Results
Vaccination phase
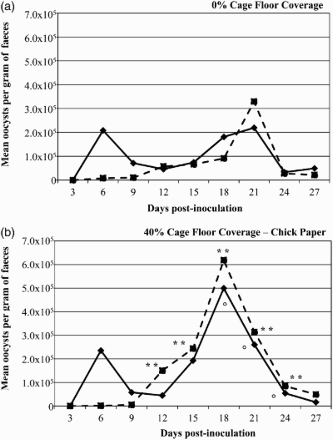
During the vaccination phase, oocyst shedding was observed at 6 DPI in the V pullets in both the 0% and 40% CFC modifications, whereas the CV birds in the same cages did not shed appreciable oocysts at that time (). V pullets in the 0% and 40% CFC modifications shed similar numbers of oocysts (2.1 × 105 and 2.4 × 105 OPG, respectively) at 6 DPI. “Peak” oocyst shedding occurred at 21 DPI for pullets reared with 0% CFC ((a)) compared to 18 DPI for the 40% CFC modification ((b)). Unlike shedding at 6 DPI, V pullets reared on 40% CFC had a significantly higher “peak” oocyst shedding (5.0 × 105 OPG) compared to the 0% CFC modification (2.2 × 105 OPG). CV pullets reared with 40% or 0% CFC had “peak” oocyst shedding at 18 and 21 DPI, respectively; CV pullets reared with 40% CFC had a significantly higher “peak” oocyst shedding (6.2 × 105 OPG) compared to pullets reared with 0% CFC (3.3 × 105 OPG). CV pullets reared with 40% CFC had significantly higher OPG from 12 to 24 DPI compared to the 0% CFC group ((b)). V pullets reared on 40% CFC had significantly higher OPG from 18 to 24 DPI compared to V pullets on 0% CFC ((a)). Faecal samples collected throughout the vaccination phase (i.e. at 9, 15, 18 and 27 DPI) demonstrated only E. acervulina, E. brunetti, E. maxima and E. tenella as determined by the nested PCR protocol outlined above.
Figure 2. Oocyst shedding throughout the vaccination phase (0–30 days of age). The mean number of oocysts per gram of faeces from 3 to 27 DPI for Lohmann-LSL pullets administered a live Eimeria vaccine of 320 total oocysts (containing E. acervulina, E. brunetti, E. maxima, E. necatrix and E. tenella oocysts) via oral-gavage at one day of age (V, solid line) or indirectly via ingestion of vaccine progeny oocysts in the environment (CV, dotted line). Pullets were housed in conventional brooder cages without floor coverage (a, 0%), or 40% covered with chick paper (b) until 30 days of age (28 birds per cage × 14 replicates per vaccine treatment group from 1 to 14 days of age, then pullets were separated into new adjacent cages of the same treatment group at 14 birds per cage × 28 replicates for vaccine treatment groups from 14 to 30 days of age). CV pullets reared on 40% coverage had significantly higher (P ≤ 0.05) OPG compared to CV pullets reared on 0% coverage (indicated by **) from 12 to 24 DPI. V pullets reared with 40% coverage had significantly higher (P ≤ 0.05) OPG compared to V pullets reared with 0% coverage (indicated by °) from 18 to 24 DPI. All significant differences are based on natural log-transformed mean oocyst per gram of faeces and a t-statistic test was used to assess differences in mean OPG between groups on a single sample day.
Challenge phase: low-dose mixed Eimeria species challenge
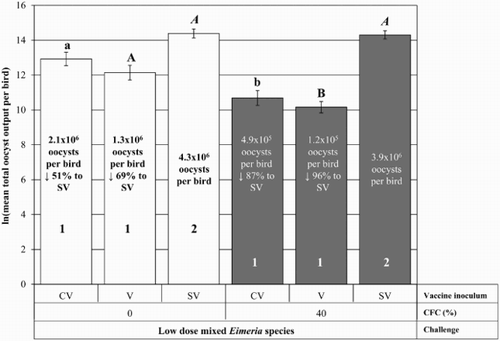
Pullets administered a low dose of mixed Eimeria species had total oocyst output that varied depending on vaccination status and CFC modification (). Pullets reared on 40% CFC had significantly lower total oocyst output following low-dose challenge than pullets reared on 0% CFC that were either CV (CV, 40% – 4.9 × 105 versus CV, 0% – 21 × 105) or V (V, 40% – 1.2 × 105 versus V, 0% – 13 × 105). Challenged CV and V pullets reared on 0% and 40% CFC had significantly lower total oocyst output than challenged SV pullets (SV, 0% – 43 × 105; and SV, 40% – 39 × 105). When reared on 0% CFC, V and CV had 69% (1.3 × 106) and 51% (2.1 × 106) reduction of mean total oocyst output per bird compared to the SV pullets (4.3 × 106), respectively. When reared on 40% CFC, V and CV had 96% (1.2 × 105) and 87% (4.9 × 105) reduction of mean total oocyst output per bird compared to the SV pullets (3.9 × 106), respectively. All of the treatment groups (0% – V, CV, SV; and 40% – V, CV, SV) shed only E. acervulina, E. brunetti, E. maxima and E. tenella as determined by the nested PCR protocol.
Figure 3. Total oocyst output per bird following low-dose mixed Eimeria species challenge infections (30–42 days of age). The natural log-transformed mean total oocyst output per bird (with standard error bars for the least squares means) following challenge infection with a low dose of mixed Eimeria species for each CFC and vaccine treatment combination (a total of 144 pullets). Within each bar the per cent reduction of mean total oocyst output per bird of pullets V or CV compared to the SV inoculation group within the same CFC modification is shown. Groups displaying different letters differ significantly (P ≤ 0.05) between CFC modifications within the same vaccine inoculation group (e.g. CV/0% versus CV/40%). Groups displaying different numbers differ significantly (P ≤ 0.05) between vaccine inoculation groups within the same CFC modification (e.g. CV/0% versus SV/0%).
Challenge phase: high-dose single or mixed Eimeria species challenges
Protection of V pullets against virulent challenge infections (Eimeria species singly or in a mixed challenge) was assessed by measuring parasite-induced lesions (, ) and mean post-challenge BW after controlling for pre-challenge BW (). Mean lesion scores and post-challenge BW of CV and V pullets challenged with E. acervulina, E. brunetti, E. maxima or E. tenella were significantly better than SV pullets challenged similarly regardless of CFC modification ( and ).
Figure 4. Lesion scores following high-dose challenges. Cumulative mean lesion scores (0–4 per region, with a maximum cumulative score of 20) for five regions of the intestinal tract (i.e. upper intestine, middle intestine, lower intestine, caeca and rectum) for each CFC and vaccine treatment (V – vaccinated; CV – contact-vaccinated; SV – sham-vaccinated) combination following high-dose challenge with the following: (a) E. acervulina; (b) E. maxima; (c) E. necatrix; (d) E. tenella; (e) E. brunetti; or, (f) sham-challenge (i.e. saline only)). Statistical significance was reported on a comparison of the reporter diagnostic region (i.e. E. acervulina – upper intestinal region; E. maxima – middle intestinal region; E. necatrix – middle intestinal region; E. tenella – caeca or E. brunetti – rectum). For each challenge, groups displaying different letters differ significantly (P ≤ 0.05) between CFC modifications within the same vaccine inoculum group (e.g. CV/0% versus CV/40%). Groups displaying different numbers differ significantly (P ≤ 0.05) between vaccine inoculum groups within the same CFC modification (e.g. CV/0% versus SV/0%).
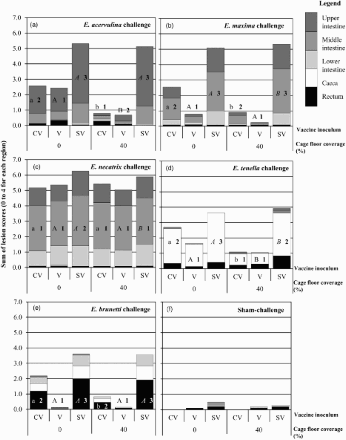
Table 3. Mean lesion scores ± standard error (SE) for least squares means of the saline only (“sham-challenge”), single and high-dose mixed Eimeria species challenged pullets for intestinal regions at 5 (E. acervulina and E. tenella), 6 (E. maxima, E. necatrix and high-dose mixed Eimeria species challenge) and 7 (E. brunetti and sham-challenged) DPCI (72 cages at 14 birds per cage – 24 cages with V and CV pullets reared on 0% CFC; 24 cages with V and CV pullets reared on 40% CFC; 14 cages with SV pullets reared on 0% CFC and 14 cages with SV pullets reared on 40% CFC).a
Table 4. Mean post-challenge BW ± standard error (SE) for least squares means, controlling for mean pre-challenge BW ± SE, for the saline only (“sham-challenged”), single and high-dose mixed Eimeria species challenged pullets at 5 (E. acervulina and E. tenella), 6 (E. maxima, E. necatrix and high-dose mixed Eimeria species challenge) and 7 (E. brunetti and sham-challenged) DPCI (a total of 72 cages at 14 birds per cage – 24 cages with V and CV pullets reared on 0% CFC; 24 cages with V and CV pullets reared on 40% CFC; 14 cages with SV pullets reared on 0% CFC and 14 cages with SV pullets reared on 40% CFC).
Lesion scores from the reporter regions and post-challenge BW of V pullets challenged with E. brunetti or E. maxima were not significantly different on different CFC modifications (; and ); in contrast, the same measures of protection were significantly worse for CV pullets reared on 0% CFC versus 40% CFC. Lesion scores from the reporter (Reid & Long, Citation1979) regions of V pullets challenged with E. acervulina or E. tenella were significantly improved in pullets reared on 40% CFC versus 0% CFC although post-challenge BW did not differ significantly. Similarly, CV pullets challenged with E. acervulina or E. tenella had significantly improved lesions scores from the species-specific reporter regions if reared on 40% CFC versus 0% CFC.
Lesion scores (, ) and post-challenge BW () indicate that no protection was elicited against E. necatrix. Mean lesion scores of the middle intestinal region (reporter region) for high-dose E. necatrix challenge (; ) were uniformly high (2.9–3.3) in all pullets (SV, V and CV pullets on both 0% and 40% CFC modifications). Similarly, post-challenge BW did not differ significantly between any groups ().
The observed lack of protection against E. necatrix in the single species challenge was also evident in the high-dose mixed Eimeria species challenged pullets; no differences were detected in lesion scores for the reporter region for E. necatrix (see “Middle Intestine” ) in the V and CV pullets compared to the SV pullets. In the other four regions of the intestinal tract, V pullets reared on 40% CFC had improved lesion scores (in three cases significantly) compared with V pullets reared on 0% CFC. Improvement in lesion scores was even more marked in the CV pullets in those regions; all CV pullets reared on 40% CFC had significantly lower lesion scores than CV pullets reared on 0% CFC. CV birds reared on 40% CFC had lesion scores that did not differ significantly from V pullets on the same CFC unlike CV birds reared on 0% CFC that had significantly higher (poorer) lesion scores in lower intestinal, caecal and rectal regions than V pullets in the same cage.
Discussion
Live Eimeria vaccine success is affected by uniformity of application (coverage and dosage) and post-vaccination oocyst cycling within a V flock (Price, Citation2012; Velkers et al., Citation2012). Cycling of oocysts produced by live vaccination is largely dependent on atmospheric conditions (i.e. temperature, relative humidity and oxygen access) suitable for proper oocyst sporulation as well as a physical environment (e.g. housing and management) that provides for prolonged availability of infective sporulated oocysts (Price, Citation2012, Citation2013, Citation2014). Examination of the beaks and mouths of chicks that have been spray vaccinated with a coloured vaccine can demonstrate that birds have had contact or ingested the coloured liquid but not quantify how many oocysts were actually ingested. By measuring the number of oocysts shed by individual birds following spray vaccination, Price et al. (Citation2015) demonstrated that spray application of a live Eimeria vaccine was not uniform; widely different doses of oocysts were apparently ingested by the vaccinates and some received no vaccine dose at all. Without conditions favourable for oocyst sporulation, there could be few vaccine progeny oocysts that will become infective for the next bird to ingest. Without availability of infective oocysts for a sufficient period, there will not be efficient cycling of the vaccine among birds in a flock. In the face of non-uniform vaccine uptake (Caldwell et al., Citation2001; Price et al., Citation2014), practical methods that encourage low-level vaccine progeny oocyst cycling become increasingly important.
In the present study, species-specific PCR confirmed shedding of E. acervulina, E. maxima, E. tenella and E. brunetti by V and CV pullets in the weeks following vaccination; however, E. necatrix was not shed by any of the pullets during that period. This explains the complete lack of protection against E. necatrix, as demonstrated by high lesion scores and low post-challenge BW, in all pullets despite the immunogenic nature of this parasite (e.g. Rose & Long, Citation1962). These observations suggest that the E. necatrix oocysts used to prepare the initial vaccine doses and the low-dose mixed Eimeria species challenge infections were dead but that the oocysts used to prepare the high-dose challenges were viable.
In the present study, V pullets shed comparable oocyst numbers at 6 DPI regardless of whether they were reared on 0% or 40% CFC. Unsurprisingly, this suggests that initial oocyst output post-vaccination is dependent solely on the initial dose of oocysts in the live vaccine (administration) rather than oocyst cycling in the environment (environmental control). In contrast, cycling of vaccine progeny oocysts for those pullets reared with 40% CFC was enhanced compared to those pullets reared with 0% CFC. The V pullets had at least two observed “peaks” of oocyst shedding regardless of whether they were reared on 0% or 40% CFC. CV pullets reared on 40% CFC had an observed OPG “peak” that coincided with a second shed (cycling) of vaccine progeny oocysts from the V groups. This overlapping of oocyst shedding is similar to observations of Velkers et al. (Citation2012) with V or CV broilers reared on litter and infected with attenuated E. acervulina. The timing of observed peaks of oocyst shed by CV pullets reared on 40% CFC occurred earlier than CV pullets reared with 0% CFC. Additionally, CV pullets reared on 40% CFC had significantly higher oocyst shed during the cycling period from 12 to 24 DPI compared to pullets reared on 0% CFC. These results suggest that CV pullets reared on 40% CFC were ingesting more oocysts and doing so earlier than the CV pullets reared on 0% CFC. The coverage material provided better availability and duration of availability of sporulated oocysts for low-level oocyst cycling to occur faster than the mesh cage floor alone.
All measurements of protection against virulent challenge should be considered, including BW, lesions scores and total oocyst output during challenge, when interpreting results of vaccination (Chapman et al., Citation2005b). In general, pullets challenged with E. acervulina or E. tenella as well as E. maxima or E. brunetti demonstrated lower mean lesion scores and higher post-challenge BW when reared on 40% CFC during the vaccination phase compared to pullets reared on 0% CFC. Mean total oocyst output per bird during the challenge period was significantly lower when pullets were reared on 40% CFC compared to 0% CFC (V – approximately 10.8 times lower; and CV – approximately 4.3 times lower, respectively). Additionally, when compared to SV pullets, pullets reared with 40% CFC had 96% (V) and 87% (CV) reduction in mean total oocyst output per bird compared to 69% (V) and 51% (CV) reduction with pullets reared on 0% CFC. The BW, lesion scores and total oocyst output results suggest that pullets reared on 40% CFC were better protected against a challenge infection compared to pullets reared on 0% CFC. Specifically, when CV pullets were reared in an environment with enhanced oocyst cycling (40% CFC), they achieved strong protection against a challenge infection despite not being vaccinated initially. V and CV pullets were protected against a challenge infection compared to SV pullets on either CFC. Overall, the results of these studies suggest that even a flock where only 50% of pullets ingested, a full vaccine dose can generate solid flock immunity if conditions that promote oocyst cycling are maintained following vaccine application.
Eimeria species differ in their ability to elicit a protective immune response; for example, E. maxima is considered highly immunogenic with a single modest infection leading to near-sterile immunity, whereas species considered less immunogenic, such as E. tenella, may require several substantial re-infections to elicit similarly robust immunity against challenge (Rose & Long, Citation1962; Rose, Citation1974; Chapman et al., Citation2005a,Citationb). For the E. brunetti and E. maxima challenged groups, the lack of a significant difference between the V pullets reared with 40% CFC and 0% CFC, especially with regard to their mean lesion scores, suggests that these species may require little within-cage cycling to elicit protection against virulent challenge. Previous studies have found that E. maxima can generate sufficient protective immunity from a single inoculation dose when administered at 3 weeks of age (Rose & Long, Citation1962). However, better protection against challenge infection has been demonstrated when at least two to four “vaccine boosting inoculation cycles” were employed (Hein, Citation1975; Chapman et al., Citation2005a), especially when chickens were inoculated starting at the day of hatch with E. maxima (Chapman et al., Citation2005a). A similar finding was noted by Hein (Citation1975) with chickens vaccinated against E. brunetti. Conversely, for the E. acervulina and E. tenella challenged groups, the significant difference between V pullets reared on 40% versus 0% CFC suggests that these species may need substantial oocyst cycling for better protection against a challenge infection. Previous studies have found that better protection against challenge infection for both E. acervulina and E. tenella was noted when at least four or more “vaccine boosting inoculation cycles” (Joyner & Norton, Citation1973; Hein, Citation1975; Joyner & Norton, Citation1976; Nakai et al., Citation1992) or continuous trickle infections (Joyner & Norton, Citation1973; Joyner & Norton, Citation1976) were employed. The need for increased oocyst cycling for these species may be due, in part, to lower immunogenicity of these species (Rose & Long, Citation1962). In commercial settings, pullets are likely to be challenged by mixed Eimeria species; thus, the cycling requirements of all Eimeria species must be considered. Consequently, a higher number of vaccine progeny oocyst cycles would be a better goal so that solid protection against challenge is elicited even for species, such as E. acervulina, that require protracted cycling to achieve this.
Live Eimeria vaccination success has been shown to be enhanced through modification of the physical environment using CFC when pullets were uniformly vaccinated via oral-gavage (Price et al., Citation2013) or vaccinated via gel-pucks (Soares et al., Citation2004). The present study demonstrated that oocysts shed by V birds are ingested by CV (originally non-vaccinated) pullets when these birds were commingled in cages. CV pullets in the present study became protected against a homologous challenge in cages lacking any floor coverage (0% CFC). Importantly, CV pullets reared on 40% CFC demonstrated significantly better protection against most homologous challenge infections (i.e. lower lesion scores, higher post-challenge BW and fewer oocysts shed during challenge) compared to CV pullets reared on 0% CFC. However, this transmission was most likely lower than would be achieved for broilers reared on litter with ample opportunity for vaccine progeny oocyst cycling (Velkers et al., Citation2012). The notable improvement in protection against multiple Eimeria species observed in the present study in the vaccinated and, even more dramatically, the CV pullets indicate that this easily adopted means to promote low-level oocyst cycling can greatly impact vaccination success. Simply modifying the physical environment by rearing birds on 40% CFC can enhance live Eimeria vaccination success significantly within historically “difficult-to-live-vaccinate” housing environments (such as pullets reared in conventional cages), even in the face of non-uniform vaccine administration or uptake by vaccinated pullets.
Acknowledgements
The technical assistance of A. Barta, J. Cobean, S. El-Sherry, M. Freeman, J. Klaas, A. Leveille, M. Paibomesai and J. Whale (University of Guelph, Guelph ON) is gratefully acknowledged. This researched formed a portion of the Ph.D. thesis for KRP.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Al-Badri, R. & Barta, J.R. (2012). The kinetics of oocyst shedding and sporulation in two immunologically distinct strains of Eimeria maxima, GS and M6. Parasitology Research, 111, 1947–1952. doi: 10.1007/s00436-012-3041-4
- Anonymous. (2003). Recommended Code of Practice for the Care and Handling of Farm Animals: Chickens, Turkeys and Breeders from Hatchery to Processing Plant. Ottawa, ON: Canadian Agri-Food Research Council.
- Bera, A.K., Bhattacharya, D., Pan, D., Dhara, A., Kumar, S. & Das, S.K. (2010). Evaluation of economic losses due to coccidiosis in poultry industry in India. Agriculture Economics Research Review, 23, 91–96.
- Caldwell, D.Y., Moore, R.W., Caldwell, D.J. & Hargis, B.M. (2001). Effect of photointensity, sound intensity, and ambient temperature on preening behavior and ingestion of spray-applied biologics. The Journal of Applied Poultry Research, 10, 99–106. doi: 10.1093/japr/10.2.99
- Chapman, H.D., Matsler, P.L., Muthavarapu, V.K. & Chapman, M.E. (2005a). Acquisition of immunity to Eimeria maxima in newly hatched chickens given 100 oocysts. Avian Diseases, 49, 426–429. doi: 10.1637/7359-032805R1.1
- Chapman, H.D., Roberts, B., Shirley, M.W. & Williams, R.B. (2005b). Guidelines for evaluating the efficacy and safety of live anticoccidial vaccines, and obtaining approval for their use in chickens and turkeys. Avian Pathology, 34, 279–290. doi: 10.1080/03079450500178378
- Charbonneau, R., Niel, L., Olfert, E., von Keyerserlingk, M. & Griffin, G. (2010). CCAC Guidelines on: Euthanasia of Animals Used in Science. Ottawa, ON: Canadian Council on Animal Care.
- Dalloul, R.A. & Lillehoj, H.S. (2005). Recent advances in immunomodulation and vaccination strategies against coccidiosis. Avian Diseases, 49, 1–8. doi: 10.1637/7306-11150R
- El-Sherry, S., Ogedengbe, M.E., Hafeez, M.A. & Barta, J.R. (2013). Divergent nuclear 18S rDNA paralogs in a turkey coccidium, Eimeria meleagrimitis, complicate molecular systematics and identification. International Journal for Parasitology, 43, 679–685. doi: 10.1016/j.ijpara.2013.03.005
- Gingerich, E. (2012). US table egg industry update. In B. Richey & K. Janicek (Eds.). Proceedings of the 116th Annual Meeting of the United States Animal Health Association (pp. 469–476). Greensboro, NC.
- Hein, H. (1975). Eimeria acervulina, E. brunetti, and E. maxima: immunity in chickens with low multiple doses of mixed oocysts. Experimental Parasitology, 38, 271–278. doi: 10.1016/0014-4894(75)90112-5
- Johnson, J. & Reid, M.W. (1970). Anticoccidial drugs: lesion scoring techniques in battery and floor-pen experiments with chickens. Experimental Parasitology, 28, 30–36. doi: 10.1016/0014-4894(70)90063-9
- Johnston, W.T., Shirley, M.W., Smith, A.L. & Gravenor, M.B. (2001). Modelling host cell availability and the crowding effect in Eimeria infections. International Journal for Parasitology, 31, 1070–1081. doi: 10.1016/S0020-7519(01)00234-X
- Joyner, L.P. & Norton, C.C. (1973). The immunity arising from continuous low-level infection with Eimeria tenella. Parasitology, 67, 333–340. doi: 10.1017/S0031182000046552
- Joyner, L.P. & Norton, C.C. (1976). The immunity arising from low-level infection with Eimeria maxima and Eimeria acervulina. Parasitology, 72, 115–125. doi: 10.1017/S0031182000043250
- Long, P.L., Millard, B.J., Joyner, L.P. & Norton, C.C. (1976). A guide to laboratory techniques used in the study and diagnosis of avian coccidiosis. Folia Veterinaria, 6, 201–217.
- McDougald, L.M., Fuller, A.L. & McMurray, B.L. (1990). An outbreak of Eimeria necatrix coccidiosis in breeder pullets: analysis of immediate and possible long-term effects on performance. Avian Diseases, 34, 485–487. doi: 10.2307/1591441
- Nakai, Y., Uchida, T. & Kanazawa, K. (1992). Immunization of young chickens by trickle infection with Eimeria tenella. Avian Diseases, 36, 1034–1036. doi: 10.2307/1591569
- Norton, C.C. & Chard, M.J. (1983). The oocyst sporulation time of Eimeria species from the fowl. Parasitology, 86, 193–198. doi: 10.1017/S0031182000050368
- Parry, S., Barratt, M.E., Jones, S., McKee, S. & Murray, J.D. (1992). Modelling coccidial infection in chickens: emphasis on vaccination by in-feed delivery of oocysts. Journal of Theoretical Biology, 157, 407–425. doi: 10.1016/S0022-5193(05)80661-7
- Price, K.R. (2012). Use of live vaccines for coccidiosis control in replacement layer pullets. The Journal of Applied Poultry Research, 21, 679–692. doi: 10.3382/japr.2011-00486
- Price, K.R. (2015). Live Eimeria vaccine use in commercial pullet rearing: environmental influences on vaccination success. (PhD dissertation). University of Guelph, Guelph, ON.
- Price, K.R., Freeman, M., Van-Heerden, K. & Barta, J.R. (2015). Shedding of live Eimeria vaccine progeny is delayed in chicks with delayed access to feed after vaccination. Veterinary Parasitology, 208, 242–245. doi: 10.1016/j.vetpar.2015.01.009
- Price, K.R., Guerin, M.T. & Barta, J.R. (2014). Success and failure: the role of relative humidity levels and environmental management in live Eimeria vaccination of cage-reared replacement layer pullets. The Journal of Applied Poultry Research, 23, 523–535. doi: 10.3382/japr.2014-00989
- Price, K.R., Guerin, M.T., Newman, L., Hargis, B.M. & Barta, J.R. (2013). Examination of a novel practical poultry management method to enhance the effect of live Eimeria vaccination for conventionally housed replacement layer pullets. International Journal of Poultry Science, 12, 175–184. doi: 10.3923/ijps.2013.175.184
- Reid, W.M. & Long, P.L. (1979). A diagnostic chart for nine species of fowl coccidia. University of Georgia, College of Agriculture Experimental Station Research report, 335, p. 25.
- Rose, M.E. (1974). The early development of immunity to Eimeria maxima in comparison with that to Eimeria tenella. Parasitology, 68, 35–45. doi: 10.1017/S0031182000045352
- Rose, M.E. (1987). Immunity to Eimeria infections. Veterinary Immunology and Immunopathology, 17, 333–343. doi: 10.1016/0165-2427(87)90152-8
- Rose, M.E. & Long, P.L. (1962). Immunity to four species of Eimeria in fowls. Immunology, 5, 79–91.
- Ryley, J.F., Meade, R., Hazelhurst, J. & Robinson, T.E. (1976). Methods in coccidiosis research: separation of oocysts from faeces. Parasitology, 73, 311–326. doi: 10.1017/S0031182000046990
- Soares, R., Cosstick, T. & Lee, E.H. (2004). Control of coccidiosis in caged egg layers: a paper plate vaccination method. The Journal of Applied Poultry Research, 13, 360–363. doi: 10.1093/japr/13.2.360
- Tennessen, T., Connor, L., de Passille, A.M., Stanford, K., von Keyerserlingk, M. & Griffin, G. (2009). CCAC Guidelines on: The Care and Use of Farm Animals in Research, Teaching and Testing. Ottawa, ON: Canadian Council on Animal Care.
- Velkers, F.C., Bouma, A., Stegeman, J.A. & de Jong, M.C. (2012). Transmission of a live Eimeria acervulina vaccine strain and response to infection in vaccinated and contact-vaccinated broilers. Vaccine, 30, 322–328. doi: 10.1016/j.vaccine.2011.10.090
- Williams, R.B. (1998). Epidemiological aspects of the use of live anticoccidials vaccines for chickens. International Journal for Parasitology, 28, 1089–1098. doi: 10.1016/S0020-7519(98)00066-6
- Williams, R.B. (2001). Quantification of the crowding effect during infections with the seven Eimeria species of the domesticated fowl: its importance for experimental designs and the production of oocysts stocks. International Journal for Parasitology, 31, 1056–1069. doi: 10.1016/S0020-7519(01)00235-1
- Zhang, J.J., Wang, L.X., Ruan, W.K. & An, J. (2013). Investigation into the prevalence of coccidiosis and maduramycin drug resistance in chickens in China. Veterinary Parasitology, 191, 29–34. doi: 10.1016/j.vetpar.2012.07.027
