ABSTRACT
Tibial dyschondroplasia (TD) is an important long bone defect of broiler chickens that disturbs the proximal growth plate and is characterized by non-vascularized cartilage, a distended growth plate and lameness. Celastrol, a medicinal root extract from the plant Tripterygium wilfordii, is reported widely as a well-known heat-shock protein 90 (Hsp90) inhibitor. Recently, Hsp90 inhibition in chondrocyte differentiation and growth-plate vascularization were effective in restoring the morphology of the growth plate. The present study was aimed at investigating Hsp90 inhibition in TD using celastrol. The broiler chicks were divided into three groups; Control; TD induced (40 mg/kg thiram) and celastrol treatment. Hsp90, vascular endothelial growth factor and Flk-1 expressions were evaluated by quantitative real-time polymerase chain reaction and the protein levels of Hsp90 were measured by Western blot analysis. Antioxidant enzymes were determined to assess the liver damage caused by thiram and the protective effects of the medicine were evaluated by levels of serum biomarkers. The expression levels of Hsp90 and vascular endothelial growth factor mRNA transcripts were increased while Flk-1 receptor was decreased in TD-affected chicks. Celastrol therapy inhibited Hsp90 mRNA and protein levels and up-regulated the expressions of receptor Flk-1 in TD-affected tibial growth plates significantly (P < 0.05) in addition to rectifying the damaging effects of thiram on the liver by decreasing the levels of aspartate aminotransferase, alanine aminotransferase and malondialdehyde and correcting the oxidative imbalance. In conclusion, administering celastrol to dyschondroplastic chicks prevented un-vascularized growth plate, lameness and reinstated angiogenesis. Celastrol may be efficacious for the treatment of TD through the inhibition of Hsp90 expression and limiting the liver damage caused by thiram in broiler chickens.
Introduction
Endochondral ossification occurs in the growth plates of long bone containing chondrocytes for bone development. During the process, the avascular tissue (cartilage) is replaced by the more vascularized tissue (bone) (Dan et al., Citation2009; Shahzad et al., Citation2015).
Tibial dyschondroplasia (TD) is a skeletal disorder of fast growing birds which presents as avascularized and non-mineralized cartilage in the growth plate (Tian et al., Citation2013). The disease has been attributed to abnormal differentiation of chondrocytes that normally lead to bone growth, cartilage vascularization and mineralization (Pines et al., Citation1998; Shahzad et al. Citation2014a). The aetiological differences in the distribution of growth factor and defective angiogenesis may mediate the development of such a lesion (Gay et al., Citation2007).
Heat-shock protein 90 (Hsp90); vascular endothelial growth factor (VEGF) and its receptor and Flk-1 play a major role in angiogenesis. Hsp90 expression has been found to be up-regulated in TD-affected growth plates of broiler chickens (Tian et al., Citation2009; Shahzad et al., Citation2015); however, the inhibition of Hsp90 activity by geldanamycin reinstated the angiogenesis in the avascularized area by up-regulating the Flk-1 gene (Herzog et al., Citation2011; Genin et al., Citation2012).
Great efforts have gone into identifying novel natural compounds targeting Hsp90 levels; and currently several Hsp90 inhibitors have been discovered that inhibit vascularization in cancer. Celastrol, a traditional Chinese medicine, has been identified as one of them. This triterpene is a root extract from Tripterygium wilfordii Hook f plant, and is one of the most promising medicinal molecules that has been used as an anti-inflammatory medicine for centuries in China. This herb has also been reported to have anti-arthritic activity. This medicine has been shown to control damage to cartilage in rats with minimal side effects (Nanjundaiah et al., Citation2012; Hu et al., Citation2013). The identification of celastrol as an important Hsp90 inhibitor has resulted in it being used as an effective treatment in several cancer models (Amolins & Blagg Citation2009; Raja et al., Citation2011; Venkatesha et al., Citation2012). Thiram, an agricultural fungicide and pesticide, is well known due to cases of toxicity in commercial poultry feed. Thiram has been reported to cause lameness in birds and to induce liver damage. The present study was designed to evaluate the effects of celastrol as an Hsp90 inhibitor, promoting angiogenesis in the avascularized lesions of thiram-induced TD as well as its protective effect on the liver.
Materials and methods
Experimental birds
A total of 250 1-day-old male broiler chicks (average initial body weight BW 47.5 ± 0.5 g) were purchased from a commercial hatchery and maintained under standard hygienic conditions taking into account all the national legislation concerning the protection of bird welfare and approval following the institutional bird welfare and committee guidelines of Huazhong Agricultural University, Wuhan, China.
Celastrol preparations
Celastrol, an active component of T. wilfordii Hook f, was purchased from Tianjin Shilan Technology Co. Ltd. Tianjin, China. The drug was dissolved in dimethyl sulfoxide and administrated at 4 mg/kg/d via the intra peritoneal (i.p) route. The dose of celastrol was based on a study of the clinical application of experimental drugs (Chu et al., Citation2014).
Establishment and treatment of thiram-induced TD
The chicks were divided into two groups; (a) control group (n = 100) which received a normal diet and (b) thiram group (n = 150) which received a normal diet with the addition of 40 mg/kg of tetramethyl thiuram disulphide (thiram) to induce TD till the end of experiment. On day 7 post-hatch, half of the birds from the thiram group were separated and designated as (c) celastrol group and administered celastrol (4 mg/kg/day) (i.p route), and the control group was administered normal saline. All the birds were fed a Vitamin D enriched diet (Vitamin D3 5000 IU/kg, Ca 1%, P 0.6%) to prevent the possibility of rickets.
Fifty birds from all the groups were euthanized by cervical dislocation (Shahzad et al., Citation2015) on days 7 and 14 and the growth plates from each tibiotarsus were dissected out, fixed in 4% paraformaldehyde or immediately frozen in liquid nitrogen and stored at −70°C for further analysis.
During the experiment, 20 blood samples from each group were collected by cardiac puncture and the serum stored at −70°C for serum alanine aminotransferase (ALT), aspartate aminotransferase (AST) level and alkaline phosphatase (ALP) activity determination. At the end of the experiment, liver samples from each group were stored at −70°C for later analysis of glutathione peroxidase (GSH-Px), superoxide dismutase (SOD) and malondialdehyde (MDA) content.
Haematoxylin and eosin staining
The tibiotarsal bone samples were fixed overnight in 4% paraformaldehyde in PBS at 4°C and decalcified in 10% ethylenediamine tetraacetic acid. After dehydration in ethanol, clearing in xylene and embedding in paraffin wax, the histological sections were cut into 4–5 µm thickness to prepare slides and the sections were stained with haematoxylin and eosin using the method according to Tian et al. (Citation2013).
RNA extraction and reverse transcription
Liquid nitrogen frozen growth plates, collected from each group, were ground in a mortar and pestle and then homogenized in TRIzol reagent (Invitrogen, Carlsbad, California, USA) to extract total RNA, which was converted to complementary DNA (cDNA) using the TransScript First-Strand cDNA synthesis kit (TransGen Biotech Co. Ltd, Beijing, China) according to the manufacturer's instructions. Reverse transcription was run at reaction temperatures of 42°C for 30 min and 85°C for 5 min in a thermocycler (Applied Biosystems, Foster City, CA, USA) and the synthesis of cDNA was performed in a total 25-µl reaction mixture consisting of oligo (dT)18, 2×TS reaction mix and 5 µg RNA.
Reverse transcription quantitative real-time PCR (RT-qPCR)
Polymerase chain reaction was performed using specific primers () for each gene of interest. The primer used for the quantitative study was designed using Primer Express® v2.0 (Applied Biosystems) software. Basic local alignment search tool BLAST was run to ensure gene specificity. Relative levels of gene expression were normalized against the reference gene GAPDH. Melting curve analysis was performed to confirm primer specificity and the absence of primer dimer formation. All the PCR reactions were run at least in quadruplicate with the StepOne Plus™ Real-Time PCR System (Applied Biosystems) using the SYBR Green RT-PCR kit (Takara, Dalian China) in a total of 20 µl reaction volume consisting of 2 µl cDNA, specific forward and reverse primers (1 µl each), 10 µl SYBR Premix Ex Taq and ROX reference dye with the following thermal cycling parameters: 95°C for 30 s, 40 amplification cycles at 95°C for 8 s, 59°C for 30 s and 72°C for 30 s. Relative quantification of each gene was performed using delta ct (ΔCt) method (Livak & Schmittgen, Citation2001). Differences between gene expression levels were analysed by t-test and differences were considered significant at P < 0.05.
Table 1. Primers used for qPCR analysis.
Western blot analysis
Growth plates were homogenized in ice-cold PBS and incubated at 4°C for 2 h. The samples were centrifuged at 15,000 × g for 10 min to collect the supernatant. Total protein concentrations were determined by the BCA method (Pierce, Rockford, USA) and the samples were stored at −70°C. Protein samples (40 µg) were separated by SDS-PAGE on 12% polyacrylamide gel at 100 mV until the dye band reached the end of the gel and were then transferred (130 mV for 1 h and 40 min) to polyvinylidene difluoride membranes (Millipore, BioSharp, Anhui, China) which were incubated in 5% skimmed milk at room temperature for 1 h. The membranes were incubated overnight at 4°C with mouse monoclonal anti-Hsp90 primary antibodies (1:1000) (Boster Biotechnology, Wuhan, China, BM1587). The membranes were washed three times with PBS Tween 20 (0.01 mol/l, pH 7.4) for 10 min each, then they were incubated with secondary antibody (1:5000 dilution) (HRP labelled goat anti-mouse or rabbit anti-goat secondary antibodies) for 1 h at room temperature. After washing, the bands were visualized by chemiluminescence (Beyotime Shanghai, China) and were exposed to light-sensitive radiography film (Carestream, Beijing, China). The images were taken using an imaging system (UVP, Upland, CA, USA).
Quantification of liver antioxidant enzymes and serum biomarkers
The activity of SOD, GSH-Px and MDA levels was assayed to study the antioxidant activity in the liver. The SOD and GSH-Px enzymes were expressed in U per milligram of protein (U/mg protein), while the MDA levels were expressed in nanomoles per gram wet tissue (nmoles/g), The values of serum ALT, AST and ALP activity were expressed as units per litre (U/l) using commercial reagent kits from Jiancheng Biochem Company Ltd., Nanjing, China, and following the protocols appropriate to each reagent kit.
Statistical analysis
Data analysis was carried out using one way ANOVA followed by the student t-test and presented as means ± standard error of means. The differences were considered statistically significant if *P < 0.05.
Results
Effect of celastrol on thiram-induced TD-affected birds
The TD was scored in affected birds according to Pines et al. (Citation2005) (). Almost all of the birds exhibited TD signs on day 4 post-hatch in the thiram-induced group (B). On slaughtering on the 7th day, enlarged growth plates, with an opaque mass on the proximal side of the tibiotarsal bones, were observed in TD-induced chicks (). In the celastrol-treated group (C), the lameness started to subside, which was more obvious after day 10 post-hatch; moreover, on the 14th day, a reduction in the tibial growth-plate size was observed when compared to the thiram-induced group (). In the control group, none of the birds displayed any sign of lameness throughout the experiment.
Figure 1. The effect of celastrol on thiram-induced TD (growth-plate width and morphology). Tibiotarsus growth plates were compared at the ages of 7 and 14 days before and after celastrol administration. Enlarged growth plate in the chicks with TD; the normal growth plate size after celastrol treatment with a decrease in growth plate width. AC, articular cartilage; GP, growth plate; TD, Tibial dyschondroplasia.
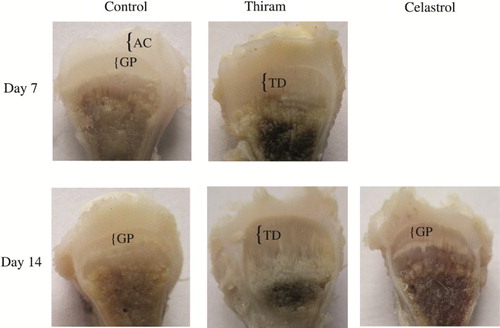
Table 2. Growth plate lesion size and TD score in control, thiram-induced and celastrol-treated groups. The results are expressed as means ± SE (n = 10).
Morphometric analysis of the growth plate
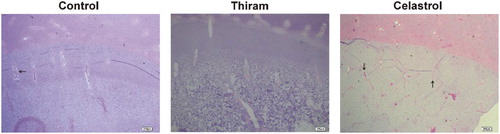
The integrity of the cartilage was assessed with haematoxylin and eosin. There were significant differences between normal and TD-affected epiphyseal growth plates of the proximal tibia. In TD-affected birds, irregular columns of chondrocytes lacking vascularization were observed. Celastrol administration resulted in massive new blood vessel formation, well-conserved cartilage columns and finally the re-establishment of a proper chondrocyte differentiation in the growth plate ().
Figure 2. Samples were collected, then tibial growth plates were stained with haematoxylin and eosin. Growth plate of TD-affected chicks was less vascularized and the columnar arrangement of the chondrocytes was lost. After celastrol administration, the columnar organization in growth plate was restored with developing blood vessels (Arrows (→) represent the BV) in the hypertrophic area (Bar = 200 µm).
Hsp90 levels in TD-affected growth plates confirmed the inhibitory effect of celastrol
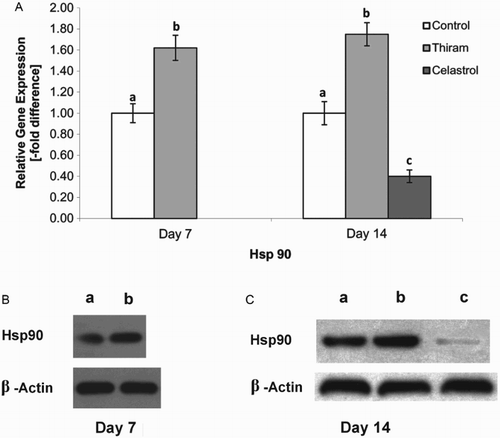
In order to examine the expression profile of genes involved in avian growth plate vascularization, we explored the relationship between TD and Hsp90 inhibition after celastrol administration. The mRNA expression levels of Hsp90, VEGF and Flk-1 receptors were observed before and after drug administration. Significantly (P < 0.05) up-regulated levels of Hsp90 were observed in TD-affected birds compared with the control group on days 7 and 14; however, the administration of celastrol caused a significant reduction in Hsp90 expression in the growth plates (P < 0.05) ((a)). The Hsp90 protein levels were also assessed by Western blot protein analysis and the results paralleled the corresponding gene expression ((b) and 3(c)).
Figure 3. Effect of inhibition of Hsp90 activity with celastrol was analysed by reverse transcription qPCR on days 7 and 14 (A).Results are expressed in arbitrary units, data represent the mean ± SE (n = 4 each group). Results are shown relative to mRNA expression levels from the control group. Control group is set to one to compare the n-fold difference, letters abc indicate significant differences (P < 0.05). Western blot analysis on day 7 (B) and day 14 (C) of normal, TD-affected and celastrol treated growth plate. Western blot analysis shows the band conforming to the Hsp90 and membranes were also probed with β-actin antibody (loading control) to confirm that equal amounts of proteins were loaded; band a, control; band b, TD-affected; band c, celastrol administration.
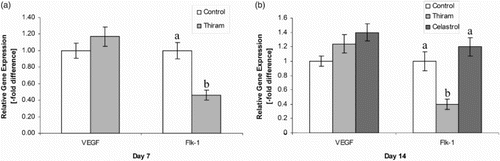
During the experiment, higher VEGF mRNA levels were observed throughout the course of the disease in the TD-induced thiram group as compared with the control group; however, the Flk-1 receptor level was significantly down-regulated in TD-affected birds (P < 0.05). Normal values were restored with celastrol administration when compared to affected birds ((a) and 4(b)).
Figure 4. Real-time quantitative PCR analysis of VEGF and FLK-1 on day 7 (a) and day 14 (b), quantification of gene amplification was done using the Ct values, all expression levels were normalized using the reference gene GAPDH. Results are shown as mean ± SE (n = 4 each group), Control group set to one thus to compare the N-fold difference. abindicate significant differences (P < 0.05).
Celastrol diminishes oxidative stress in the liver
A significant decrease (P < 0.05) in SOD and GSH-Px antioxidant enzymes and serum ALP activity along with an increase in ALT, AST and MDA content in the liver were observed in the thiram-fed birds as compared with the controls ( and ). However, the intra peritoneal administration of celastrol resulted in a significant increase in SOD and GSH-Px enzymes and ALP activity with a decrease in ALT, AST enzymes and MDA content (P < 0.05) ( and ).
Table 3. The effect of thiram (40 mg/kg) and celastrol (4 mg/kg/d) on ALT, AST and ALP activities, Values (mean ± SD) were compared by one way ANOVA.
Table 4. Effect of celastrol (4 mg/kg/d) on SOD, GSH-Px activities and MDA contents on 14 days post-hatch, Values (mean ± SD) were compared by one way ANOVA.
Discussion
Normal avian growth plates consist of long columns of chondrocytes that are well vascularized, each zone being more cellular when compared with mammalian growth plates. TD is a bone abnormality and the lesion is characterized by an irregular white, opaque, unmineralized and unvascularized mass of cartilage present in the proximal end of the tibia (Shim et al., Citation2012). TD has been attributed to cell death, lack of blood supply, degenerative changes and the inhibition of angiogenesis via VEGF and its receptors (Rath et al., Citation2005).
Thiram, one of the dithiocarbamates, is an organic compound which is normally used as a pesticide and exhibits cytotoxic properties. It interferes with the metabolism and development of chondrocytes resulting in TD in fast growing birds (Rath et al., Citation2007). In this experiment, a vitamin D-enriched diet was provided so as to ensure that the TD was not caused by a vitamin D deficiency as a vitamin D-deficient diet has been reported to cause this disease (Ben-Bassat et al., Citation1999; Nield et al., Citation2006; Shahzad et al., Citation2015).
In cancer therapy, the clinical inhibition of Hsp90 is widely used to stop vascularization within the neoplasm. Some previous reports have demonstrated a role of Hsp90 in avian growth plates in which Hsp90 inhibition resulted in vascularization of the cartilage core in TD through its effect on the VEGF-receptor system, ultimately eliminating the lameness observed clinically. To the best of our knowledge, this is the first study to investigate the role of celastrol on its Hsp90 inhibition activity in birds. The results showed that the administration of celastrol resulted in cartilage angiogenesis in TD-afflicted birds by inhibiting Hsp90 expression, resulting in the restoration of the normal avian growth plate.
Hsp90 together with VEGF and VEGF-receptors are pro-angiogenic regulators (Herzog et al., Citation2011). VEGF and its receptor Flk-1 are required for bone development and growth, especially in bone angiogenesis, chondrocyte differentiation and membranous ossification (Zhang et al., Citation2013). VEGF, an important regulator of vasculogenesis, plays a central role through its receptors; Flk-1 which stimulates endothelial cell mitogenesis and blood vessel growth (Neufeld et al., Citation1999). We compared the VEFG and Flk1 mRNA expressions of cartilage from normal and TD-affected birds; and our findings showed the up-regulated expression of VEGF in TD-affected birds; however, the Flk-1 receptor levels were down-regulated in these birds. By administering celastrol, the mRNA expression of Flk-1 was restored in thiram-induced TD birds and the clinical lameness started subsiding. The increase in VEGF and reduced Flk-1 mRNA expressions in TD-affected birds in our experiment were similar to findings from previous studies (Herzog et al., Citation2011; Velada et al., Citation2011; Genin et al., Citation2012; Shahzad et al., Citation2015). From this study it would appear that it is the levels of Hsp-90 and Flk-1 receptors that determine the degree of vascularization in the affected area rather than the level of VEGF. Similar findings have also been reported by Herzog et al. (Citation2011) and Genin et al. (Citation2012) in which synthetic drug Hsp-90 inhibition resulted in the development of blood vessels in affected growth plate and a decrease in lameness.
Thiram, apart from causing lameness in birds, has also been reported to cause liver damage with increased MDA content and levels of AST and ALT in the blood together with reduced liver antioxidant activity (Gupta and Amma, Citation1993; Li et al., Citation2007; Shahzad et al., Citation2014b). In our study, the release of aminotransferases (AST and ALT) into the blood, due to the thiram-induced liver damage, was prevented by the administration of celastrol. Moreover, thiram, as an oxidative agent, increased the oxidative stress on the liver, ultimately leading to a decrease in antioxidant enzymes (SOD and GSH-Px). This results in the lipid peroxidation of cell membrane and the release of MDA content (Marikovsky, Citation2002). In our study, these biomarkers, used to assess liver function, were altered in thiram-fed chicks; however, after celastrol administration, the levels were restored.
Lack of calcification and the failure of chondrocyte hypertrophy in the growth plate, one of the characteristic lesions of TD, are associated with a reduced serum ALP activity. Celastrol administration restored the normal tibial growth plate and increased the serum ALP activity. Serum ALP was found deficient in TD lesion and reappeared in that area also equally in blood after the lesion was abrogated (Genin et al., Citation2012; Shahzad et al., Citation2014b).
In conclusion, this is the first study which describes the inhibition of Hsp90 activity by celastrol resulting in angiogenesis in the avascularized area of the chicken growth plate with TD. It also demonstrates the liver protective properties of celastrol.
Additional information
Funding
References
- Amolins, M.W. & Blagg, B.S. (2009). Natural product inhibitors of Hsp90: potential leads for drug discovery. Mini Reviews in Medicinal Chemistry, 9, 140–152. doi: 10.2174/138955709787316056
- Ben-Bassat, S., Genina, O., Lavelin, I., Leach, R.M. & Pines, M. (1999). Parathyroid receptor gene expression by epiphyseal growth plates in rickets and tibial dyschondroplasia. Molecular and Cellular Endocrinology, 149, 185–195. doi: 10.1016/S0303-7207(98)00231-7
- Chu, C., He, W., Kuang, Y., Ren, K. & Gou, X. (2014). Celastrol protects kidney against ischemia–reperfusion-induced injury in rats. Journal of Surgical Research, 186, 398–407 doi: 10.1016/j.jss.2013.07.048
- Dan, H., Simsa-Maziel, S., Hisdai, A., Sela-Donenfeld, D. & Monsonego Ornan, E. (2009). Expression of matrix metalloproteinases during impairment and recovery of the avian growth plate. Journal of Animal Science, 87, 3544–3555. doi: 10.2527/jas.2009-2068
- Gay, C.V., Gilman, V.R. & Leach, R.M., Jr. (2007). Immunolocalization of vascularization factors in normal, tibial dyschondroplasia and rachitic cartilage. Avian Pathology, 36, 445–451. doi: 10.1080/03079450701591387
- Genin, O., Hasdai, A., Shinder, D. & Pines, M. (2012). The effect of inhibition of heat-shock proteins on thiram-induced tibial dyschondroplasia. Poultry Science, 91, 1619–1626. doi: 10.3382/ps.2012-02207
- Gupta, M. & Amma, M.K. (1993). Alterations in hepatic biochemistry of mice intoxicated with MIC, carbaryl and thiram. Journal of Applied Toxicology, 13, 33–37. doi: 10.1002/jat.2550130108
- Herzog, A., Genin, O., Hasdai, A., Shinder, D. & Pines, M. (2011). Hsp90 and angiogenesis in bone disorders – lessons from the avian growth plate. AJP: Regulatory, Integrative and Comparative Physiology, 301, R140–R147.
- Hu, Y., Wang, S., Wu, X., Zhang, J., Chen, R., Chen, M. & Wang, Y. (2013). Chinese herbal medicine-derived compounds for cancer therapy: a focus on hepatocellular carcinoma. Journal of Ethnopharmacology, 149, 601–612. doi: 10.1016/j.jep.2013.07.030
- Li, J., Bi, D., Pan, S. & Zhang, Y. (2007). Effect of diet with thiram on liver antioxidant capacity and tibial dyschondroplasia in broilers. British Poultry Science, 48, 724–728. doi: 10.1080/00071660701665858
- Livak, K.J. & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods, 25, 402–408. doi: 10.1006/meth.2001.1262
- Marikovsky, M. (2002). Thiram inhibits angiogenesis and slows the development of experimental tumours in mice. British Journal of Cancer, 86, 779–787. doi: 10.1038/sj.bjc.6600078
- Nanjundaiah, S.M., Venkatesha, S.H., Yu, H., Tong, L., Stains, J.P. & Moudgil, K.D. (2012). Celastrus and its bioactive celastrol protect against bone damage in autoimmune arthritis by modulating osteoimmune cross-talk. Journal of Biological Chemistry, 287, 22216–22226. doi: 10.1074/jbc.M112.356816
- Neufeld, G., Cohen, T., Gengrinovitch, S. & Poltorak, Z. (1999). Vascular endothelial growth factor (VEGF) and its receptors. Federation of American Societies for Experimental Biology Journal, 13, 9–22.
- Nield, L.S., Mahajan, P., Joshi, A. & Kamat, D. (2006). Rickets: not a disease of the past. American Family Physician, 74, 619–626.
- Pine, M., Hasdai, A. & Monsonego-Ornan, M. (2005) Tibial dyschondroplasia – tools, new insights and future prospects. World's Poultry Science Journal, 61, 285–297. doi: 10.1079/WPS200454
- Pines, M., Knopov, V., Genina, O., Hurwitz, S., Faerman, A., Gerstenfeld, L.C. & Leach, R.M. (1998). Development of avian tibial dyschondroplasia: gene expression and protein synthesis. Calcified Tissue International, 63, 521–527. doi: 10.1007/s002239900568
- Raja, S.M., Clubb, R.J., Ortega-Cava, C., Williams, S.H., Bailey, T.A., Duan, L. & Band, H. (2011). Anticancer activity of celastrol in combination with ErbB2-targeted therapeutics for treatment of ErbB2-overexpressing breast cancers. Cancer Biology & Therapy, 11, 263–276. doi: 10.4161/cbt.11.2.13959
- Rath, N.C., Huff, W.E. & Huff, G.R. (2007). Thiram-induced changes in the expression of genes relating to vascularization and tibial dyschondroplasia. Poultry Science, 86, 2390–2395. doi: 10.3382/ps.2007-00219
- Rath, N.C., Richards, M.P., Huff, W.E., Huff, G.R. & Balog, J.M. (2005). Changes in the tibial growth plates of chickens with thiram-induced dyschondroplasia. Journal of Comparative Pathology, 133, 41–52. doi: 10.1016/j.jcpa.2005.01.005
- Shahzad, M., Gao, J., Qin, P., Liu, J., Wang, Z., Zhang, D. & Li, J. (2014a). Expression of genes encoding matrilin-3 and cyclin-I during the impairment and recovery of chicken growth plate in tibial dyschondroplasia. Avian Diseases, 58, 468–473. doi: 10.1637/10781-012614-ResNote.1
- Shahzad, M., Liu, J., Gao, J., Wang, Z., Zhang, D., Nabi, F. & Li, J. (2014b). Hsp-90 inhibitor geldanamycin attenuates liver oxidative stress and toxicity in thiram-induced tibial dyschondroplasia. Pakistan Veterinary Journal, 34, 545–547.
- Shahzad, M., Liu, J., Gao, J., Wang, Z., Zhang, D., Nabi, F. & Li, J.k. (2015). Differential expression of extracellular matrix metalloproteinase inducer (EMMPRIN/CD147) in avian tibial dyschondroplasia. Avian Pathology, 44, 13–18. doi: 10.1080/03079457.2014.987210
- Shim, M.Y., Karnuah, A.B., Anthony, N.B., Pesti, G.M. & Aggrey, S.E. (2012). The effects of broiler chicken growth rate on valgus, varus, and tibial dyschondroplasia. Poultry Science, 91, 62–65. doi: 10.3382/ps.2011-01599
- Tian, W.X., Li, J.K., Qin, P., Wang, R., Ning, G.B., Qiao, J.G., Li, H.Q., Bi, D.R., Pan, S.Y. & Guo, D.Z. (2013). Screening of differentially expressed genes in the growth plate of broiler chickens with tibial dyschondroplasia by microarray analysis. BMC Genomics, 14, 276. doi: 10.1186/1471-2164-14-276
- Tian, W.X., Zhang, W.P., Li, J.K., Bi, D.R., Guo, D.Z., Pan, S.Y, Zhang, Y.H. & Qin, P. (2009). Identification of differentially expressed genes in the growth plate of broiler chickens with thiram-induced tibial dyschondroplasia. Avian Pathology, 38, 161–166. doi: 10.1080/03079450902737789
- Velada, I., Capela-Silva, F., Reis, F., Pires, E., Egas, C., Rodrigues-Santos, P. & Barros, M.T. (2011). Expression of genes encoding extracellular matrix macromolecules and metalloproteinases in avian tibial dyschondroplasia. Journal of Comparative Pathology, 145, 174–186. doi: 10.1016/j.jcpa.2010.12.008
- Venkatesha, S.H., Astry, B., Nanjundaiah, S.M., Yu, H. & Moudgil, K.D. (2012). Suppression of autoimmune arthritis by celastrus-derived celastrol through modulation of pro-inflammatory chemokines. Bioorganic & Medicinal Chemistry, 20, 5229–5234. doi: 10.1016/j.bmc.2012.06.050
- Zhang, J.P., Deng, Y.F., Zhou, Z.L. & Hou, J.F. (2013). Expression and identification of recombinant chicken vascular endothelial growth factor in Pichia pastoris and its role in the pathogenesis of tibial dyschondroplasia. Poultry Science, 92, 3214–3227. doi: 10.3382/ps.2013-03420
