ABSTRACT
Mycoplasma gallisepticum is the most important pathogenic avian Mycoplasma species and causes chronic respiratory disease in poultry. In addition, the prevalence of Mycoplasma synoviae is of increasing concern in several EU member states. We investigated the prevalence of M. gallisepticum in commercial poultry (5220 layers, 1224 broilers and 1020 meat turkeys), 56 racing pigeons and 890 wild birds (Order Anseriformes, Galliformes, Pelecaniformes, Accipitriformes, Gruiformes, Charadriiformes, Columbiformes, Strigiformes, Falconiformes and Passeriformes). Broilers and wild birds were also evaluated for Mycoplasma synoviae. Dependent on the bird lifespan and the nature of the sample, different diagnostic tests were used including the rapid plate agglutination test, enzyme-linked immunosorbent assay (ELISA), polymerase chain reaction and real-time polymerase chain reaction. A low prevalence of M. gallisepticum was found in both layers (0.9%; 95% CI: 0.7–1.2%) and broilers (2.7%; 95% CI: 1.9–3.8%) possibly due to reduced vertical transmission by breeder farms, which are under official surveillance. None of the samples from turkeys or racing pigeons tested positive. In wild birds, we found five birds were positive (1.7%; 95% CI: 0.7–3.9%): one wood pigeon, two grey herons, one mallard and one Eurasian magpie. For M. synoviae a high prevalence was found in broilers (12.9%: 95% CI: 11.1–14.9%). Four samples collected by hunters gave a positive result for M. synoviae (4%: 95% CI: 1.6–9.8%): one carrion crow and three wood pigeons. In addition, 12 house sparrows were found to be positive (3%; 95% CI: 1.7–5.2%). Wild birds probably play a limited role as a reservoir but we cannot exclude a possible impact on transmission of Mycoplasmas.
Introduction
Mycoplasma gallisepticum is a pathogenic mycoplasma species in poultry and causes major economic losses (Ley et al., Citation1997; Levisohn & Kleven, Citation2000). It is the aetiological agent of chronic respiratory disease and can result in a variety of different signs such as breathing difficulty, nasal discharge, sinusitis, airsacculitis, decrease in egg production and increase in embryo mortality in layer parents and commercial layers (Mohammed et al., Citation1987). Reduction of weight gain and downgrading of carcass quality in broilers is also reported (Stipkovits & Kempf, Citation1996; Bradbury, Citation2007). M. gallisepticum is also infectious for turkeys and can cause severe sinusitis and airsacculitis (Jordan, Citation1975). There have also been reports of turkeys developing neurological signs due to meningoencephalitis (Cordy & Adler, Citation1965; Wyrzykowski et al., Citation2013). Although infections may also remain without clinical signs, they can make birds more prone to secondary infections with bacteria such as Escherichia coli, and viruses such as Newcastle disease, infectious bronchitis and infectious laryngotracheitis (Gross, Citation1990; Kleven, Citation1998). Since M. gallisepticum can spread via vertical transmission, eradication of infected breeder flocks is currently the main mitigation method used. Vaccination can play a role in preventing respiratory disease as it can protect against the development of tracheal and nasal sinus lesions. It has also been shown to reduce fall in egg production. Protection against air sac lesions is not always consistent and more importantly, there is no prevention of transmission through the egg (Whithear, Citation1996). For these reasons and because vaccination can cause false positive reactions in serologic testing it is not the most suitable method in prevention of infections (Whithear, Citation1996; Evans et al., Citation2005). Despite current eradication measures, outbreaks in breeder flocks still occur and it is not well known how M. gallisepticum finds its way into the poultry farms. Previous studies have described M. gallisepticum in many different bird species, which indicates that they can act as a reservoir for commercial poultry. Isolation of M. gallisepticum from tracheal swabs of racing pigeons (Columba livia) has been successful but none of the birds studied showed clinical signs. However, they could play a role in transmission since they are potential carriers of the organism (Bencina et al., Citation1987; Nagatomo et al., Citation1997; Tsai & Lee, Citation2006; Gharaibeh & Hailat, Citation2011). Many other species studied such as rooks (Corvus frugilegus), carrion crows (Corvus corone), jack daws (Corvus monedula), house sparrows (Passer domesticus), and members of the family Anatidae such as ducks and geese also seem to be possible carriers (El-Shabiny et al., Citation2005; Pennycott et al., Citation2005; Stipkovits & Szathmary, Citation2012). In some species clinical disease has been demonstrated. Partridges, pheasants and peafowl have shown similar respiratory distress and conjunctivitis as found in chickens (Cookson & Shivaprasad, Citation1994; Ganapathy & Bradbury, Citation1998; Bencina et al., Citation2003). Another bird species that is very sensitive to M. gallisepticum infection is the house finch (Haemorhous mexicanus), which mostly presents with severe conjunctivitis and increased mortality (Luttrell et al., Citation1998, Citation2001; Faustino et al., Citation2004). This problem has been studied for many years in the United States where severe infections occur mainly in eastern North America (Dhondt et al., Citation2005). Farmer et al. (Citation2005) tested the susceptibility of other wild songbirds by infecting them with a house finch strain and discovered that birds mainly from the family of Fringillidae developed clinical signs. The tufted titmouse (Baeolophus bicolor) was the only member of the family Paridae to show clinical signs.
M. synoviae has always been considered less important than M. gallisepticum in poultry but during the last decade its importance has been highlighted in several studies and there is an increased consciousness to generate M. synoviae-free poultry (Feberwee et al., Citation2008; Landman, Citation2014). There seems to be a large variability in the virulence of M. synoviae strains (Lockaby et al., Citation1998, Citation1999; Lockaby & Hoerr, Citation1999). In both chickens and turkeys, M. synoviae can cause similar respiratory problems as M. gallisepticum as well as affecting the egg shell quality with typical eggshell apex abnormalities and decreased egg production (Feberwee et al., Citation2009; Catania et al., Citation2010; Ranck et al., Citation2010). Furthermore, arthropathic and amyloid-inducing strains may cause severe economic losses due to growth retardation and lameness induced by synovitis (Kleven et al., Citation1975; Landman & Feberwee, Citation2001; Kleven, Citation2008). In previous studies, M. synoviae has been identified in other bird species such as pigeons, ducks and Japanese quails (Coturnix japonica) but none of them showed clinical signs (Bencina et al., Citation1987, Citation1988; Nagatomo et al., Citation1997; Tsai & Lee, Citation2006).
We investigated the prevalence of mycoplasmas in commercial poultry (layers, broilers and meat turkeys), racing pigeons and individuals of natural populations obtained by hunting or sampled at bird rescue centres or during directed field campaigns, in order to determine possible reservoirs of these bacteria.
Materials and methods
Category of bird and sampling method
Commercial poultry
Out of 260 layer farms, 759 broiler farms and 42 turkey farms active in the 2012 Belgian National Animal Identification and Registration System (Sanitel) database, owned by the Federal Agency for the Safety of the Food Chain (FASFC), a random selection of farms was made, proportional to the farm density per province using SAS 9.2 (SAS Institute Inc., Cary, NC, USA). A selection of five replicate datasets was created, so that in case a farm had stopped or refused to collaborate, a different farm could be sampled.
During 2013 and 2014, the owners of the randomly selected farms (layers, broilers and meat turkeys) were asked to participate voluntarily and if agreeable, then 60 blood samples were collected per layer and turkey flock and 12 tracheal swabs were collected per broiler flock. Only one flock per farm was sampled. A different sampling method was chosen for broilers than for layers based on their short lifespan. The build-up of antibodies in Mycoplasma infections is very slow and it can take several weeks until seroconversion is reached and because broilers are slaughtered at the age of 6–7 weeks, the time to reach seroconversion is limited and can possibly lead to false negative results.
Racing pigeons
Nine owners of racing pigeons (56) presenting their birds at the veterinary clinic of the University of Ghent were asked to join the study. From each racing pigeon, one blood sample and one choanal swab were collected.
Wild birds
Hunters, contacted by the Flemish hunter association Hubertus, voluntarily collected 100 tracheal swabs from game birds (Order Anseriformes (36), Galliformes (7), Columbiformes (31) and Passeriformes (26)).
Blood samples from birds (197) kept in seven different bird rescue centres were collected (Order Anseriformes (26), Galliformes (8), Pelecaniformes (4), Accipitriformes (1), Gruiformes (2), Charadriiformes (16), Columbiformes (77), Strigiformes (27), Falconiformes (2) and Passeriformes (34)). All wild birds sampled by hunters and at bird rescue centres are listed in . Additionally, sera from geese (Anser anser (22), Branta canadensis (74)) and carrion crows (96), collected during a survey investigating West-Nile virus in 2012 in the region of Brussels, Bruges and the province Walloon Brabant and stored at our research facility, were included in this study. We also included results from conjunctival (401) and choanal (397) swabs collected during a directed field campaign from wild house sparrows in the region of Antwerp, Ghent and Leuven (2013–2014).
Table 1. Wild birds sampled by hunters and sampled at bird rescue centres in Belgium in 2013–2014.
Diagnosis of M. gallisepticum and M. synoviae
Rapid plate agglutination (RPA) test
The 60 serum samples from each layer and meat turkey flock were first analysed by RPA test. In brief, a drop of M. gallisepticum antigen (MG-RPA test, Soleil Diagnostics, Cantenay-Epinard, France) was laid on a glass plate and next to it, a drop of serum. These were then mixed by rocking the plate gently and reactions were read according to the manufacturer's instructions. If a positive result was observed, the sera were diluted, decomplemented and retested.
Enzyme-linked immunosorbent assay
All serum samples from layer and meat turkey flocks that gave a positive result with RPA were retested with a blocking ELISA kit (Svanovir® Mg-Ab kit, Boehringer Ingelheim Svanova, Uppsala, Sweden) within 5 days of sampling. This was done to exclude false positive reactions with RPA.
Sera from racing pigeons, birds from bird rescue centres and the sera collected from geese and carrion crows were tested with the same ELISA kit according to the manufacturer's instructions.
Polymerase chain reaction
DNA extraction was performed on each tracheal swab (VWR transport swab Amies charcoal 116C, VWR International, Radnor, PA, USA) from game birds, using a commercial DNA extraction kit (QIAamp® DNA mini kit, QIAGEN, Hilden, Germany) according to the manufacturer's recommendations but with modifications. Briefly, swabs were placed in microtubes containing 180 µl buffer ATL (tissue lysis buffer; QIAGEN) and 20 µl proteinase K and incubated at 56°C for 15 min. After centrifugation 200 µl of buffer AL (lysis buffer; QIAGEN) was added and the microtubes were then incubated at 70°C for 10 min. After incubation, 210 µl of ethanol 100% was added to the lysate. The samples were then washed and centrifuged following the manufacturer's recommendations. PCR was performed on the DNA extracts with a commercial PCR kit (ADIAVET™ Myco AV, Biomérieux, Craponne, France) testing for the presence of M. gallisepticum, M. synoviae and Mycoplasma meleagridis DNA, following the manufacturer's instructions. Amplification was performed using a GeneAmp 9700 thermal cycler (Applied Biosystems, Foster City, CA, USA). Detection of amplified products was performed on a 1.3% agarose gel, containing SYBR® Safe DNA gel stain (Invitrogen, Life technologies, Grand Island, NY, USA), in TBE 0.5× at a constant voltage of 70 V for 1.5 hours and visualized under ultraviolet light.
Samples from house sparrows were analysed using a different PCR. Briefly, DNA was extracted from conjunctival and choanal swabs with the extraction kit Prepman® Ultra (Applied Biosystems). A PCR was performed using GPO-1 and MGSO genus primers (1 µM) with mastermix (12.5 µl), one µl of DNA and 6.5 µl of distilled water. A cycling programme of 5 min at 95°C, 35 cycles of 1 min at 94°C, 30 sec at 58°C and 1.5 min at 72°C, followed by 5 min at 72°C was performed.
Real-time PCR
Since broilers are slaughtered at the age of 6–7 weeks, a diagnostic method that detects infections in an earlier phase than RPA or ELISA was necessary. Therefore samples were analysed using a multiplex real-time PCR (RT-PCR). This also allowed us to gather data on the prevalence of M. synoviae in broilers.
DNA was extracted from each tracheal swab from broilers using the DNA extraction kit as described above. RT-PCR was performed on the DNA extracts for the detection of the presence of M. gallisepticum and M. synoviae DNA (ADIAVET™ Myco AV, Biomérieux) using an ABI Prism 7500 thermal cycler (Applied Biosystems), following the manufacturer's instructions. In each well of a 96-well microplate (MicroAmp optical 96-well reactor plate, (Applied Biosystems), 23 µl of the prepared amplification solution was added together with 2 µl of DNA extract. The following cycling conditions were used, 2 min at 50°C, 10 min at 95°C, followed by 30 sec at 95°C for 45 cycles and 1 min and 30 sec at 60°C.
Statistical analysis
All prevalence results and 95% confidence intervals were estimated using “Estimated true prevalence with an imperfect test”, a tool provided by Epitools (Sergeant, Citation2016). The estimated value that was chosen in this study was the apparent prevalence (Wilson CL: Brown et al., Citation2001). The within-flock prevalence was based on the average positives within positive flocks and the average flock size in positive flocks, estimated using the same tool as described above.
Results
Mycoplasma gallisepticum
displays all the locations where samples were taken and where M. gallisepticum-negative (black) and M. gallisepticum-positive (red) birds were found. Eighty-seven layer farms were first tested with RPA. Out of these layer farms, six (6.9%; 95% CI: 3.2–14.2%) demonstrated a positive result. To exclude any possible false positive reactions, positive serum samples were retested with ELISA and only two layer farms (2.3%; 95% CI: 0.6–8%) remained positive after confirmation. The within-flock prevalence in positive layer farms was 39.2% (95% CI: 28.6–52.6%). Samples from 102 broiler farms were analysed with RT-PCR and eight farms (7.8%; 95% CI: 4–14.7%) showed a positive result. The within-flock prevalence in broiler flocks was 34.4% (95% CI: 13.8–60.9%).
Figure 1. Map of Belgium showing all M. gallisepticum-negative (black) locations and M. gallisepticum-positive (red) locations in 2013–2014.
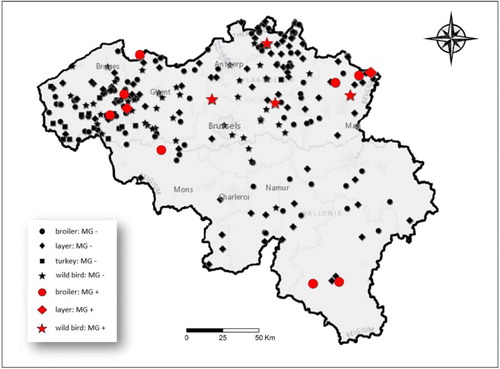
None of the 17 turkey farms that participated showed presence of antibodies for M. gallisepticum. Furthermore, in racing pigeons we did not detect any positive birds. The estimated bird prevalence in commercial poultry and racing pigeons is shown in together with the results from wild birds. Various wild birds () were investigated and one wood pigeon (Columba palumbus), one mallard (Anas plathyrhynchos), one Eurasian magpie (Pica pica) and two grey herons (Ardea cinerea) tested positive (1.7%; 95% CI: 0.7–3.9%). None of the 96 serum samples from carrion crows and 96 samples from greylag geese and Canadian geese showed a positive antibody titre for M. gallisepticum after ELISA testing.
Table 2. Prevalence of M. gallisepticum in commercial poultry, racing pigeons and wild birds in Belgium in 2013–2014.
shows all sites positive for M. gallisepticum. Eleven locations tested positive in Flanders and three in Wallonia. Two of these farms were located in the same province. shows the farm prevalence of broilers per province.
Table 3. Flock prevalence of M. gallisepticum per province in broilers in Belgium in 2013–2014.
Mycoplasma synoviae
shows all positive locations for M. synoviae (red). Samples from 102 broiler farms were analysed. Twenty-seven farms were positive (26.5%; 95% CI: 18.9–35.8%) and the within-flock prevalence in broiler farms was 48.8% (95% CI: 25.4–74.6%). shows the bird prevalence of M. synoviae in broilers compared to wild birds and house sparrows. Of the samples from wild birds, one carrion crow and three wood pigeons (4%; 95% CI: 1.6–9.8%) tested positive for M. synoviae after PCR amplification. We also obtained positive results from 12 conjunctival swabs and 5 choanal swabs from wild house sparrows.
Figure 2. Map of Belgium showing all M. synoviae-negative (black) locations and M. synoviae-positive (red) locations in 2013–2014.
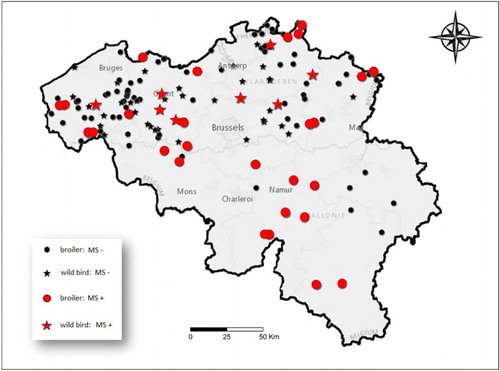
Table 4. Prevalence of M. synoviae in broilers, wild birds and house sparrows in Belgium in 2013–2014.
Discussion
Our findings show a low estimated prevalence of M. gallisepticum in and within both layer and broiler farms. Only at the farm level, we did detect a prevalence for M. gallisepticum that is somewhat higher in broilers than in layers which is probably due to the use of RT-PCR, which is a more sensitive detection method. Due to the different diagnostic methods used for layers and broilers, we have to remain cautious when comparing these values. These low prevalences may be explained by the reduction of vertical transmission due to the mandatory surveillance programme in breeder poultry flocks that aims to prevent and control spread from breeder hens to their offspring and protect national and international trade markets (EU, Citation2009, Citation2011). Our findings appear to correspond with other studies in Western Europe that found a low prevalence of M. gallisepticum in layers, broilers and meat turkeys which consistently decreased after including layers and meat turkeys in the official surveillance programme (Feberwee & Landman, Citation2012). It can be argued that collecting only 12 tracheal swabs per flock is not sufficient to declare a flock positive or negative and therefore could result in a possible underestimation of the prevalence in broilers at the farm level. Furthermore, we only sampled one flock per farm which could result in an underestimation of the true prevalence if biosecurity measures are well applied. However, we assumed, based on a study by Feberwee et al. (Citation2005) which demonstrated that the reproduction ratio R0 of M. gallisepticum is higher than 1, that once there is an infection in a flock it will spread quickly within the whole flock. The within-flock prevalence in both layers and broilers was nearly 40–50%, meaning that at the moment of sampling approximately half of the chickens within a positive flock were affected.
For meat turkeys, we were only able to test a small number of farms due to the low number of active farms in Belgium. None of the samples collected were positive and since there are few data available on meat turkeys in other European countries we are unable to confirm if our findings corresponded, although in the Netherlands, where meat turkeys are included in the official surveillance programme, there has been a decrease in positive flocks (Feberwee & Landman, Citation2012).
In our study none of the racing pigeons was M. gallisepticum-positive. Previous studies conducted in racing pigeons also showed very few positive birds (Keymer et al., Citation1984; Tsai & Lee, Citation2006). In these studies only Mycoplasma species that are typically associated with Columbiformes, such as Mycoplasma columbinum, Mycoplasma columborale and Mycoplasma columbinasale, were demonstrated in high frequency. In contrast to the racing pigeons, in the group of wild birds, one wood pigeon was found positive for M. gallisepticum. Although the prevalence in wood pigeons is low, it could be advised to take precautions as these birds prevail through the country and thus could potentially infect nearby farms. In other wild birds, the number of positive birds was also low. The presence of M. gallisepticum was demonstrated in one mallard, two grey herons and one Eurasian magpie. This is the first description of the presence of M. gallisepticum in grey herons or Eurasian magpies.
By using a multiplex RT-PCR kit, we also obtained data on the prevalence of M. synoviae in broilers. We found about one quarter of farms was positive, which is substantially higher than for M. gallisepticum. Again we mention that there is a possible underestimation of the prevalence at the farm level as stated above. Similar results on M. synoviae have been published by Feberwee et al. (Citation2008) in the Netherlands who found a seroprevalence of 35%, 6% and 6% in broiler parent, broiler parent rearing and broiler farms, respectively.
Four wild birds were M. synoviae-positive, one carrion crow and three wood pigeons. We also found 12 positive house sparrows. Previous studies have shown the presence of both M. gallisepticum and M. synoviae in a broad range of wild birds such as geese, pheasants, partridges, house finches, falcons, buzzard, house sparrows and many other avian species (Buntz et al., Citation1986; Poveda et al., Citation1990a, Citation1990b; Hoffman et al., Citation1997; Ganapathy & Bradbury, Citation1998; Bradbury et al., Citation2001; Luttrell et al., Citation2001; Pennycott et al., Citation2005; Lierz et al., Citation2008; Loria et al., Citation2008; Vitula et al., Citation2011; Stipkovits & Szathmary, Citation2012; Dhondt et al., Citation2014). Based on our results we consider the role of pigeons and other wild birds as a reservoir for pathogenic mycoplasmas for poultry to be minor, but they can probably act as transmitters of the bacteria. Previous studies in the USA concerning the endemic situation of M. gallisepticum in house finches have demonstrated that the strains originate from one single source and are distinct from poultry strains (Delaney et al., Citation2012; Tulman et al., Citation2012; Hochachka et al., Citation2013; Dhondt et al., Citation2014). Thus it still remains unsure which bird species pose a threat to other species.
Most mycoplasmas tend to be host specific, although certain avian Mycoplasma species such as M. gallisepticum and M. synoviae have been found in many different orders of birds. There does seem to be a preference for the Galliformes (Kleven, Citation1998) but the reason for this remains to be elucidated. Infections can spread through airborne routes, or by direct or indirect contact. Therefore, the proximity to the poultry site of other Galliformes such as backyard fowl and fancy breed chickens can be a potential threat of spreading infection into commercial holdings. During the same period as our prevalence study in commercial poultry, a similar study in fancy breed chickens was performed in Belgium (Haesendonck et al., Citation2014). These birds were kept for ornamental purposes and often brought to exhibitions, therefore posing a risk for disease transmission (de Wit et al., Citation2004). Haesendonck et al. (Citation2014) demonstrated a very high prevalence of both M. gallisepticum and M. synoviae with 73.2% and 96.4% of flocks, respectively, being positive, suggesting that this group of birds might act as a potential reservoir for Mycoplasma. In another study in Switzerland, similar results were obtained (Wunderwald & Hoop, Citation2002). Owners of these birds may also be a source of infection for commercial poultry rearing, and strict biosecurity measures should be kept against people entering farms.
In conclusion, our findings seem to be consistent with other European studies which suggest that eradication programmes have decreased the prevalence of M. gallisepticum in commercial poultry dramatically by drastically reducing vertical transmission. In Belgium for M. synoviae no such programme has been established yet, and our results show that there might be a threat of this bacterium to general poultry health. The question remains as to which birds pose a threat to which other birds. The low prevalence found in wild birds suggests that they probably play a limited role as reservoirs but we cannot exclude a possible impact on the transmission of Mycoplasma species. Further work is needed to establish the link between infections in fancy breed chickens, wild birds and commercial poultry.
Acknowledgements
We would like to thank Animal Health Care Flanders and ARSIA for their skillful technical assistance in collecting and analysing the samples. We would also like to thank all collaborating hunters and staff of the bird rescue centres for their expertise and assistance. And finally, the Department of Virology at our research facility for the serum collection of wild crows and geese.
Additional information
Funding
References
- Bencina, D., Dorrer, D. & Tadina, T. (1987). Mycoplasma species isolated from six avian species. Avian Pathology, 16, 653–664. doi: 10.1080/03079458708436413
- Bencina, D., Mrzel, I., Rojs, O.Z., Bidovec, A. & Dovc, A. (2003). Characterisation of Mycoplasma gallisepticum strains involved in respiratory disease in pheasants and peafowl. The Veterinary Record, 152, 230–234. doi: 10.1136/vr.152.8.230
- Bencina, D., Tadina, T. & Dorrer, D. (1988). Natural infection of ducks with Mycoplasma synoviae and Mycoplasma gallisepticum and Mycoplasma egg transmission. Avian Pathology, 17, 441–449. doi: 10.1080/03079458808436462
- Bradbury, J.M. (2007). Biosecurity and vaccination control Mycoplasma infections. World Poultry, 23, 35–36.
- Bradbury, J.M., Yavari, C.A. & Dare, C.M. (2001). Mycoplasmas and respiratory disease in pheasants and partridges. Avian Pathology, 30, 391–396. doi: 10.1080/03079450120066395
- Brown, L.D., Cai, T.T. & DasGupta, A. (2001). Interval estimation for a binomial proportion. Statistical Science, 16, 101–133.
- Buntz, B., Bradbury, J.M., Vuillaume, A. & Rousselot-Paillet, D. (1986). Isolation of Mycoplasma gallisepticum from geese. Avian Pathology, 15, 615–617. doi: 10.1080/03079458608436320
- Catania, S., Bilato, D., Gobbo, F., Granato, A., Terregino, C., Iob, L. & Nicholas, R.A.J. (2010). Treatment of eggshell abnormalities and reduced egg production caused by Mycoplasma synoviae infection. Avian Diseases, 54, 961–964. doi: 10.1637/9121-110309-Case.1
- Cookson, K.C. & Shivaprasad, H.L. (1994). Mycoplasma gallisepticum infection in chukar partridges, pheasants, and peafowl. Avian Diseases, 38, 914–921. doi: 10.2307/1592135
- Cordy, D.R. & Adler, H.E. (1965). Brain and muscle lesions caused by Mycoplasma gallisepticum in turkey poults. The American Journal of Veterinary Research, 26, 186–190.
- Delaney, N.F., Balenger, S., Bonneaud, C., Marx, C.J., Hill, G.E., Ferguson-Noel, N., Tsai, P., Rodrigo, A. & Edwards, S.V. (2012). Ultrafast evolution and loss of CRISPRs following a host shift in a novel wildlife pathogen, Mycoplasma gallisepticum. PLoS Genetics, 8, e1002511. doi: 10.1371/journal.pgen.1002511
- Dhondt, A.A., Altizer, S., Cooch, E.G., Davis, A.K., Dobson, A., Driscoll, M.J.L., Hartup, B.K., Hawley, D.M., Hochachka, W.M., Hosseini, P.R., Jennelle, C.S., Kollias, G.V., Ley, D.H., Swarthout, E.C.H. & Sydenstricker, K.V. (2005). Dynamics of a novel pathogen in an avian host: mycoplasmal conjunctivitis in house finches. Acta Tropica, 94, 77–93. doi: 10.1016/j.actatropica.2005.01.009
- Dhondt, A.A., DeCoste, J.C., Ley, D.H. & Hochachka, W.M. (2014). Diverse wild bird host range of Mycoplasma gallisepticum in eastern North America. PloS One, 9, e103553. doi: 10.1371/journal.pone.0103553
- El-Shabiny, L.M., Shaker, M.M. & Ouda, S.E. (2005). The application of a recent technique for diagnosis of Mycoplasma gallisepticum infection from migratory quail. Veterinary Medical Journal Giza, 53, 143–152.
- EU. (2009). COUNCIL DIRECTIVE 2009/158/EC of 30 November 2009 on animal health conditions governing intra-Community trade in, and imports from third countries of, poultry and hatching eggs. Official Journal of the European Communities, 55, L343/374–L/113.
- EU. (2011). COMMISSION DECISION of 1 April 2011 amending Annexes II to IV to Council Directive 2009/158/EC on animal health conditions governing intra-Community trade in, and imports from third countries of, poultry and hatching eggs. Official Journal of the European Communities, 54, L90/27–L90/49.
- Evans, J., Leigh, S., Branton, S., Collier, S., Pharr, G. & Bearson, S. (2005). Mycoplasma gallisepticum: current and developing means to control the avian pathogen. Journal of Applied Poultry Research, 14, 757–763. doi: 10.1093/japr/14.4.757
- Farmer, K.L., Hill, G.E. & Roberts, S.R. (2005). Susceptibility of wild songbirds to the house finch strain of Mycoplasma gallisepticum. Journal of Wildlife Diseases, 41, 317–325. doi: 10.7589/0090-3558-41.2.317
- Faustino, C.R., Jennelle, C.S., Connolly, V., Davis, A.K., Swarthout, E.C., Dhondt, A.A. & Cooch, E.G. (2004). Mycoplasma gallisepticum infection dynamics in a house finch population: seasonal variation in survival, encounter and transmission rate. Journal of Animal Ecology, 73, 651–669. doi: 10.1111/j.0021-8790.2004.00840.x
- Feberwee, A. & Landman, W. (2012). The successful implementation of Mycoplasma gallisepticum monitoring and control programmes in Dutch commercial poultry: a declining seroincidence during an eleven year period. Abstracts of the 19th Congress of the International Organization for Mycoplasmology (p. 153). Toulouse, France.
- Feberwee, A., Mekkes, D.R., Klinkenberg, D., Vernooij, J.C.M., Gielkens, A.L.J. & Stegeman, J.A. (2005). An experimental model to quantify horizontal transmission of Mycoplasma gallisepticum. Avian Pathology, 34, 355–361.
- Feberwee, A., de Vries, T.S. & Landman, W.J. (2008). Seroprevalence of Mycoplasma synoviae in Dutch commercial poultry farms. Avian Pathology, 37, 629–633. doi: 10.1080/03079450802484987
- Feberwee, A., de Wit, J.J. & Landman, W.J. (2009). Induction of eggshell apex abnormalities by Mycoplasma synoviae: field and experimental studies. Avian Pathology, 38, 77–85. doi: 10.1080/03079450802662772
- Ganapathy, K. & Bradbury, J.M. (1998). Pathogenicity of Mycoplasma gallisepticum and Mycoplasma imitans in red-legged partridges (Alectoris rufa). Avian Pathology, 27, 455–463. doi: 10.1080/03079459808419369
- Gharaibeh, S. & Hailat, A. (2011). Mycoplasma gallisepticum experimental infection and tissue distribution in chickens, sparrows and pigeons. Avian Pathology, 40, 349–354. doi: 10.1080/03079457.2011.582480
- Gross, W.B. (1990). Factors affecting the development of respiratory disease complex in chickens. Avian Diseases, 34, 607–610. doi: 10.2307/1591252
- Haesendonck, R., Verlinden, M., Devos, G., Michiels, T., Butaye, P., Haesebrouck, F., Pasmans, F. & Martel, A. (2014). High seroprevalence of respiratory pathogens in hobby poultry. Avian Diseases, 58, 623–627. doi: 10.1637/10870-052314-ResNote.1
- Hochachka, W.M., Dhondt, A.A., Dobson, A., Hawley, D.M., Ley, D.H. & Lovette, I.J. (2013). Multiple host transfers, but only one successful lineage in a continent-spanning emergent pathogen. Proceedings of the Royal Society B: Biological Sciences, 280, 20131068. doi: 10.1098/rspb.2013.1068
- Hoffman, R.W., Luttrell, M.P., Davidson, W.R. & Ley, D.H. (1997). Mycoplasmas in wild turkeys living in association with domestic fowl. Journal of Wildlife Diseases, 33, 526–535. doi: 10.7589/0090-3558-33.3.526
- Jordan, F.T. (1975). Avian mycoplasma and pathogenicity – a review. Avian Pathology, 4, 165–174.
- Keymer, I.F., Leach, R.H., Clarke, R.A., Bardsley, M.E. & Mcintyre, R.R. (1984). Isolation of Mycoplasma spp. from racing pigeons (Columba livia). Avian Pathology, 13, 65–74. doi: 10.1080/03079458408418509
- Kleven, S.H. (1998). Mycoplasmas in the etiology of multifactorial respiratory disease. Poultry Science, 77, 1146–1149. doi: 10.1093/ps/77.8.1146
- Kleven, S.H. (2008). Mycoplasmosis. In Y.M. Saif, A.M. Fadly, J.R. Glisson, L.R. McDougald, L.K. Nolan & D.E. Swayne (Eds.), Diseases of Poultry 12th edn (pp. 805–807). Ames, IA: Blackwell Publishing Professional.
- Kleven, S.H., Fletcher, O.J. & Davis, R.B. (1975). Influence of strain of Mycoplasma synoviae and route of infection on development of synovitis or airsacculitis in broilers. Avian Diseases, 19, 126–135. doi: 10.2307/1588963
- Landman, W.J. (2014). Is Mycoplasma synoviae outrunning Mycoplasma gallisepticum? A viewpoint from the Netherlands. Avian Pathology, 43, 2–8. doi: 10.1080/03079457.2014.881049
- Landman, W.J. & Feberwee, A. (2001). Field studies on the association between amyloid arthropathy and Mycoplasma synoviae infection, and experimental reproduction of the condition in brown layers. Avian Pathology, 30, 629–639. doi: 10.1080/03079450120092125
- Levisohn, S. & Kleven, S.H. (2000). Avian mycoplasmosis (Mycoplasma gallisepticum). Revue scientifique et technique (International Office of Epizootics), 19, 425–442.
- Ley, D.H., Berkhoff, J.E. & Levisohn, S. (1997). Molecular epidemiologic investigations of Mycoplasma gallisepticum conjunctivitis in songbirds by random amplified polymorphic DNA analyses. Emerging Infectious Diseases, 3, 375–380. doi: 10.3201/eid0303.970318
- Lierz, M., Hagen, N., Hernadez-Divers, S.J. & Hafez, H.M. (2008). Occurrence of mycoplasmas in free-ranging birds of prey in Germany. Journal of Wildlife Diseases, 44, 845–850. doi: 10.7589/0090-3558-44.4.845
- Lockaby, S.B. & Hoerr, F.J. (1999). Virulence of Mycoplasma synoviae in poultry: a review. World's Poultry Science Journal, 55, 175–185. doi: 10.1079/WPS19990012
- Lockaby, S.B., Hoerr, F.J., Lauerman, L.H. & Kleven, S.H. (1998). Pathogenicity of Mycoplasma synoviae in broiler chickens. Veterinary Pathology, 35, 178–190. doi: 10.1177/030098589803500303
- Lockaby, S.B., Hoerr, F.J., Lauerman, L.H., Smith, B.F., Samoylov, A.M., Toivio-Kinnucan, M.A. & Kleven, S.H. (1999). Factors associated with virulence of Mycoplasma synoviae. Avian Diseases, 43, 251–261. doi: 10.2307/1592615
- Loria, G.R., Ferrantelli, E., Giardina, G., Li Vecchi, L., Sparacino, L., Oliveri, F., McAuliffe, L. & Nicholas, R.A. J. (2008). Isolation and characterization of unusual Mycoplasma spp. from captive Eurasian Griffon (Gyps fulvus) in Sicily. Journal of Wildlife Diseases, 44, 159–163. doi: 10.7589/0090-3558-44.1.159
- Luttrell, M.P., Stallknecht, D.E., Fischer, J.R., Sewell, C.T. & Kleven, S.H. (1998). Natural Mycoplasma gallisepticum infection in a captive flock of house finches. Journal of Wildlife Diseases, 34, 289–296. doi: 10.7589/0090-3558-34.2.289
- Luttrell, M.P., Stallknecht, D.E., Kleven, S.H., Kavanaugh, D.M., Corn, J.L. & Fischer, J.R. (2001). Mycoplasma gallisepticum in house finches (Carpodacus mexicanus) and other wild birds associated with poultry production facilities. Avian Diseases, 45, 321–329. doi: 10.2307/1592971
- Mohammed, H.O., Carpenter, T.E. & Yamamoto, R. (1987). Economic impact of Mycoplasma gallisepticum and M. synoviae in commercial layer flocks. Avian Diseases, 31, 477–482. doi: 10.2307/1590727
- Nagatomo, H., Kato, H., Shimizu, T. & Katayama, B. (1997). Isolation of mycoplasmas from fantail pigeons. The Journal of Veterinary Medical Science/The Japanese Society of Veterinary Science, 59, 461–462. doi: 10.1292/jvms.59.461
- Pennycott, T.W., Dare, C.M., Yavari, C.A. & Bradbury, J.M. (2005). Mycoplasma sturni and Mycoplasma gallisepticum in wild birds in Scotland. The Veterinary Record, 156, 513–515. doi: 10.1136/vr.156.16.513
- Poveda, J.B., Carranza, J., Miranda, A., Garrido, A., Hermoso, M., Fernandez, A. & Domenech, J. (1990a). An epizootiological study of avian mycoplasmas in southern Spain. Avian Pathology, 19, 627–633. doi: 10.1080/03079459008418718
- Poveda, J.B., Giebel, J., Kirchhoff, H. & Fernandez, A. (1990b). Isolation of mycoplasmas from a buzzard, falcons and vultures. Avian Pathology, 19, 779–783. doi: 10.1080/03079459008418729
- Ranck, M.F., Schmidt, V., Philipp, H.-C., Voss, M., Kacza, J., Richter, A., Fehlhaber, K. & Krautwald-Junghanns, M.-E. (2010). Mycoplasma synoviae-associated egg-pole shell defects in laying hens. Berliner und Münchener tierärztliche Wochenschrift, 123, 111–118.
- Sergeant, E.S.G. (2016). Epitools epidemiological calculators. AusVet Animal Health Services and Australian Biosecurity Cooperative Research Centre for Emerging Infectious Disease.
- Stipkovits, L. & Kempf, I. (1996). Mycoplasmoses in poultry. Revue scientifique et technique (International Office of Epizootics), 15, 1495–1525.
- Stipkovits, L. & Szathmary, S. (2012). Review: Mycoplasma infection of ducks and geese. Poultry Science, 91, 2812–2819. doi: 10.3382/ps.2012-02310
- Tsai, H.J. & Lee, C.Y. (2006). Serological survey of racing pigeons for selected pathogens in Taiwan. Acta Veterinaria Hungarica, 54, 179–189. doi: 10.1556/AVet.54.2006.2.5
- Tulman, E.R., Liao, X., Szczepanek, S.M., Ley, D.H., Kutish, G.F. & Geary, S.J. (2012). Extensive variation in surface lipoprotein gene content and genomic changes associated with virulence during evolution of a novel North American house finch epizootic strain of Mycoplasma gallisepticum. Microbiology, 158, 2073–2088. doi: 10.1099/mic.0.058560-0
- Vitula, F., Peckova, L., Bandouchova, H., Pohanka, M., Novotny, L., Jira, D., Kral, J., Ondracek, K., Osickova, J., Zendulkova, D., Rosenbergova, K., Treml, F. & Pikula, J. (2011). Mycoplasma gallisepticum infection in the grey partridge Perdix perdix: outbreak description, histopathology, biochemistry and antioxidant parameters. BMC Veterinary Research, 7, 34. doi: 10.1186/1746-6148-7-34
- Whithear, K. (1996). Control of avian mycoplasmoses by vaccination. Revue scientifique et technique (International Office of Epizootics), 15, 1527–1553.
- de Wit, J.J., van Eck, J.H., Crooijmans, R.P. & Pijpers, A. (2004). A serological survey for pathogens in old fancy chicken breeds in central and eastern part of The Netherlands. Tijdschrift voor Diergeneeskunde, 129, 324–327.
- Wunderwald, C. & Hoop, R.K. (2002). Serological monitoring of 40 Swiss fancy breed poultry flocks. Avian Pathology, 31, 157–162. doi: 10.1080/03079450120118649
- Wyrzykowski, B., Albaric, O., Moreau, S., Nguyen, F., Fleurance, R., Belluco, S., Wyers, M. & Colle, M.-A. (2013). Retrospective study of Mycoplasma gallisepticum meningoencephalitis in six turkey flocks in western France. Journal of Comparative Pathology, 148, 173–177. doi: 10.1016/j.jcpa.2012.06.006
