ABSTRACT
An infection trial and a production trial over 35 days were conducted in parallel to study the influence of feeding crimped kernel maize silage (CKMS) on the intestinal Campylobacter jejuni colonization and broiler performance, respectively. The CKMS was used at dietary inclusion levels of 15% and 30% in maize-based diets. Broilers were orally inoculated with 2 × 105 log cfu/ml C. jejuni on day 14. Four birds from each pen were randomly selected and killed by cervical dislocation on days 3, 6, 9, 14 and 21 post infection and intestinal contents from ileum, caeca and rectum as well as liver samples were taken. Body weight and feed consumption of broilers were registered on days 13, 22 and 35. On day 35, litter dry matter (DM) was measured and the condition of the foot pads was evaluated. There was no significant effect of CKMS on the colonization of C. jejuni. Body weight of the broilers supplemented with 15% CKMS was comparable with the control maize-based feed, whereas addition of 30% CKMS reduced broiler body weight (P < 0.001). However, DM intake and feed conversion ratio were the same in all three dietary treatments. Furthermore, the foot pad condition of broilers significantly improved with the inclusion of CKMS on broiler diets as a result of a higher DM content in the litter material. It is concluded that CKMS did not influence intestinal Campylobacter colonization, but improved the foot pad health of broilers.
Introduction
Campylobacter infection is the most frequently reported human food-borne gastroenteritis in industrialized countries, with 80–90% of infections being attributed to Campylobacter jejuni, due to the consumption of contaminated undercooked poultry meat and other poultry products (Lin, Citation2009; Hermans et al., Citation2011b, Citation2012). Despite being an enormous health problem in humans, broilers are the natural host for Campylobacter and are often colonized by 104–105 colony-forming units (cfu)/g content in the upper intestine and 108 cfu/g content or more in the lower intestine, mainly in the caeca (Hermans et al., Citation2011a). The probability of colonization increases with the age of the broilers, and around 60–80% of them are found to be positive at slaughter age worldwide. Infected livestock, rodents, flies, contaminated water, and lack of biosecurity measures are the probable sources of primary infection (Hald et al., Citation2004, Citation2007; Hermans et al., Citation2011b, Citation2012). Thus, on-farm control of Campylobacter in poultry is very important in relation to food safety and public health (Sahin et al., Citation2002; Lin, Citation2009).
Various methods including hygiene and biosecurity measures, drinking water treatment with organic acids, vaccination and application of prebiotics and probiotics in the feed have been evaluated to prevent Campylobacter colonization in poultry, however, none of these methods have been very successful (Hermans et al., Citation2011b). Besides these, the feeding of fermented wet feed was found to decrease the shedding of Campylobacter in broilers, which may have been due to low feed pH and high content of organic acids (Heres et al., Citation2003b). However, fermented compound feed with a porridge-like consistency has been associated with poor litter quality and loss of nutrients such as synthetic amino acids, especially lysine (Canibe & Jensen, Citation2003; Engberg et al., Citation2009). Therefore, the feeding strategy for poultry can be changed by supplementing a compound feed with fermented grains to avoid the negative impacts of feeding fermented a whole compound feed.
In line with this, fermented grain in the form of ground or crimped ensiled maize is now a common practice in pig nutrition as a basic ingredient for fermented liquid feed. Due to a rise in temperature in Northern Europe, maize can now be grown to full ripeness in Denmark and is harvested in November with an approximate moisture content of 40%. To achieve storage stability, the maize is ensiled, which is cheaper than artificial drying of the grains. In addition to the reduced production cost, ensiled maize may further improve the gut health of poultry keeping the positive results of feeding fermented feed in mind. However, to the best of our knowledge, the scientific literature is lacking on crimped kernel maize silage (CKMS) and its role on gut microbiota. Therefore, the influence of CKMS on broiler performance and on the intestinal colonization of C. jejuni was investigated applying a broiler infection model.
Materials and methods
Experimental design
The experiments were carried out following the guidelines of The Animal Experiments Inspectorate (Danish Ministry of Environment and Food, Danish Veterinary and Food Administration, Ref. No. 2013-15-2934-00819) regarding animal experimentation and care of animals under study. Two experiments, a production trial and an infection study, were carried out in parallel with Ross 308 male broilers from day 1 to day 35. For the infection study, a total of 450-day-old male broiler chicks were randomly distributed to three dietary treatments. Each treatment consisted of five replicate pens with 30 birds per pen. For the production trial, 300-day-old male broiler chicks were reared which were also divided randomly into three dietary treatments and were distributed to 15 pens (20 birds per pen) with five replicate pens per treatment. In both studies, replicate pens were distributed randomly in the broiler house. All birds were wing-tagged and reared in pens with a floor area of 1.7 m2, covered by the same amount of wood shavings as bedding material in all the pens. The two trials were carried out in two individual and isolated rooms equipped with the facilities for automatic control of temperature, light and humidity. For the first three days, the temperature was maintained at 33°C and thereafter gradually reduced by 0.6°C per day until it reached 21°C. The temperature was then maintained at 21°C for rest of the experiment. The relative humidity was 45% in week 1, 50% in week 2, 55% in the week 3 and 60% in weeks 4 and 5. Light was provided for 24 h on day 1, 23 h on days 2–5, 16 h on days 6–13 and 19.5 h during the remaining period.
Characterization of crimped kernel maize
The CKMS used in this study was obtained from a commercial pig producer in Southern Denmark. The maize was harvested, crimped and ensiled in November 2014 for approximately 8 weeks with the addition of an organic acids mixture containing formic acid, propionic acid, benzoic acid and ammonium formate (Kemira AIV Pro®, Kemira, Finland). The CKMS was stored in 20-kg evacuated plastic bags at −20°C to maintain a similar quality throughout the feeding trial. According to the method described by Engberg et al. (Citation2004), CKMS was analysed for dry matter (DM), pH and microbial counts [coliform bacteria, lactic acid bacteria (LAB), yeast and mould]. In short, DM was analysed following freeze-drying of the samples. Coliform bacteria were enumerated on MacConkey agar (Merck KGaA, Darmstadt, Germany 1054650500) incubated at 38°C for 24 h and LAB were counted on MRS agar (Merck KGaA, 1106600500) incubated in an anaerobic cabinet at 38°C for 48 h. Yeast and mould were enumerated on Malt Chloramphenicol Agar incubated aerobically at 25°C for 48 h. The Malt Chloramphenicol Agar plates were prepared as described by Engberg et al. (2004).
The content of crude protein (CP, N × 6.25) in the CKMS was determined using the DUMAS method (Helrich, Citation1990). The ash was analysed according to the method described by Helrich (Citation1990), and fat was extracted with diethyl ether after acid hydrolysis (Stoldt, Citation1952). The analyses of starch, non-starch polysaccharides (NSP) and lignin were conducted using the method as described (Knudsen, Citation1997). The short-chain fatty acids and lactic acids were analysed as described (Jensen et al., Citation1995).
Composition of experimental diets
Three dietary treatments were included in the experiments. The compositions of the starter and all grower diets are given in . The starter pelleted diet (1–7 days), containing 265.8 g/kg CP and 13.54 MJ/kg gross energy, was the same for all dietary treatments. In the growing period (8–35 days), the birds were fed with three different grower diets: All three grower diets contained maize as the major feed ingredient and were formulated without addition of enzymes and coccidiostat. Group 1 received a diet containing 58% maize (maize-based feed, MBF). Groups 2 and 3 received MBF supplemented with 15% CKMS (CKMS-15) and 30% CKMS (CKMS-30), respectively. In CKMS-15 and CKMS-30, the maize was replaced by the CKMS on a DM basis.
The CKMS was thawed overnight and mixed with the pelleted grower feeds before being fed to the birds. All three grower diets were formulated taking the DM content of CKMS into consideration to obtain the same nutritional composition after mixing. The ready-mixed grower diets were analysed for DM, CP, ash, fat, starch, organic acids, NSP and lignin following the methods mentioned above. The total sugar and phosphorus were analysed as described by Jacobsen (Citation1981) and Carlson and Poulsen (Citation2003), respectively. The gross energy was measured using a LECO AC 300 automated calorimeter system 789-500 (LECO, St Joseph, MI, USA). The apparent metabolisable energy (AME) was calculated using the formula: AME (MJ/kg) = 0.3431 × %Fat + 0.1551 × %Crude protein + 0.1669 × %Starch + 0.1301 × %Total sugar (Fisher et al., Citation2000). The numbers of LAB in the ready-mixed grower diets were counted on MRS agar as described above.
Campylobacter jejuni infection
Abeyta-Hunt-Bark (AHB) media was prepared by mixing 4% Heart Infusion agar (Difco Laboratories, Le Pont de Claix, France 244400) and 0.2% Yeast extracts (Merck KGaA, Darmstadt, Germany 1.03753) in distilled water (autoclave 15 min at 121°C). It was further added with Campylobacter growth supplement (1 amp/500 ml; Oxoid Limited, Basingstoke, England SR-0232), sodium cefoperazone (2 ml/500 ml; Sigma-Aldrich, Steinheim, Germany C4292), 4 ml rifampicin (2 ml/500 ml; Sigma, 13292461) and Amphotericin B, solubilized (2 ml/500 ml; Sigma, A9528). Cloacal swabs from the broilers (six birds/pen) were taken at day 13 on AHB media and incubated at 41°C for 48 h to ensure that the birds were negative for C. jejuni before infection. A similar procedure was also followed in the broilers from the production trial. Inoculation was carried out with C. jejuni (strain DVI-sc181), which had been isolated from intestinal content of infected broilers. After overnight incubation in Brain-heart infusion medium (Merck KGaA, 1104930500) at 41°C, the birds were inoculated individually by oral gavage of 1 ml of culture suspension (2 × 105 cfu/ml) at day 14 using a syringe.
On days 3, 6, 9, 14 and 21 post infection (p.i.), four birds were randomly selected from each pen and killed by cervical dislocation to collect the intestinal contents from ileum (the intestinal segment caudal to Meckel's diverticulum), caeca and rectum. From each bird within the same pen, equal amounts of intestinal content from each segment were collected and pooled by segment at each sampling time. The liver tissue was also collected at days 3, 6, 9, 14 and 21 p.i. At the end of experiment (day 35), the status of C. jejuni was checked in the broilers from the production trial by taking the cloacal swabs as mentioned above to determine whether cross infection had occurred.
Enumeration of Campylobacter jejuni
Two to–3 g of the contents from ileum, caecum and rectum were weighed and subsequently 1:10 serial dilutions were made in microaerophilic phosphate buffered saline and incubated on AHB media at 41°C for 48 hours for quantitative enumeration. For the liver tissue, 2–3 g of sample was taken aseptically after having sterilized the liver surface in a flame. The enumeration of C. jejuni was done on AHB agar at 41°C for 48 hours as described above for the intestinal samples. Only the liver samples from 3 days p.i. were enumerated in Bolton broth to check whether they were positive or negative for C. jejuni.
Production trial
Fresh feed and water were provided to the birds ad libitum throughout the trial. The body weight of the birds was measured individually at days 13, 22 and 35. The feed intake per pen was also recorded on the same days. Samples of the freshly prepared feed as well as pooled samples of feed remnants were used to determine the DM content in the feed for the calculation of DM intake and feed conversion ratio (FCR) of the birds. Dead birds were removed daily after registration of date, wing number and body weight. Body weight and DM intake of the dead birds were also considered when calculating FCR. All calculations regarding DM intake, and FCR were done on a DM basis.
Litter quality and foot pad scoring
Litter samples were collected from the same five different areas in all the pens and freeze-dried for the analysis of litter DM content. Foot pad scoring was done on the right foot of all birds from each pen after killing them by cervical dislocation on day 35. The foot pads were examined for lesions, and the severity of the lesions was scored as follows: score 0: no lesion, score 1: mild lesions and score 2: severe lesions as described by Ekstrand et al. (Citation1998). The number of score 0 feet was multiplied by 0, the number of score 1 feet was multiplied by 0.5 and the number of score 2 feet was multiplied by 2 to calculate the total cumulative lesion scores and their relative frequency.
Statistical analysis
A statistical analysis of the comparison of the colonization of C. jejuni was performed using the General Linear Models procedure of the SAS® according to the following general model: Yij = μ + αi + βj + (αβ)ij + εij, where Yij is the observed dependent variable, μ is the overall mean, αi is the effect of treatments (MBF, CKMS-15 and CKMS-30), βj is the effect of days, (αβ)ij is the interaction between treatment and days and εij is the random error.
The results of body weight, DM intake, FCR, foot pad score and litter DM were compared according to the general model: Yi = μ + αi + εi, where Yi is the observed dependent variable, μ is the overall mean, α is the effect of treatments (MBF, CKMS-15 and CKMS-30) and εi is the random error.
Results are given as least square means (LSMeans) with a pooled standard error (SE). Probability values below or equal to 0.05 were accepted to indicate significant differences between means.
Results
Biochemical and microbial analyses of CKMS and compound feeds
The pH and DM content of CKMS were 3.95 and 59.3%, respectively (). The LAB count in CKMS was determined to be 7.8 log cfu/g. Coliform bacteria, yeasts and mould were below the detection limit (<3.0 log cfu/g). Furthermore, concentrations of acetic acid (57.6 mmol/kg) and lactic acid (179.1 mmol/kg) were higher in CKMS compared to other organic acids. As presented in , the dietary DM content was higher in MBF (88.7%) compared to the diets containing CKMS-15 (84.5%) and CKMS-30 (80.4%). The contents of protein, ash and phosphorus per kg DM were similar in all diets. The fat content was slightly higher in diets containing CKMS than MBF (55 g/kg vs. 61–64 g/kg), however, the AME was similar in all three grower diets. Organic acid concentrations and LAB counts were higher in CKMS-30 followed by CKMS-15 and MBF ().
Table 1. Biochemical and microbial composition of CKMS.
Table 2. Composition of the starter and grower diets.
Counts of C. jejuni in cloacal swabs and intestinal contents
All the birds were determined to be negative for the shedding of C. jejuni before the inoculation. The broilers in the production trial that were used as negative controls remained negative for C. jejuni throughout the experiment. Counts of C. jejuni in the contents of ileum, caecum and rectum, and liver tissue did not vary between the three dietary treatments (). From all the intestinal segments, C. jejuni was isolated at day 3 p.i. with a count ranging from 6 to 8 log cfu/g which increased to 7–9 log cfu/g at day 21 p.i. A significant increase in the C. jejuni counts was observed on day 6 p.i. in all segments, and continued to increase until the end of the experiment (P < 0.001). All liver samples were positive for C. jejuni at day 3 p.i. A similar increase in the counts of C. jejuni was also determined in the liver tissue (P = 0.008) as the broilers grew older. However, no significant difference in the colonization level of C. jejuni was observed between the three dietary treatments in all intestinal segments or in liver tissues.
Table 3. Number of colony forming units (log cfu/g) of C. jejuni in intestinal content and liver tissue of broilers fed with maize-based diets at 3, 6, 9, 14 and 21 days post infection (p.i.)
Production performance
The body weight of the broilers between the dietary treatments MBF and CKMS-15 did not differ, but the body weight of broilers fed CKMS-30 was significantly lower throughout the growing period (). At day 35 (P < 0.001), body weight of the broilers in CKMS-30 (2354 g/bird) was lowered by 153 g/bird and 232 g/bird compared to the broilers in CKMS-15 and MBF, respectively. Except for day 13, the DM intake did not vary between dietary treatments. During the entire growth period, no significant difference was observed for FCR between the three dietary groups. The mortality of the broilers was low (2–3%) during the production trial and did not differ between the dietary treatments (P = 0.94).
Table 4. Body weight, feed DM intake and FCR of the broilers fed with maize-based diets on13, 22 and 35 days.
Litter DM and foot pad lesions
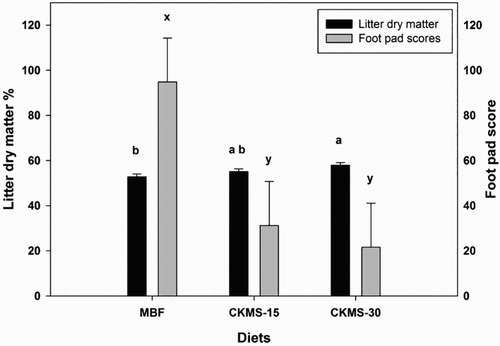
The DM content in the litter (P = 0.026) increased significantly with the addition of CKMS in the feed, and was highest in CKMS-30 (57.9%) compared to MBF (52.8%) and CKMS-15 (55.0%) (). The foot pad lesions further reflected the DM content of the litter. The foot pad score was significantly lower (P = 0.042) in the broilers supplemented with CKMS-15 (31.2) and CKMS-30 (21.6) compared with the very high score in birds receiving MBF (94.8).
Figure 1. Litter dry matter and foot pad lesions of broilers fed with MBF, maize-based feed with 15% crimped kernel maize silage (CKMS-15), and maize-based feed with 30% crimped kernel maize silage (CKMS-30) on day 35. LSMeans with different lower case letters (a & b for litter dry matter and x & y for foot pad scores) differ significantly (P < 0.05).
Discussion
Fermented feed has been shown to be beneficial for maintaining a healthy gastrointestinal tract due to its characteristics such as low pH, high numbers of lactobacilli and high concentrations of lactic acid and acetic acid (Canibe & Jensen, Citation2003, Citation2012; Engberg et al., Citation2009). The low pH of the fermented feed with a higher concentration of organic acids is suggested to acidify the upper digestive tract improving its barrier function especially against the acid sensitive pathogens. In line with this, a reduction in the susceptibility and shedding of Escherichia coli, Salmonella and Campylobacter in poultry were reported earlier with the feeding of fermented compound feed (Heres et al., Citation2003a, 2003b, Citation2004; Engberg et al., Citation2009).
The results of this experiment indicated no difference in the colonization of C. jejuni between the control MBF and the diets supplemented with CKMS. Unlike previous experiments (Heres et al., Citation2003b; Engberg et al., Citation2009) applying wet fermented compound feed, this study supplemented CKMS as a feed supplement to a dry compound feed. Broilers receiving wet fermented compound feed were reported to be less susceptible to intestinal colonization with C. jejuni (Heres et al., Citation2003b). The discrepancy in the result was probably due to the dissimilarity in the set-up of both studies. Another possible explanation could be the difference in the LAB counts between the wet fermented compound feed and diets containing CKMS. The LAB counts in CKMS-15 (6.4 log cfu/g) and CKMS-30 (6.9 log cfu/g) were approximately 1000 times lower than in fermented compound feed as previously reported by Engberg et al. (Citation2009). Keeping the high numbers of lactobacilli around 109 cfu/g of crop contents in mind, the contribution of CKMS containing about 107 cfu/g diet added at dietary levels of 30% will increase the lactobacilli population by only 1% in the crop. The numbers of LAB in the crop play an important role in preventing colonization from the undesirable and pathogenic bacteria in the lower digestive tract through a competitive exclusion mechanism (Juven et al., Citation1991). Therefore, the lower numbers of LAB in the feed supplemented with CKMS in comparison to wet fermented compound feed may be insufficient to increase the LAB population in the crop to induce a sufficient competitive exclusion at this location.
Other studies conducted with acidified feed using organic acids such as lactic acid (5.7%) and acetic acid (0.7%) (Heres et al., Citation2004), and acetic acid (1–2%) and sorbate (0.1%) (Skånseng et al., Citation2010) report a decrease in the colonization of Campylobacter in the lower digestive tract. It was further demonstrated that acidified drinking water with commercial organic acid (Selko-DWB, Selko Co., Tilburg, The Netherlands) prevented the spreading of Campylobacter in broilers (Chaveerach et al., Citation2004). Thus, organic acids play an important role in the prevention of Campylobacter infection. However, the concentration of organic acids such as lactic acid (160–250 mmol/kg) and acetic acid (20–30 mmol/kg) was higher in the wet fermented feed (Engberg et al., Citation2009) compared to feed supplemented with CKMS (), which may explain the ineffectiveness of CKMS on Campylobacter colonization.
In this study, a steady increase in the numbers of Campylobacter was observed as the broilers grew older (). Similar to this, Heres et al. (Citation2004) had reported that the number of colonized broilers increased after inoculation. This may be due to the use of high inoculation dose (105–107 cfu/ml) in both experiments. Furthermore in both studies, either the number of C. jejuni or the number of infected broilers had increased rapidly from days 5 to 6 p.i., demonstrating that C. jejuni can be easily established in the gut and increase their counts rapidly after infection. This emphasizes the importance of a Campylobacter prevention strategy in broilers as Campylobacter infections will be rather difficult to control once they have colonized in the gut. Any of the non-colonized broilers were found positive with respect to the shedding of C. jejuni at the end of the experiment which was attributed to the strict hygienic procedure maintained during the experiment. Therefore, it can be suggested that the cross-contamination or the transmission of the C. jejuni between the farms can be controlled through strict biosecurity measures.
The results of the current study showed comparable body weights in the control group (MBF) and the group fed with CKMS-15. However, a significant reduction in the body weight of broilers was determined when feeding the diet containing 30% CKMS. The body weight of broilers in CKMS-30 was 153–231 g lower than that of broilers receiving CKMS-15 and MBF, respectively. These results support our previous results (Ranjitkar et al., Citation2015). It has been shown that fermented feed has a positive influence on body weight with the feeding of 10% rapeseed meal (Chiang et al., Citation2010), fermented wet feed (water:feed ratio = 1.3:1) (Missotten et al., Citation2013), and 20–60% fermented dry barley or wheat (Skrede et al., Citation2003). In this feeding trial, DM intake and FCR in all three dietary treatments were similar (), suggesting that the lower body weight of broilers fed CKMS-30 was not due to the lower DM intake. Therefore, the decreased digestibility of the nutrients as a result of the dilution of digestive enzymes due to high moisture content in the diets supplemented with 30% CKMS () may have resulted in a reduced body weight of broilers. However, further studies will be needed to confirm this hypothesis.
Apart from the production performances of broilers, bird welfare is an emerging issue in the poultry meat industry, which is due to increased consumer concerns regarding bird welfare in intensive broiler production (Jonge & Trijp, Citation2013; Vanhonacker & Verbeke, Citation2014). The prevalence and severity of foot pad lesions are used as an indicator to estimate the general housing conditions and welfare standards of birds in different parts of the world. The moisture content in the litter is directly correlated with the development and severity of foot pad lesions in broilers (Ekstrand et al., Citation1997; Wang et al., Citation1998; Shepherd & Fairchild, Citation2010). In this connection, the foot pad scores of broilers fed CKMS-15 and CKMs-30 were significantly lower than those of broilers fed MBF, which is attributed to a higher DM content of the litter in the birds fed CKMS (). There was only a slight difference in the litter DM content between MBF (53%) and CKMS-15 (55%), but the foot pad scores in both groups were significantly different. Therefore, even the minor variation in the litter DM content seems to have a large impact on the development of foot pad lesions. As reported earlier (Ranjitkar et al., Citation2015), this suggests that, besides the litter DM content, other factors associated with the feeding of CKMS, might have impact on the development of foot pad lesions.
In conclusion, the supplementation of maize-based diets with CKMS did not significantly influence the intestinal colonization with C. jejuni in broilers The results of this study also supported the importance of biosecurity measures on the prevention of Campylobacter transmission between farms. Apart from the depressed body weight gain in broilers fed CKMS-30, no other negative effects on production performance were observed in relation to the dietary supplementation of CKMS. The increased litter DM content and improved foot pad health clearly suggested that the supplementation of CKMS in broiler diets has advantageous with respect to broiler welfare.
Acknowledgements
The authors are grateful to Trine Poulsen, Thomas Repsdorf and Karin Durup for their skilful technical assistance. Mette Eriksen is thanked for taking care of the experimental animals.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Canibe, N. & Jensen, B.B. (2003). Fermented and nonfermented liquid feed to growing pigs: effect on aspects of gastrointestinal ecology and growth performance. Journal of Animal Science, 81, 2019–2031.
- Canibe, N. & Jensen, B.B. (2012). Fermented liquid feed-microbial and nutritional aspects and impact on enteric diseases in pigs. Animal Feed Science and Technology, 173, 17–40. doi: 10.1016/j.anifeedsci.2011.12.021
- Carlson, D. & Poulsen, H.D. (2003). Phytate degradation in soaked and fermented liquid feed-effect of diet, time of soaking, heat treatment, phytase activity, pH and temperature. Animal Feed Science and Technology, 103, 141–154. doi: 10.1016/S0377-8401(02)00288-2
- Chaveerach, P., Keuzenkamp, D.A., Lipman, L.J.A. & Van Knapen, F. (2004). Effect of organic acids in drinking water for young broilers on Campylobacter infection, volatile fatty acid production, gut microflora and histological cell changes. Poultry Science, 83, 330–334. doi: 10.1093/ps/83.3.330
- Chiang, G., Lu, W.Q., Piao, X.S., Hu, J.K., Gong, L.M. & Thacker, P.A. (2010). Effects of feeding solid-state fermented rapeseed meal on performance, nutrient digestibility, intestinal ecology and intestinal morphology of broiler chickens. Asian-Australian Journal of Animal Sciences, 23, 263–271. doi: 10.5713/ajas.2010.90145
- Ekstrand, C., Algers, B. & Svedberg, J. (1997). Rearing conditions and foot-pad dermatitis in Swedish broiler chickens. Preventive Veterinary Medicine, 31, 167–174. doi: 10.1016/S0167-5877(96)01145-2
- Ekstrand, C., Carpenter, T.E., Andersson, I. & Algers, B. (1998). Prevalence and control of foot-pad dermatitis in broilers in Sweden. British Poultry Science, 39, 318–324. doi: 10.1080/00071669888845
- Engberg, R.M., Hedemann, M.S., Steenfeldt, S. & Jensen, B.B. (2004). Influence of whole wheat and xylanase on broiler performance and microbial composition and activity in the digestive tract. Poultry Science, 83, 925–938.
- Engberg, R.M., Hammershøj, M., Johansen, N.F., Abousekken, M.S., Steenfeldt, S. & Jensen, B.B. (2009). Fermented feed for laying hens: effects on egg production, egg quality, plumage condition and composition and activity of the intestinal microflora. British Poultry Science, 50, 228–239. doi: 10.1080/00071660902736722
- Fisher, C., Moughan, P.J., Verstegen, M.W.A. & Visser-Reyneveld, M.I. (2000). Advances in feed evaluation for poultry. Feed Evaluation: Principles and Practice, 243–268.
- Hald, B., Skovgård, H., Bang, D.D., Pedersen, K., Dybdahl, J., Jespersen, J.B. & Madsen, M. (2004). Flies and Campylobacter infection of broiler flocks. Emerging Infectious Diseases, 10, 1490–1492. doi: 10.3201/eid1008.040129
- Hald, B., Sommer, H.M. & Skovgård, H. (2007). Use of fly screens to reduce Campylobacter spp. introduction in broiler houses. Emerging Infectious Diseases, 13, 1951–1953. doi: 10.3201/eid1312.070488
- Helrich, K.C. (1990). Official Methods of Analysis of the AOAC. Volume 1, 777.
- Heres, L., Engel, B., Urlings, H.A.P., Wagenaar, J.A. & Van Knapen, F. (2004). Effect of acidified feed on susceptibility of broiler chickens to intestinal infection by Campylobacter and Salmonella. Veterinary Microbiology, 99, 259–267. doi: 10.1016/j.vetmic.2003.12.008
- Heres, L., Engel, B., Van Knapen, F., De Jong, M.C., Wagenaar, J.A. & Urlings, H.A. (2003a). Fermented liquid feed reduces susceptibility of broilers for Salmonella enteritidis. Poultry Science, 82, 603–611. doi: 10.1093/ps/82.4.603
- Heres, L., Engel, B., Van Knapen, F., Wagenaar, J.A. & Urlings, B.A. (2003b). Effect of fermented feed on the susceptibility for Campylobacter jejuni colonisation in broiler chickens with and without concurrent inoculation of Salmonella enteritidis. International Journal of Food Microbiology, 87, 75–86. doi: 10.1016/S0168-1605(03)00055-2
- Hermans, D., Pasmans, F., Messens, W., Martel, A., Van Immerseel, F., Rasschaert, G., Heyndrickx, M., Van Deun, K. & Haesebrouck, F. (2012). Poultry as a host for the zoonotic pathogen Campylobacter jejuni. Vector-Borne and Zoonotic Diseases, 12, 89–98. doi: 10.1089/vbz.2011.0676
- Hermans, D., Van Deun, K., Martel, A., Van Immerseel, F., Messens, W., Heyndrickx, M., Haesebrouck, F. & Pasmans, F. (2011a). Colonization factors of Campylobacter jejuni in the chicken gut. Veterinary Research, 42, 10–1186. doi: 10.1186/1297-9716-42-82
- Hermans, D., Van Deun, K., Messens, W., Martel, A., Van Immerseel, F., Haesebrouck, F., Rasschaert, G., Heyndrickx, M., & Pasmans, F. (2011b). Campylobacter control in poultry by current intervention measures ineffective: urgent need for intensified fundamental research. Veterinary Microbiology, 152, 219–228. doi: 10.1016/j.vetmic.2011.03.010
- Jacobsen, E. (1981). Sukker og stivelse (LHK)- ny analysemetode. Meddelelser fra Bioteknisk Institut A.T.V., 98, 39–54.
- Jensen, M.T., Cox, R.P. & Jensen, B.B. (1995). Microbial production of skatole in the hind gut of pigs given different diets and its relation to skatole deposition in backfat. Animal Science, 61, 293–304. doi: 10.1017/S1357729800013837
- Jonge, J. & Trijp, H.C. (2013). The impact of broiler production system practices on consumer perceptions of animal welfare. Poultry Science, 92, 3080–3095. doi: 10.3382/ps.2013-03334
- Juven, B.J., Meinersmann, R.J. & Stern, N.J. (1991). A review: antagonistic effects of lactobacilli and pediococci to control intestinal colonization by human enteropathogens in live poultry. Journal of Applied Bacteriology, 70, 95–103. doi: 10.1111/j.1365-2672.1991.tb04433.x
- Knudsen, K.E.B. (1997). Carbohydrate and lignin contents of plant materials used in animal feeding. Animal Feed Science and Technology, 67, 319–338. doi: 10.1016/S0377-8401(97)00009-6
- Lin, J. (2009). Novel approaches for Campylobacter control in poultry. Foodborne Pathogens and Disease, 6, 755–765. doi: 10.1089/fpd.2008.0247
- Missotten, J.A., Michiels, J., Dierick, N., Ovyn, A., Akbarian, A. & De Smet, S. (2013). Effect of fermented moist feed on performance, gut bacteria and gut histo-morphology in broilers. British Poultry Science, 54, 627–634. doi: 10.1080/00071668.2013.811718
- Ranjitkar, S., Karlsson, A.H., Petersen, M.A., Bredie, W.L., Petersen, J.S. & Engberg, R.M. (in press). The influence of feeding crimped kernel maize silage on broiler production, nutrient digestibility and meat quality. British Poultry Science.
- Sahin, O., Morishita, T.Y. & Zhang, Q. (2002). Campylobacter colonization in poultry: sources of infection and modes of transmission. Animal Health Research Reviews, 3, 95–105. doi: 10.1079/AHRR200244
- Shepherd, E.M. & Fairchild, B.D. (2010). Footpad dermatitis in poultry. Poultry Science, 89, 2043–2051. doi: 10.3382/ps.2010-00770
- Skånseng, B., Kaldhusdal, M., Moen, B., Gjevre, A., Johannessen, G.S., Sekelja, M., Trosvik, P. & Rudi, K. (2010). Prevention of intestinal Campylobacter jejuni colonization in broilers by combinations of in feed organic acids. Journal of Applied Microbiology, 109, 1265–1273. doi: 10.1111/j.1365-2672.2010.04766.x
- Skrede, G., Herstad, O., Sahlstrøm, S., Holck, A., Slinde, E. & Skrede, A. (2003). Effects of lactic acid fermentation on wheat and barley carbohydrate composition and production performance in the chicken. Animal Feed Science and Technology, 105, 135–148. doi: 10.1016/S0377-8401(03)00055-5
- Stoldt, W. (1952). Vorschlag zur vereinheitlichung der fettbestimmung in lebensmitteln. Fette und Seifen, 54, 206–207. doi: 10.1002/lipi.19520540406
- Vanhonacker, F. & Verbeke, W. (2014). Public and consumer policies for higher welfare food products: Challenges and opportunities. Journal of Agricultural and Environmental Ethics, 27, 153–171. doi: 10.1007/s10806-013-9479-2
- Wang, G., Ekstrand, C. & Svedberg, J. (1998). Wet litter and perches as risk factors for the development of foot pad dermatitis in floor-housed hens. British Poultry Science, 39, 191–197. doi: 10.1080/00071669889114
