ABSTRACT
Trichodinid ciliophorans are opportunistic parasites of many species of fish, amphibians, and molluscs, but yet never reported in association with lesions in birds. Postmortem and histopathological evaluation of a commercial adult Toulouse gander and female goose, and a wild Mallard drake revealed the presence of severe pathological parasitic colonization of their reproductive tracts. Histopathological findings included moderate to severe granulocytic inflammation, acanthosis, accentuation of the rete pegs, and proliferative hyperplastic squamous metaplasia of the mucosa of the ejaculatory ducts and groove, sulcus spermaticus, glandular part of the phallus (cavum penis), and oviduct in association with large numbers of ciliated protozoa anchored to the tissues or free in the lumen. These protozoa had characteristic morphological features analogous to the family of Trichodinidae. The source of this parasitism could not be determined. To our knowledge, this is the first report of trichodinosis associated with pathology in birds.
Introduction
Trichodinids are ciliated protozoa, 50–100 µm in diameter, of the family Trichodinidae, which can infest the skin and gills of many species of amphibians, fish, and mollusc invertebrates (Basson et al., Citation1990; Noga, Citation1996; Collymore et al., Citation2013). One of their characteristic features is the presence of a skeletal ring with radially arranged denticles, readily apparent in ventral view (Basson & Van As, Citation1989; Basson et al., Citation1990; Noga, Citation1996; Mitchell, Citation2007; Collymore et al., Citation2013). These organisms are motile, have a saucer-to-bell-shaped body that is about 84.5 ± 8.2 µm in diameter, a highly developed basal adhesive disc with denticulated (48–64) blades, and a dorsal zone of cilia arranged in a spiral. Traditionally, morphologic characteristics, such as the number, arrangement, and shape of the denticles, have been used to identify Trichodinidae at the species level using silver-staining methods (Basson & Van As, Citation1989; Basson et al., Citation1990; Noga, Citation1996; Mitchell, Citation2007).
Although their presence in low numbers is not related to disease, in poor water quality and overcrowding conditions they may be responsible for chronic morbidity and mortality in adult fish and high mortality in juveniles (Noga, Citation1996). Trichodinids utilize a sucking disc to anchor to their hosts and feed on suspended organic material and bacteria. Adherence and suction on the epithelium can induce morphological and inflammatory alterations and may result in high morbidity and mortality in highly infested young fish (Noga, Citation1996; Mitchell, Citation2007). Trichodina along with other genera, such as Vauchomia, Paratrichodina, and Trichodoxa, can colonize the urinary bladder, oviducts, or gastrointestinal tracts of fish, but there is no definitive proof of their pathogenicity in these organs (Mueller, Citation1938; Noga, Citation1996; Mitchell, Citation2007; Collymore et al., Citation2013). Their life cycle is direct, and they reproduce asexually by binary fission and sexually by conjugation. Trichodinids are transmitted primarily by direct contact with infected birds and they may be transmitted during copulation. These species can survive for a few hours in water outside the host and can also be transmitted via contaminated water and fomites (Mueller, Citation1938). Unlike most species of birds, Anseriformes (ducks, geese, and swans) have an elaborate corkscrew-like intromittent phallus in the males and a spiral vagina in the females (Hodges, Citation1974; Fujihara et al., Citation1976; King & McLelland, Citation1984; Etches, Citation1996; Brennan et al., Citation2007; Brennan et al., Citation2010). At rest, the phallus is inverted within the phallic sac (saccus phalli) in the right ventral wall of the cloaca (King & McLelland, Citation1984; Brennan et al., Citation2010). Semen is emitted from the vasa deferentia to the ejaculatory ducts after passing through a diverticulum (dilated portion of vas deferens). Then, it is ejected into a triangular ejaculatory groove which directs the flow into the sulcus spermaticus at the base of the phallus. In contrast to mammals, the semen is transported in an open groove (sulcus spermaticus or phallic sulcus) which runs on the dorsal surface of the phallus delimited by the fibrolymphatic bodies. In the Anseriformes, the centre of the phallus presents an elastic ligament and a blind-ending tubular cavity (cavum penis) which is surrounded by folds of mucus-secreting pseudostratified columnar epithelium (glandular part of the phallus). This deep blind-ending portion of the tube is non-erectile and is connected with the sulcus spermaticus through an opening at the apex of the phallus. Copulation generally takes place in water in ducks and wild geese, but on land in domestic fowl (Lake, Citation1957; Hodges, Citation1974; Szép et al., Citation1974; Fujihara et al., Citation1976; King & McLelland, Citation1984; Marius-Jestin et al., Citation1987; Etches, Citation1996; Brennan et al., Citation2007, Brennan et al., Citation2010). In goose venereally-transmitted diseases, infection can involve the phallus, vagina, and cloaca leading to infertility and drop in egg production (Szép et al., Citation1974; Szép et al., Citation1979; King & McLelland, Citation1984; Stipkovits et al., Citation1984; Stipkovits & Szathmary, Citation2012).
This report describes the parasitic infestation of the reproductive tract of a commercial Toulouse gander and female goose and of a wild Mallard drake submitted, respectively, to the Turlock and Fresno branches of the California Animal Health and Food Safety (CAHFS) laboratory system and the William R. Pritchard Veterinary Medical Teaching Hospital (VMTH) of the University of California, Davis (UC Davis). Gross and microscopic lesions of the reproductive tract, bacteriology and serology and morphological features of the Trichodinid ciliophorans are presented in this paper.
Materials and methods
Specimen collection, clinical and pathological examination
At the beginning of February 2015, one live male adult Toulouse goose (case no. 1) was submitted to the Turlock branch of the CAHFS laboratory system for necropsy evaluation. The bird was humanely euthanized with carbon dioxide. The diagnosis of the trichodinosis in this commercial Toulouse gander prompted a retrospective investigation of diagnostic reports which lead to the discovery of two other unpublished cases in California. The first report (case no. 2), dated March 1991, originated from the carcass of a wild, adult Mallard drake (Anas platyrhynchos) submitted to the UC Davis, VMTH, while an April 2005 report originated from the carcass of a 2-year-old commercial Toulouse female goose (case no. 3) submitted to the CAHFS, Fresno branch. Case nos. 1 and 3 originated from the same farm. The Toulouse is a domesticated breed of geese originally from France and derived from the Graylag goose (Anser anser). The diagnostic protocols for each case consisted of clinical history, necropsy and histopathology. Additional testing for the Toulouse gander (case no. 1) included bacteriology and biotechnology. Epidemiologic information of the Toulouse flock was also collected, such as number, species and breed of birds on the ranch, number of houses, pens and type of housing system, and type of production.
Case no. 1: Toulouse gander
The adult Toulouse gander belonged to a major duck and goose operation in California. At this farm, all gander genitalia were checked in preparation for the 2015 breeding season and all the individuals presenting pathological alterations compromising their ability to mate were removed. The gander submitted for necropsy was selected due to marked phallic alterations. The farm was a multi-age and multi-breed operation, raising several breeds of geese and ducks for the production of hatching eggs and other poultry products. At the time, there were approximately 3000 goose and 6000 duck breeders in total on the property. All of the goose eggs and most of the duck eggs produced were hatched and sold to individuals, feed stores or commercial growers throughout the United States. Some duck eggs were sold as fresh or hatching eggs and others were sold in the ethnic market in California as salted or embryonated (Balute) eggs. All the birds were brooded indoors. The different breeds of goslings and ducklings were raised together indoors in a semi-intensive system on deep litter with daily access to a pasture. Before the onset of sexual maturity, birds were sexed and moved to outdoor pens. Each pen housed about 500–700 birds and consisted of dirt and grass flooring and wire fencing and roofing. This specific Toulouse flock consisted of approximately 470 birds, out of which there were approximately 400 females and 70 males (∼1:6 ratio). A few feet of grass were present separating the pen from an adjacent canal. No stagnant water pools or ponds were observed inside the pens or houses. Birds were provided with food and drinking water ad libitum. Feed was produced locally and distributed to all the breeder flocks on site.
Case no. 2: Mallard drake
This was a wild, male Mallard that was found on the side of the road unable to walk in the vicinity of UC Davis, Yolo County, CA, in March of 1991, and was picked up by a passerby. It was reportedly exhibiting central neurological signs and bilateral leg paresis and had an ulcerated wound and feather loss on the tail. It was presented to the Zoological Medicine Service of the VMTH for evaluation and treatment. Radiographs did not reveal a cause for the neurological signs, but a mass was identified in the dorsal abdomen. On laparoscopy, the mass was determined to be an enlarged spleen. Foci of pallor suggestive of necrosis were seen in one testis and on the liver. The bird was euthanized, and organs were sampled by the clinician and submitted to the pathology service for histological examination.
Case no. 3: Toulouse female goose
One 2-year-old Toulouse female goose carcass was submitted in 2005 from the same commercial producer as the Toulouse gander. The bird was collected among the daily mortality of the flock for routine monitoring. An increase in mortality was not reported. No information regarding the number of birds on the ranch at the time nor other information could be retrieved.
Pathology
Complete necropsies were performed and tissue samples for histopathologic examination included testis, epididymis, vas deferens, ejaculatory ducts and groove, phallus, cloaca, ovary, oviduct, liver, spleen, trachea, lung, air sac, intestine, pancreas, kidney, adrenal, proventriculus, thyroid, parathyroid, nerves, skin, bones, and skeletal muscles. All tissue sections collected during necropsy were fixed in 10% neutral buffered formalin, routinely processed, and embedded in paraffin. Sections of 4 μm (CAHFS) and 5 μm thickness (VMTH) were stained with haematoxylin and eosin (HE) and examined by light microscopy. In the tissue sections from case no. 1, special stains including Gram, Periodic Acid Schiff, Congo Red, Giemsa, Gomori's Methenamine Silver and Hurtado's Leptospira Silver were used to determine the presence of bacteria, fungi, and parasites. In case no. 3, special stains, specifically Congo Red and Gomori's Methenamine Silver and Hurtado's Leptospira silver were also applied.
Additional diagnostic tests
Swabs from trachea, liver, testes, and phallus (seminal duct) of the gander (case no. 1) and liver and peritoneum of the female goose (case no. 3) were plated onto 5% sheep blood and MacConkey agar (Remel, Lenexa, KS, USA) and incubated at 37°C with 7% CO2 for a minimum of 48 h. In addition, swabs from trachea, testes, and phallus of the gander were cultured for Mycoplasma spp. in both Modified-Ortmayer and Frey's broths and plates (Biological Media Services, School of Veterinary Medicine, University of California, Davis, CA, USA) and incubated at 37°C with 7% CO2 for a minimum of 7 days. Swabs from the caeca of the female goose were placed in Tetrathionate broth enriched with iodine and brilliant green and inoculated at 48 h onto Xylose Lysine Tergitol-4 and MacConkey agar for salmonella isolation. No cultures were obtained from the wild duck (case no. 2).
Sera from five live geese from the gander flock (case no. 1) were tested by haemagglutination inhibition (HI) to detect antibody titres to avian influenza (AI), Newcastle disease (ND) virus, Mycoplasma gallisepticum (MG) and Mycoplasma synoviae (MS). Cloacal swabs collected from the two commercial geese (cases nos. 1 and 3) were tested for AI and ND by real time reverse transcriptase polymerase chain reaction. Water samples from two of the six drinkers from the commercial Toulouse flock (case no. 1) were collected in sterile plastic tubes for researching parasites. The tubes were centrifuged at 213 × g for 10 min and the supernatant removed. The bottom of the tube was sampled, placed on a glass slide and examined under the optical microscope. From case no. 1, one formalin fixed tissue section (size: ∼0.5 cm × 0.3 cm) of the ejaculatory duct was analysed by scanning electron microscopy at the Electron Microscopy Laboratory of the Department of Pathology and Laboratory Medicine (UC-Davis).
Results
Gross findings
Case no. 1: Toulouse gander
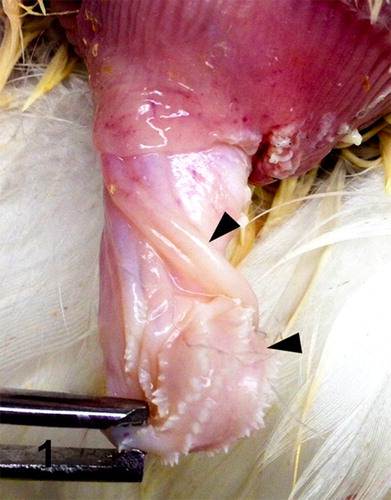
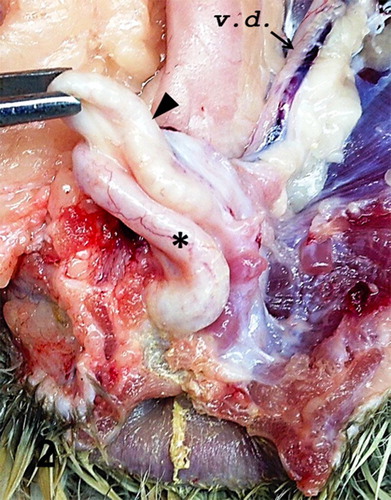
The bird was submitted alive, in a good state of nutrition and hydration. Lesions were confined to the phallus and compromised the physical integrity of the organ. The phallus was moderately reduced in size, flaccid, oedematous, and whitish in colour with accentuated and congested capillaries visible on the surface. Apical phallic loops were atrophied with alteration of the typical spiral architecture. The right fibrolymphatic body was prominent over the sulcus spermaticus, on the external surface of the phallus (). On sectioning, the ejaculatory ducts were prominent, visibly thickened, with congestion, reddish colouration of the mucosa and exuding whitish, opaque, viscous material (). There was mild testicular atrophy, the right side being smaller and measuring ∼1.8 cm longitudinally and ∼1.5 cm in diameter compared with the left which was 2.5 cm × 2.0 cm. No other macroscopic lesions were observed.
Case no. 2: Mallard drake
The report of the gross examination of the submitted tissues listed the presence of multifocal whitish areas of presumptive necrosis in the liver, splenomegaly, and large testes measuring 7.5 cm × 3.0 cm × 3.5 cm and 7.0 cm × 3.0 cm × 2.5 cm. One testis had a focus of pallor (presumed necrosis). There were similar pale areas in a section of liver.
Case no. 3: female goose
The bird was mildly emaciated and the internal visceral organs were moderately autolysed. There was accumulation of a large amount of watery, milky white exudate and ascites with small to large flakes of exudate in the abdominal cavity, consistent with yolk peritonitis. The oviduct was enlarged, the serosa thinned and filled with a flaky white exudate, yolk material, and milky white fluid similarly described in the peritoneum. The ovary was not active.
Microscopic findings
Histology of the urogenital tracts of the birds revealed large numbers of protozoan parasites attached to the mucosa or filling the lumen of the ejaculatory ducts, ejaculatory groove, glandular part of the phallus, and the sulcus spermaticus (phallic sulcus) in both male birds and of the oviduct in the female goose (case nos. 1–3) (–). The parasites had the typical trichodinid bell-shape or ovoid appearance, with a large circular, concave, adhesive disk, 50–70 µm in diameter and characteristic indentations formed by rays of denticles (–). The conformation of the denticles was highlighted by the silver-staining technique which revealed their species-related features (). The macronuclei were basophilic with HE staining, horseshoe shaped or fragmented and located at the centre of the body of the protozoa. Small indentations were evident at the site of attachment of the parasites on the mucosal surface of the phallic structures and salpinx, associated with mostly heterophilic inflammation. The parasites were numerous in all birds and often attached by their adhesive disks to the inflamed mucosa. In both male birds (case nos. 1 and 2), the pseudostratified columnar epithelium of the ejaculatory duct and glandular part of the phallus was substituted by proliferative hyperplastic squamous metaplasia associated with the parasitic colonization. Contrarily, the epithelium lining the inner folds of the ejaculatory ducts and glandular cavity of the phallus was still intact and consistent with pseudostratified columnar epithelium. There was acanthosis with accentuation of the rete pegs of the stratified squamous epithelium of the sulcus spermaticus associated with the ciliates. The gander (case no. 1) had more severe changes, including multifocal hyperplasia of the mucosal epithelium of the ejaculatory ducts and sulcus spermaticus, hydropic to ballooning degeneration and necrosis at the site of attachment of the protozoa. Superficial layers of the mucosa were sloughing into the lumen and joining a fibrinonecrotic exudate originating from the moderate superficial heterophilic inflammatory reaction. In the female goose (case no. 3), there was thickening of the mucosa of the oviduct due to severe infiltration of heterophils mixed with fibrin, plasma cells, and giant cells into the lamina propria and surface epithelium. In the lumen, admixed with the cellular debris and inflammatory exudate were trichodinid trophozoites (). The lamina propria of the salpinx and the walls of the vessels were strongly positive for amyloid which exhibited apple green birefringence (Congo Red special stain) on polarized light. In the drake (case no. 2), the inflammatory reaction associated with the parasites was mild and consisted of mostly superficial eosinophilic and heterophilic inflammation. In some sections of the gander (case no. 1) genitalia, granulocytes extended deep into the submucosa and joined a moderate to severe, diffuse lymphoplasmacytic reaction (). Multifocal lymphoid nodules were present in the submucosa and lamina propria at several levels of phallus and cloaca of the gander (case no. 1), whereas they were observed sporadically in the drake (case no. 2). Occasionally, lymphoid nodules in the submucosa of the phallus at the sulcus spermaticus and connective tissue at the lymph folds were undergoing single-cell necrosis of the lymphocytes and central caseation. The gander's testes were undergoing mild to moderate degeneration consisting of formation of numerous spermatid multinucleated cells, disorganization of the spermatogenic columns and presence of few spermatozoa (case no. 1). In the vasa efferentia and epididymal tubes, the lumen of the ducts were reduced in size and were occupied more by sloughed cells and spermatid giant cells, than spermatozoa. No lesions were seen in the vasa deferentia and ureters. The kidneys had mild mononuclear cell infiltration in the interstitium. In the drake's testes, the seminiferous tubules were large in diameter with active spermatogenesis (case no. 2). One section of the testes had a focal extensive area of acute coagulative necrosis and hemorrhaging. Extensive coagulative necrosis of the drake renal parenchyma was also present in some sections. In the male birds (case nos. 1 and 2), trichodinid parasites were found in sections of phallus, ejaculatory duct, groove, cavity of the phallus, and sulcus spermaticus.
Figure 3. Phallus; goose, case no. 1. Large numbers of trichodinid ciliates associated with acanthosis, superficial heterophilic and submucosal lymphoplasmacytic inflammation. HE. Bar = 75 µm.
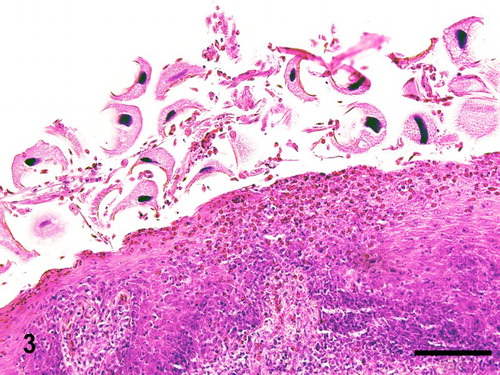
Figure 4. Phallus; goose, case no. 1. Severe hyperplastic squamous metaplasia of the mucosa of the glandular body of the phallus associated with large numbers of trichodinid protozoa in the lumen. Moderate heterophilic and lymphoplasmacytic inflammation of the mucosa. HE. Bar = 230 µm.
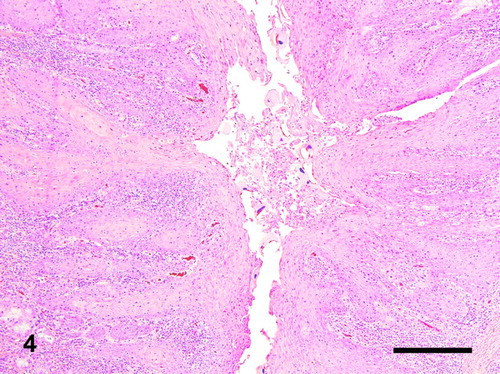
Figure 5. Phallus; duck, case no. 2. Glandular (left) and cutaneous (right) part of the phallus. Large numbers of trichodinids are filling the lumen (arrowheads). HE. Bar = 700 µm.
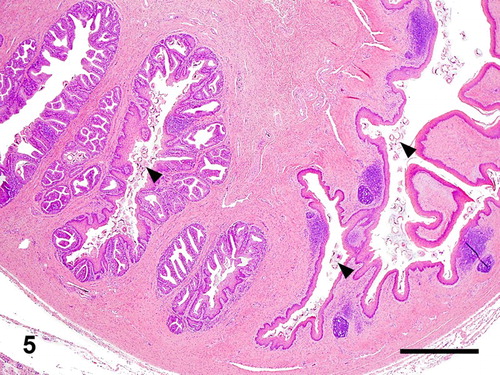
Figure 6. Oviduct; goose, case no. 3. Severe salpingitis associated with large numbers of trichodinids. Bar = 105 µm.
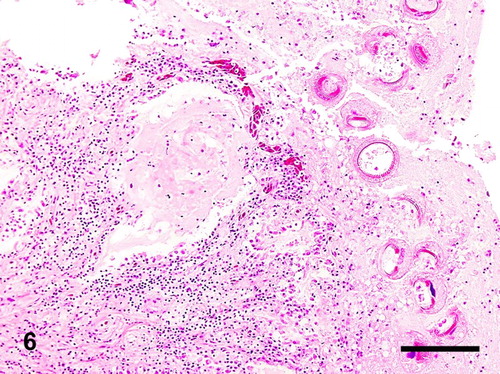
Figure 7. Phallus; goose, case no. 1. The denticles of the trichodinids are highlighted by the silver staining. Hurtado's Leptospira silver. Bar = 70 µm.
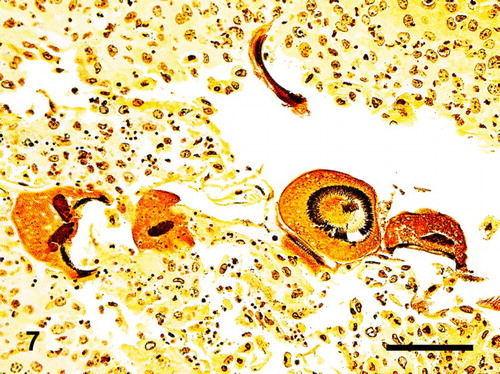
Figure 8. Phallus; goose, case no. 1. Trichodinids adhering to the surface mucosa of the phallus through their adhesive disc and denticles. Bar = 50 µm.
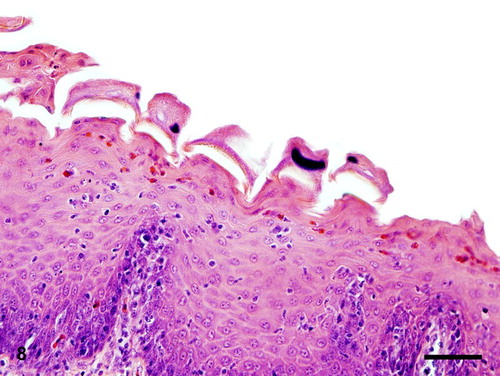
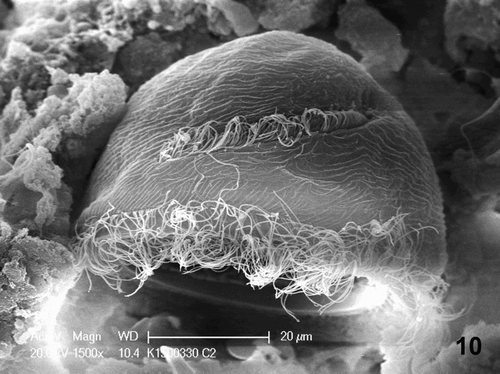
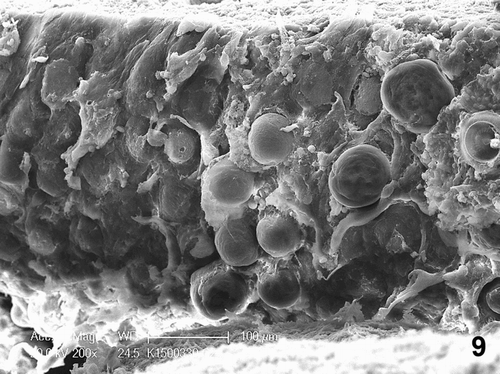
Figure 9. Scanning electron microscopy showing circular indentations on the mucosal surface of the phallus associated with numerous trichodinid ciliates. Bar = 100 µm.
Inflammation and lymphoid hyperplasia were found outside of the urogenital tract. In case no. 1, superficial granulocytic inflammation and fibrinonecrotic exudate were noted on the proventricular mucosa. Minor multifocal lymphocytic infiltrations were noted in the liver, lung, pancreas, thyroid, adrenal, vascular body, and testes. No lesions or inflammatory reaction were noted in heart, trachea, skeletal muscles, nerves, and parathyroid of the gander (case no. 1). In the drake (case no. 2), there was enlargement of the spleen due to hyperplasia of the reticular sheaths. Foci of acute coagulative necrosis in the liver as well as similar foci in one testis and kidney were associated with acute vascular necrosis and minimal inflammation.
Additional diagnostic tests
Mycoplasma species were isolated from the gander's phallic culture, whereas Escherichia coli was also isolated from both phallus and cloaca (case no. 1). No growth was observed from aerobic cultures of liver, trachea and testis from case no. 1 and liver and peritoneum of the female goose (case no. 3). All sera from case no. 1 tested negative for ND, MG, and MS. Cloacal swabs from case nos. 1 and 3 were negative for AI by reverse transcriptase polymerase chain reaction. No parasites were found in the water samples taken from the water drinkers (case no. 1). Scanning electron microscopy revealed numerous bell-shaped ciliated protozoa attached to the mucosa of the ejaculatory duct. The surface mucosa reported the formation of numerous multifocal circular indentations and sloughing of the epithelium associated with large numbers of trichodinids ().
Discussion
Characteristic morphological features of the geese and duck parasites of the reproductive tract were consistent with members of the Trichodinidae family. Based on histopathological analysis, the acute lesions and inflammatory reaction in the reproductive tracts of the males and the female waterfowl appeared to be associated with morphologically similar trichodinid ciliates. Indentations in the mucosal surface were formed by anchoring of the organisms, frequently still present in situ. The mucosa on the surface of the ejaculatory ducts and glandular part of the phallus was undergoing hyperplasia and squamous metaplasia. Squamous metaplasia may have occurred as a regenerative or protective response of the epithelium to the damage inflicted by the attachment of protozoa. Large numbers of parasites may exacerbate the severity of epithelial damage. However, the geese (case nos. 1 and 3) had more severe lesions and inflammatory reaction compared to the drake (case no. 2) despite similar number of parasites observed in all three birds. Concurrent diseases may also play a role in the severity of lesions observed in the gander (case no. 1). The Mycoplasma strains isolated in conjunction with lesions compatible with mycoplasmosis, namely lymphoid follicular hyperplasia, may have played a role in the development of phallic lesions in the gander. Mycoplasma isolates have been identified as putative agents of venereal diseases in geese and ducks (Stipkovits et al., Citation1984; Marius-Jestin et al., Citation1987; Behr et al., Citation1990; Stipkovits & Szathmary, Citation2012). Lesions suggestive of mycoplasmosis were not observed in the other two birds (case nos. 2 and 3). These had lesions in other organs compatible with septicaemia or endotoxaemia. The role of these protozoan parasites in the development of the egg peritonitis in the female goose (case no. 3) and infarctive lesions in the drake (case no. 2) has not been elucidated. Since the duration of the parasitism and the species of trichodinids were not determined in the three cases, the parasites could have affected each bird differently with respect to the severity of the lesions.
Trichodinidae have been reported from the intestine, urinary and reproductive tract of fish and other aquatic animals (e.g. amphibian larvae, molluscs, and terraceous invertebrates) (Noga, Citation1996; Mitchell, Citation2007; Collymore et al., Citation2013). To our knowledge, this is the first published report of T. ciliophorans in birds. Wild waterfowl frequently have access to different types of stagnant water and physical contact with highly contaminated water sources may be a common source of infection. While this may have occurred in the wild Mallard drake (case no. 2) there was no reported access to ponds or stagnant water in the commercial geese (case nos. 1 and 3). Drinking water was provided by bell drinkers, which could not be excluded as a possible source of infection by water analysis. The introduction in the commercial goose flocks may have been through the ingestion of an intermediate host infected by contact with the stagnant water of the canal flowing a few feet from the pens. Subsequently, parasitic colonization may have occurred via the shared opening of genital and digestive tracts. The presence of trichodinids in both males and a female suggests the plausibility of sexual transmission in waterfowls. Infected birds may have been introduced in the commercial flocks and transmitted the trichodinids through mating. Sexual transmission of urinary or genital trichodinids has been hypothesized in fish due to the low persistence of the parasite in the environment (Collymore et al., Citation2013). Even in aquatic settings, trichodinids do not survive more than few hours outside their hosts (Noga, Citation1996; Mitchell, Citation2007; Collymore et al., Citation2013). This seems to support the importance of direct transmission. In addition, wild waterfowl and other aquatic birds may serve as a natural reservoir of these organisms, carrying them long distances and contaminating aquatic environments. Since the lower reproductive tract is not always examined histologically in routine avian necropsies, little has been done on the histology of normal or abnormal genitalia in waterfowl (Szép et al., Citation1974; King & McLelland, Citation1984; Brennan et al., Citation2010). In addition, trichodinids are difficult to diagnose as they are loosely attached to the tissue and slough easily from histologic sections during processing (Mueller, Citation1938). More research is needed to investigate the prevalence of these organisms in birds, specifically wild waterfowl, aquatic, and marine birds. Research is also required to elucidate the role of Trichodinidae in the development of venereal disease in birds, as in these cases their presence was associated with the pathology of the genital tract and may have contributed to mortality. These organisms may serve as initiators of secondary bacterial infections due to the damage they afflict to the mucosae. In conclusion, more work is required to determine their importance in birds and their relationship with the other documented goose venereal diseases.
Acknowledgements
The authors thank Drs Kathleen Funk and Peter F. Moore, the pathology resident and supervising pathologist respectively on the Mallard case from the VMTH as well as the staff of the histology laboratory at the VMTH. The authors want to acknowledge the work of Dr Portia Cortes who was the avian medicine resident on the female Toulouse goose case at the CAHFS lab of Fresno.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Basson, L. & Van As, J.G. (1989). Differential diagnosis of the genera in the family Trichodinidae (Ciliophora: Peritrichida) with the description of a new genus ectoparasitic on freshwater fish from southern Africa. Systematic Parasitology, 13, 153–160. doi: 10.1007/BF00015224
- Basson, L., Van As, J.G. & Fishelson, L. (1990). A new species of Trichodina (Ciliophora: Peritrichia) from the intestine of the surgeonfish Acanthurus xanthopterus. International Journal for Parasitology, 20, 785–787. doi: 10.1016/0020-7519(90)90012-C
- Behr, K.P., Hinz, K.H. & Rottmann, S. (1990). Phallus-inflammation of ganders: clinical observations and comparative bacteriological examinations of healthy and altered organs. Zentralblatt für Veterinärmedizin. Reihe B, 37, 774–776.
- Brennan, P.L.R., Clark, C.J. & Prum, R.O. (2010). Explosive eversion and functional morphology of the duck penis supports sexual conflict in waterfowl genitalia. Proceedings of the Royal Society B: Biological Sciences, 277, 1309–1314. doi: 10.1098/rspb.2009.2139
- Brennan, P.L.R., Prum, R.O. & McCracken, K.G., Sorenson, M.D., Wilson, R.E. & Birkhead, T.R. (2007). Coevolution of male and female genital morphology in waterfowl. PLoS One, 2, e418. doi: 10.1371/journal.pone.0000418
- Collymore, C., White, J.R. & Lieggi, C. (2013). Trichodina xenopodus, a ciliated protozoan, in a laboratory-maintained Xenopus laevis. Comparative Medicine, 63, 310–312.
- Etches, R.J. (1996). The Male. In R.J. Etches (Ed.), Reproduction in Poultry 1st edn (pp. 208–233). Wallingford: Cab International.
- Fujihara, N., Nishiyama, H. & Nakashima, N. (1976). Studies on the accessory reproductive organs in the drake: 4. Effects of androgen on the ejaculatory groove region of the drake. Poultry Science, 55, 1324–1330. doi: 10.3382/ps.0551324
- Hodges, R.D. (1974). The Reproductive System. In R.D. Hodges (Ed.), The Histology of the Fowl 1st edn (pp. 300–326). London: Academic Press.
- King, A.S. & McLelland, J. (1984). Cloaca and Vent. In A.S. King & J. McLelland (Eds.), Birds, Their Structure and Function 2nd edn (pp. 187–199). London: Baillière Tindall.
- Lake, P.E. (1957). The male reproductive tract of the fowl. Journal of Anatomy, 91, 116–129.
- Marius-Jestin, V., Le Menec, M., Thibault, E., Moisan, J.C. & L'Hospitalier, R. (1987). Normal phallus flora of the gander. Zentralblatt für Veterinärmedizin. Reihe B, 34, 67–78.
- Mitchell, M.A. (2007). Parasites of Amphibians. In D.G. Baker (Ed.), Flynn's Parasites of Laboratory Animals 2nd edn (pp. 118–171). Ames, IA: Blackwell Publisher.
- Mueller, J.F. (1938). A new species of Trichodina (ciliate) from the urinary tract of the Muskalonge, with a repartition of the genus. Journal of Parasitology, 24, 251–258. doi: 10.2307/3272296
- Noga, E.J. (1996). Trichodinosis. In L. Duncan (Ed.), Fish Diseases: Diagnosis and Treatment 1st edn (pp. 99–101). St. Louis, MO: Mosby Publisher.
- Stipkovits, L., Bové, J.M., Rousselot, M., Larrue, P., Labat, M. & Vuillaume, A. (1984). Studies on Mycoplasma infection of laying geese. Avian Pathology, 14, 57–68. doi: 10.1080/03079458508436207
- Stipkovits, L. & Szathmary, S. (2012). Mycoplasma infection of ducks and geese. Poultry Science, 91, 2812–2819. doi: 10.3382/ps.2012-02310
- Szép, I., Pataky, M. & Bögre, J. (1979). Recent practical and experimental observations on the infectious inflammatory diseases of cloaca and penis in geese. Acta veterinaria Academiae Scientiarum Hungaricae, 27, 195–202.
- Szép, I., Pataky, M. & Nagy, Gy. (1974). An infectious inflammatory disease of cloaca and penis in geese (goose gonorrhea): epizootological, clinical, pathological observations and control. Acta Veterinaria Academiae Scientiarum Hungaricae, 24, 347–354.