ABSTRACT
One hundred and six Clostridium perfringens field strains, isolated from diseased turkeys in Italy between 2006 and 2015, were toxinotyped by polymerase chain reaction. Strains were derived from intestines (87), livers (17) and subcutaneous tissues (2). In addition to the four major toxins, strains were also screened for NetB toxin, enterotoxin and beta2 toxin encoding genes. The intestinal gross lesions of turkeys with enteric disorders were statistically studied with respect to the presence of C. perfringens beta2 toxin encoding gene and coccidia in the gut. All the isolates belonged to the toxinotype A and were netB negative. Enterotoxin (cpe) and beta2 toxin (cpb2) encoding genes were detected in two (2.63%) and 76 (71.69%) strains, respectively. Toxinotype results agree with the few published reports concerning the genetic characterization of C. perfringens of turkey origin. On the contrary, the presence of netB and cpb2 genes differs from the results of a previous study where these genes were detected respectively in 6.6% and in 0.5% of the tested strains. Necrotic enteritis in turkeys was not statistically correlated either to the presence of cpb2 gene, or to the synergistic effect operated by coccidia, even though a high percentage of birds with these protozoa in the gut showed necrotic enteritis lesions (64.29%).
Introduction
Clinical necrotic enteritis (NE) is a severe intestinal disease of poultry characterized by fibrinonecrotic lesions with formation of diphtheritic pseudomembranes and high mortality rates. For this reason, NE can be considered the most clinically dramatic bacterial enteric disease of poultry. It is caused by Clostridium perfringens (CP), a Gram-positive, anaerobic, spore-forming rod-shaped microorganism widely present in the environment and in the intestine of humans and birds. This microorganism has been historically classified into five toxinotypes (A–E) based on the production of the four major toxins alpha, beta, epsilon and iota. All toxinotypes produce alpha toxin and, in addition, toxinotype B produces beta and iota toxins, toxinotype C produces beta toxin, toxinotype D produces epsilon toxin and toxinotype E produces iota toxin. Although this classification has allowed assignment of some toxinotypes to specific diseases in mammals, in chickens both NE-related and NE-unrelated strains belong to toxinotype A (Keyburn et al., Citation2006). In 2008 Keyburn and coworkers discovered a new “pore-forming toxin” named NetB, that is considered essential for the appearance of NE in chickens (Keyburn et al., Citation2008).
CP beta2 toxin and enterotoxin are two additional virulence factors responsible for intestinal diseases in animals and humans, respectively. The beta2 toxin seems to play an important role in the pathogenesis of NE in pigs (Klaasen et al., Citation1999), in horse enterocolitis (Herholz et al., Citation1999) and in cattle enterotoxaemia (Manteca et al., Citation2002). while the enterotoxin is associated with food toxico-infections in human (Brynestad & Granum, Citation2002).
In comparison with NE in chickens, few studies analysed in depth NE in turkeys with respect to the age of occurrence, virulence factors of the CP strains involved and the role of some potential predisposing factors. Histopathological findings in turkey intestines revealed deeper and more severe lesions than in broiler chickens (Gazdzinski & Julian, Citation1992).
In the present study, we report the toxinotyping results of CP field strains isolated from diseased meat and breeder turkeys in Italy. The results were statistically analysed with respect to the severity of the intestinal lesions (NE vs. catarrhal enteritis) and the presence of coccidial oocysts.
Materials and methods
Sampling
Between 2006 and 2015, carcasses collected in 106 diseased turkey flocks (87 meat and 19 breeder) were submitted to the Diagnostic Laboratory “Tre Valli” for post-mortem and collateral diagnostic examinations. The birds, aged 1–55 weeks, were selected on the basis of the appearance of enteric disorders and/or the observation of an unusual increase of the mortality rate in the flock.
CP isolation
On the basis of the gross lesions observed, intestinal contents, livers and subcutaneous exudates were cultured for isolation of CP. They were plated onto sheep blood agar (Oxoid, Basingstoke, UK) and incubated at 37°C for 24 h under anaerobic conditions (Anaerogen, Oxoid). Identification was performed by means of the biochemical commercial miniaturized kits rapid ID 32 A (bioMèrieux, Marcy l'Etoile, France).
Parasitological investigation
In the presence of gastrointestinal lesions, a microscopic semi-quantitative evaluation of the coccidian oocysts was performed. Scrapings were collected from the intestinal tracts with macroscopic lesions referable to catarrhal or NE and observed under the microscope (100×). Scores were assigned on the basis of the microscopic observations: score 1 (1–50 oocysts per microscope field) and score 2 (>50 oocysts).
CP molecular characterization
Only one strain per bird and per flock was subjected to the biomolecular characterization. All the selected strains were submitted to automatic DNA extraction using the MagMAX Total Nucleic Acid Extraction Kit (Thermofisher Scientific, Life Technologies, Vilnius, Lithuania) and Microlab Starlet instrument (Hamilton, Bonaduz, Switzerland).
Toxinotyping was performed by the biomolecular detection of cpa, cpb1, cpetx and cpi genes encoding, respectively, for the four major toxins alpha, beta, epsilon and iota (Baums et al., Citation2004). Moreover, the presence of enterotoxin (cpe), NetB (netB) and beta2 (cpb2) encoding genes was investigated (Yoo et al., Citation1997; Garmory et al., Citation2000; Keyburn et al., Citation2008). The reference CP strains used as positive polymerase chain reaction (PCR) controls for the targeted genes were: ATCC 27324 (toxinotype E and enterotoxin), CCUG 2036 (toxinotype C), CCUG 2037 (toxinotype D) and ATCC 10543 (toxinotype A and beta2 toxin). One CP strain courtesy of Prof. Van Immersel (Ghent University, Belgium), was used as positive control for NetB encoding gene.
Statistical analysis
The Fisher exact test was applied to study possible correlations between NE and the coccidia scores. The same test was also used to investigate the possible correlations between the severity of the macroscopic gastrointestinal lesions and the presence of cpb2-positive CP strains infection.
Results
Among the 106 CP included in the study, 45 were isolated from intestines with catarrhal enteritis, 42 from intestines with NE, 17 from livers with hepatic lipidosis/necrosis and two from sero-haemorrhagic subcutaneous exudate of turkeys with gangrenous dermatitis (, ).
Figure 1. Gross lesion categorization. (a) catarrhal enteritis, (b) NE (“Turkish towels”), (c) lipidosis, (d) necrotic hepatitis and (e) gangrenous dermatitis.
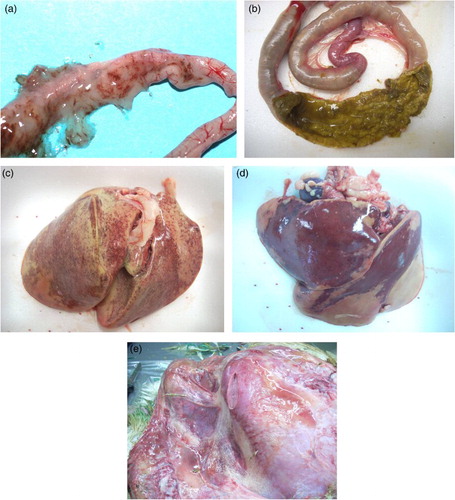
Table 1. Classification of clinical cases with respect to macroscopic lesions, age of birds and site of CP isolation.
The majority of strains isolated from intestines (66.98%) was collected in birds aged from 10 to 60 days. Gangrenous dermatitis was observed in two turkey breeder flocks of 22 and 32 weeks of age. Necrotic hepatitis was detected in three cases; one occurred in meat turkeys of one day of age while another was at 9 days and the third at 92 days. Twelve cases of hepatic lipidosis were recorded in turkey breeders of ages between 17 and 29 weeks. In those birds the livers were simultaneously infected by CP and E. coli.
Genetic characterization revealed that all the isolates belonged to the toxinotype A and did not harbour the netB gene. shows the detailed results concerning the virulence genetic factors investigated in relation to the gross lesions observed.
Table 2. Distribution of the toxin encoding genes investigated among the CP isolates with respect to the macroscopic lesions.
Sixty-four out of 87 CP strains isolated from the intestines were cpb2 positive. The distribution of those strains among the intestinal isolates was not statistically correlated with the presence of catarrhal or necrotic intestinal lesions (P = 0.080). This gene was also detected in 10 out the 17 strains isolated from the livers and in both strains isolated from the subcutis of birds with gangrenous dermatitis.
Among the 87 intestinal clinical cases, coccidia were more frequently observed in birds aged from 31 to 60 days with catarrhal or NE (). In particular, 18 cases of coccidiosis were detected in turkeys with NE and 10 in birds with catarrhal enteritis. This difference was not statistically significant (P = 0.065) and the same result was obtained considering the coccidian scores 1 and 2 (P = 0.105).
Table 3. Number and age (days) of birds with coccidian score 1 or 2 among the subjects with catarrhal and NE.
Discussion
Toxinotyping results obtained with this study are in agreement with those reported in other similar studies concerning CP strains of turkey origin, where only the toxinotype A was detected (Erol et al., Citation2008; Saita et al., Citation2009; Lyhs et al., Citation2013; Aras & Hadimli, Citation2015).
The major differences concern the percentage of cpb2-positive strains recorded, in comparison with the results of a previous similar Finnish survey (Lyhs et al., Citation2013). In the above mentioned study conducted on 212 CP strains isolated from turkeys, the cpb2 gene was detected only in one strain.
Although this research was conducted in Finland, the differences with our results cannot be explained only on an epidemiological basis. Indeed, PCR protocols adopted for cpb2 were different. Lyhs et al., (2013) used that described by Baums et al. (Citation2004) for cpb2 detection whereas we used that described by Garmory et al. (Citation2000). In the past we noted conflicting results on cpb2 detection by using both PCR protocols, but those discrepancies were clarified by the genetic sequencing which confirmed the correctness of the second PCR protocol (data not shown).
The CP cpb2-positive strain results were not statistically correlated to the macroscopic lesions. This gene was also identified in CP strains isolated from liver and subcutaneous tissue of turkeys not affected by enteric disorders. This result does not confirm a role for beta2 toxin turkey enteric diseases. In addition, the possible pathogenic role of CP cpb2-positive strains in turkey gangrenous dermatitis and hepatic pathological findings could not be elucidated by this study due to the scarce number of the limited from those sample types.
Coccidia were detected in a higher percentage of birds with NE (64.29%) compared with birds with catarrhal enteritis. This difference was not statistically significant due to the inadequate number of birds with coccidia, which reduced the potency of the statistical test.
All tested strains were netB negative, includeding the ones isolated from NE lesions, whereas 6.6% of Finnish strains isolated from turkeys with NE harboured this gene but not all the strains isolated from NE lesions were netB-positive. In an analogous study conducted among CP of turkey origin in Italy, the netB gene was not detected in any of the 25 tested strains (Saita et al., Citation2009). This finding suggests that NetB is not an essential virulence factor for the occurrence of NE in turkeys and new unknown virulence factors should be investigated in clinical CP strains isolated from well-defined lesions.
In contrast, in a previous Italian survey on 107 CP isolates from diseased and healthy chickens, the netB encoding gene was detected in 93% of CP strains isolated from chickens with enteric disorders (Drigo et al., Citation2009).
In addition to the possible existence of unknown virulence factors of CP strains implicated in turkey NE, predisposing factors such as dietary composition, coccidiosis, stress conditions, immunosuppressive diseases, mycotoxins and flock density could play a major role in the appearance of the disease in turkeys, as reported for chickens (Timbermont et al., Citation2011). In particular, turkeys may be affected by the immunosuppressive adenovirus responsible for haemorrhagic enteritis. Despite the name, this virus does not consistently cause evident haemorrhagic intestinal lesions, but the immunosuppressive effect is well documented (Rautenschlein & Sharma, Citation2000).
Despite reports of NE in turkeys that date back over 20 years, the disease is still a problem in turkey flocks (Gazdzinski & Julian, Citation1992; Droual et al., Citation1994, Citation1995). The present study demonstrates that NE in turkeys is not related to the CP toxin genes investigated. New unknown toxins might be involved in the pathogenesis of the disease in this bird species and they should be studied in clinical isolates. The lack of an experimental model to reproduce the disease in turkeys, makes it difficult to study effective counter measures to prevent or face the disease.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Aras, Z. & Hadimli, H.H. (2015). Detection and molecular typing of Clostridium perfringens isolates from beef, chicken and turkey meats. Anaerobe, 32, 15–17. doi: 10.1016/j.anaerobe.2014.11.004
- Baums, C.G., Schotte, U., Amtsberg, G. & Goethe, R. (2004). Diagnostic multiplex PCR for toxin genotyping of Clostridium perfringens isolates. Veterinary Microbiology, 100, 11–16. doi: 10.1016/S0378-1135(03)00126-3
- Brynestad, S. & Granum, P.E. (2002). Clostridium perfringens and foodborne infections. International Journal of Food Microbiology, 74, 195–202. doi: 10.1016/S0168-1605(01)00680-8
- Drigo, I., Agnoletti, F., Bacchin, C., Guolo, A., Cocchi, M., Bonci, M., & Bano, L. (2009). Diffusion of Clostridium perfringens NetB positive strains in healthy and diseased chickens. Italian Journal of Animal Science, 8, 761–764. doi: 10.4081/ijas.2009.761
- Droual, R., Farver, T. & Bickford, A. (1995). Relationship of sex, age, and concurrent intestinal disease to necrotic enteritis in turkeys. Avian Diseases, 39, 599–605. doi: 10.2307/1591814
- Droual, R., Shivaprasad, H. & Chin, R. (1994). Coccidiosis and necrotic enteritis in turkeys. Avian Diseases, 38, 177–183. doi: 10.2307/1591854
- Erol, I., Goncuoglu, M., Ayaz, N., Bilir Ormanci, F. & Hildebrandt, G. (2008). Molecular typing of Clostridium perfringens isolated from turkey meat by multiplex PCR. Letters in Applied Microbiology, 47, 31–34. doi: 10.1111/j.1472-765X.2008.02379.x
- Garmory, H., Chanter, N., French, N., Bueschel, D., Songer, J. & Titball, R. (2000). Occurrence of Clostridium perfringens β2-toxin amongst animals, determined using genotyping and subtyping PCR assays. Epidemiology and Infection, 124, 61–67. doi: 10.1017/S0950268899003295
- Gazdzinski, P. & Julian, R. (1992). Necrotic enteritis in turkeys. Avian Diseases, 36, 792–798. doi: 10.2307/1591787
- Herholz, C., Miserez, R., Nicolet, J., Frey, J., Popoff, M., Gibert, M., Gerber, H., & Straub, R. (1999). Prevalence of beta2-toxigenic Clostridium perfringens in horses with intestinal disorders. Journal of Clinical Microbiology, 37, 358–361.
- Keyburn, A.L., Boyce, J.D., Vaz, P., Bannam, T.L., Ford, M.E., Parker, D., Di Rubbo, A., Rood, J.I. & Moore, R.J. (2008). NetB, a new toxin that is associated with avian necrotic enteritis caused by Clostridium perfringens. PLoS Pathogens, 4, e26. doi: 10.1371/journal.ppat.0040026
- Keyburn, A.L., Sheedy, S.A., Ford, M.E., Williamson, M.M., Awad, M.M., Rood, J.I. & Moore, R.J. (2006). Alpha-toxin of Clostridium perfringens is not an essential virulence factor in necrotic enteritis in chickens. Infection and Immunity, 74, 6496–6500. doi: 10.1128/IAI.00806-06
- Klaasen, H.L., Molkenboer, M.J., Bakker, J., Miserez, R., Häni, H., Frey, J., Popoff, M.R. & van den Bosch, J.F. (1999). Detection of the β2 toxin gene of Clostridium perfringens in diarrhoeic piglets in the Netherlands and Switzerland. FEMS Immunology & Medical Microbiology, 24, 325–332.
- Lyhs, U., Perko-Makela, P., Kallio, H., Brockmann, A., Heinikainen, S., Tuuri, H. & Pedersen K. (2013). Characterization of Clostridium perfringens isolates from healthy turkeys and from turkeys with necrotic enteritis. Poultry Science, 92, 1750–1757. doi: 10.3382/ps.2012-02903
- Manteca, C., Daube, G., Jauniaux, T., Linden, A., Pirson, V., Detilleux, J., Ginter, A., Coppe, P., Kaeckenbeeck, A. & Mainil, J.G. (2002). A role for the Clostridium perfringens β2 toxin in bovine enterotoxaemia? Veterinary Microbiology, 86, 191–202. doi: 10.1016/S0378-1135(02)00008-1
- Rautenschlein, S. & Sharma, J.M. (2000). Immunopathogenesis of haemorrhagic enteritis virus (HEV) in turkeys. Developmental & Comparative Immunology, 24, 237–246. doi: 10.1016/S0145-305X(99)00075-0
- Saita, M., Bano, L. & Gallazzi, D. (2009). Pathogenicity markers of Clostridium spp. in commercial turkeys. Italian Journal of Animal Science, 8, 781–784. doi: 10.4081/ijas.2009.781
- Timbermont, L., Haesebrouck, F., Ducatelle, R. & Van Immerseel, F. (2011). Necrotic enteritis in broilers: an updated review on the pathogenesis. Avian Pathology, 40, 341–347. doi: 10.1080/03079457.2011.590967
- Yoo, H.S., Lee, S.U., Park, K.Y. & Park, Y.H. (1997). Molecular typing and epidemiological survey of prevalence of Clostridium perfringens types by multiplex PCR. Journal of Clinical Microbiology, 35, 228–232.
