ABSTRACT
Salmonella enterica serovar Enteritidis (SE) is a public health concern and infected chickens serve as a reservoir that potentially transmits to humans through food. Although SE seldom causes systemic disease in chickens, virulent SE strains can colonize in intestines and lead a persistent infection of the liver. The liver is the primary organ for lipid metabolism in chickens and the site for production and assembly of main components in yolk. We performed a time-course experiment using LMH-2A cells that were infected with SE and co-incubated with β-oestradiol to evaluate if SE infection affected lipid metabolism and subsequently changed lipoprotein formation for egg yolk. The results indicated that lipid accumulation significantly increased in infected LMH-2A cells while the viability of these cells was only slightly decreased. The mRNA expressions of lipid transportation and most lipogenetic genes including sterol regulatory element binding protein 1, acetyl-CoA carboxylase, fatty-acid synthase, long-chain-fatty-acid-CoA ligase 1, peroxisome proliferator-activated receptor-γ, and very-low-density lipoproteins (VLDLs) II were significantly up-regulated while the expression of lipogenetic-related stearoyl-CoA denaturase 1 was down-regulated. Moreover, decline in lipid transportation of hepatocytes was evidenced by the down-regulation of oestrogen receptor α which promotes VLDLy formation, an increase of intra-cellular accumulation of Apoprotein B (ApoB) protein, and a decrease of cellular excretion of VLDL protein. Conclusively, SE infection could elevate lipid synthesis and reduce lipid transportation in the chicken hepatocytes. These changes may lead excessive lipid accumulation in liver and slower lipoprotein deposition in yolk.
Introduction
Salmonella enterica serovar Enteritidis (SE) infection is an important public health problem and also affects the profitability of the poultry industry. SE has been found in internal tissues of infected chickens (e.g. intestine, liver, spleen, lung, ovary, and oviduct) (Hoop & Pospischil, Citation1993). Infection with SE increases chick mortality and disrupts egg formation to reduce egg production in laying hens without obvious clinical signs (Shivaprasad et al., Citation1990; Wang et al., Citation2014). SE can be recovered from 53% of chicken liver, and SE can persist in the liver as a chronic infection for up to 5 weeks after oral inoculation of Leghorn hens (Gast & Holt, Citation2000). Other pathological lesions in include degeneration, steatosis and necrosis in S. Agona infected-rabbit liver (Prokopowicz & Ananko, Citation1979). SE infection in hens also down-regulates genes involved in metabolic and inflammatory pathways in the liver (Coble et al., Citation2012).
The liver is an important organ for lipid metabolism and the metabolic processes include de novo fatty-acid synthesis, triacylglycerol synthesis, fatty-acid oxidation, and lipid transportation. Excessive amounts of consumed glucose are shunted into de novo fatty-acid synthesis by stearoyl-CoA denaturase-1 (SCD-1) to synthesize long-chain fatty acids (Kaestner et al., Citation1989). The long-chain fatty acids trigger triacylglycerol synthesis and fatty-acid oxidation, which are controlled by long-chain-fatty-acid-CoA ligase-1 (ACSL-1) (Li et al., Citation2009). The synthesized triacylglycerol is further transported as very-low-density lipoproteins (VLDLs) to ovary as well as to other organs.
Induction and maintenance of egg productivity are dependent upon the expression of genes related to lipid metabolism and the generation of egg yolk lipid that is mostly composed of VLDLs (Burghelle-Mayeur et al., Citation1989; Yen et al., Citation2009). In laying hens, abundant VLDL is secreted from the liver, and only VLDLs with a 25–44 nm particle diameter (i.e. VLDLy) are used for yolk deposition for egg maturation (Yang et al., Citation2013). VLDLy contains 23 units of VLDL II and one unit of Apoprotein B (ApoB) that acts as a ligand to oocyte triacylglycerol deposition (Bujo et al., Citation1997; Walzem et al., Citation1999). In laying hens, formation of VLDLy results from the production of ApoB and VLDL II which are produced via de novo fatty-acid synthesis in the liver and the recombination of hepatic VLDL stimulated by oestrogen (Burghelle-Mayeur et al., Citation1989; Walzem et al., Citation1999). VLDLy is released into the plasma and transported to the ovary for vitellogenesis.
Results of our previous studies indicated that infection with SE retards steroidogenesis of chicken ovarian granulosa cells ex vivo. The resultant decrease in progesterone production may be the cause of decreased egg production in hens infected with SE (Wang et al., Citation2013a, Citation2014). Lipid metabolism in the liver has also been regarded as having an important role in egg maturation and production. Reduced pyruvate levels and down-expression of the genes regulating lipid metabolism occur in the chicken liver after infection with salmonellas (Coble et al., Citation2012; Freeman & Chubb, Citation1964). We performed an in vitro time-course study to identify whether SE infection affects lipid metabolism in the chicken hepatocyte. LMH-2A cells, the Gallus gallus hepatoma cell line, were treated with oestrogen to mimic hormone regulation during SE infection in this study. The objectives were to investigate lipid metabolism and other possible physiological processes in the LMH-2A cells during SE infection, and to elucidate the possible roles underlying reduced egg production in layer hens.
Materials and methods
Bacterial preparation
Salmonella enterica serovar Enteritidis ATCC 13076 (American Type Culture Collection, Manassas, VA, USA) was used in this study and has previously been characterized in studies of the interactions between SE and egg production (Wang et al., Citation2013a, Citation2014). Bacteria were cultured in brain heart infusion broth (Difco, Detroit, MI, USA) at 37°C for 18 h, and then centrifuged (5 min, 1500 × g). The supernatant was removed, and the pellet was washed and re-suspended in phosphate-buffered saline (PBS, pH 7.0) of 108 colony-forming units (cfu/ml).
Cell culture and treatments
The LMH-2A (ATCC CRL-2118) cell line is sensitive to oestrogen stimulation and can be used to model oestrogen-mediated ApoB synthesis and secretion pathways (Hermann et al., Citation1997, Citation2003). LMH-2A cells were maintained with Waymouth MB 753/1 medium (Sigma, Saint Louis, MO, USA) supplemented with 10% foetal bovine serum in 0.1% type A gelatine (Sigma) coated 75T flasks in a humidified incubator with 5% CO2 at 37°C. For cell seeding, 2 × 106 cells per well of 6-well gelatine-coated plates for evaluating gene expression and lipoprotein production, as well as 2 × 104 cells per well of 96-well gelatine-coated plates for MTT assay and lipid accumulation stain were applied.
After the LMH-2A cells in plates were cultured for 24 h, the cells of 70–80% confluence in each well were confirmed. The culture plates were randomly divided into the treatment and control groups. The treatment group was incubated at approximately 50:1 multiplicity of infection with the SE suspension as previously described (Shah et al., Citation2012). The control group was treated with equal volumes of PBS. All plates were treated with β-oestradiol (Sigma) dissolved in 99% ethanol (final cultured concentration at 50 nM) (Hermann et al., Citation2003). The plates were centrifuged at 300 × g for 5 min. After 2 h of incubation at 5% CO2, 37°C, gentamicin (final concentration at 100 μg/ml, Sigma) was added to every plate. They were then incubated for an additional 2 h for the 4 h post-infection (4 hours post-infection (hpi)), 22 h for the 24 hpi, or 46 h for the 48 hpi.
Lipid accumulation
After LMH-2A cells were infected with the bacteria, the cells of each group were washed with PBS and fixed using 4% paraformaldehyde. Then Nile Red stain (final concentration at 1 μg/ml; Sigma) was used for cellular lipid droplet staining and Hoechst 33,342 nucleic acid stain (final concentration at 2 μg/ml; Sigma) was used for cell nucleus staining. Lipid accumulation was estimated using fluorescence intensity (SpectraMax M5™ microfluorometer, Molecular Devices, Sunnyvale, CA, USA). The wavelengths used were 530 nm for excitation and 592 nm for emission for the Nile Red, and 351 nm for excitation and 460 nm for emission for the Hoechst 33,342, stained samples. After the Nile Red fluorescence units were normalized relative to the Hoechst 33,342 fluorescence units, the relative fluorescence intensity values were used for semi-quantitative comparisons of each group (Cury-Boaventura et al., Citation2004). The microphotographs (200×) were taken using a fluorescence microscope (IX70 Fluorescence Microscope Cutaway Diagram, Olympus, Tokyo, Japan).
Cell viability
After the LMH-2A cells were treated as previously described, cell viability was evaluated using an MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; Sigma) colorimetric assay. First, culture medium was removed, and 200 μl MTT was added to every well to achieve a final concentration of 5 mg/ml. The cells were then incubated at 37°C for 1.5 h, and the MTT reagent was removed. Dimethyl sulfoxide (200 μl; DMSO; Sigma) was then added to each well and mixed thoroughly. Absorbance values at wavelengths of 570 and 690 nm were measured using a microplate absorbance reader (Sunrise™, TECAN, Männedorf, Switzerland) to analyse cell viability.
Gene expression and production of lipoproteins
To validate gene regulation, quantitative reverse transcription polymerase chain reaction (qRT-PCR) was performed using an iQ5 optical system (BioRad, Hercules, CA, USA) and SsoFast™ EvaGreen® supermix (BioRad). The total RNA used in the qRT-PCR was collected from three biological repeats. cDNA synthesis was performed using the iScrip cDNA synthesis kit (BioRad) according to the manufacturer’s protocols. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and β-actin were used for the internal standard.
The oestrogen receptor α (ERα), acetyl-CoA carboxylase (ACC), fatty-acid synthase (FAS), sterol regulatory element binding protein-1 (SREBP-1), peroxisome proliferator-activated receptor-γ (PPAR-γ), SCD-1, ACSL-1, VLDL II, and ApoB primers were designed using the nucleotide BLAST database (http://www.ncbi.nlm.nih.gov/blast) (). A total of 50 ng cDNA and 0.5 μM of each primer were used in the following reaction conditions: enzyme activation at 95°C for 30 s, followed by 5 s denaturation at 95°C and 10 s annealing, and extension at 58°C for 40 cycles. Each reaction was carried out in three experimental replicates. Melting curve analyses were performed to confirm the products. The relative expression folds of the target genes were calculated with the 2−ΔΔCt method where Ct denotes cycle threshold. (Livak & Schmitten, Citation2001).
Table 1. The qRT-PCR primer sequences.
After the cells were treated as previously mentioned, the cell lysate and the supernatant were collected for evaluation of the ApoB accumulation and the VLDL secretion, respectively using ELISA kits (MyBioSource, San Diego, CA, USA) according to the manufacturer’s protocols. The absorbance was detected using a microplate absorbance reader (TECAN) at a wavelength of 450 nm.
Statistics
Mean fold-change ± standard error of the mean (SEM) values were calculated using the control group for comparison. The Student’s t-test was used to determine whether there was a statistically significant (P < 0.05) difference between infected and non-infected cells.
Results
Lipid accumulation and cell viability
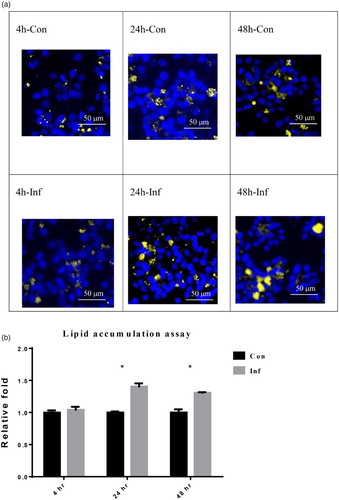
In (a), the lipid contents were observed under a fluorescence microscope. Blue staining indicates the cell nucleus whereas yellow staining indicates the cellular lipid droplet. Hence, the greater level of yellow staining in the infection groups indicates that increased lipid accumulation occurred in the LMH-2A cells after they were infected with SE. Semi-quantitative comparison by relative fluorescence intensity values from (a) showed that lipid accumulation in LMH cells increased significantly (P < 0.05) to 1.40 ± 0.05-fold and 1.31 ± 0.01-fold at 24 and 48 hpi, respectively when compared to the non-infected LMH-2A cells ((b)). However, the amount of lipid accumulation was not changed in the infected LMH-2A cells at 4 hpi. The results of MTT assay showed that LMH-2A cell viability slightly decreased to 98%, 95%, and 92% at 4, 24, and 48 hpi, respectively during SE infection.
Figure 1. Lipid accumulation in LMH-2A cells evaluated using a fluorescent stain at 4, 24, and 48 hpi with SE. (a) The lipid contents were observed under a fluorescence microscope at 4, 24, and 48 hpi. The blue areas indicate cell nucleus staining (Hoechst 33342 stain) and the yellow areas indicate cellular lipid droplet staining (Nile Red stain). (b) Semi-quantitative comparisons of lipid accumulation from (a) between groups of SE-infected (Inf) LMH-2A cells and control (Con) LMH-2A cells co-incubated with β-oestradiol. Error bars indicate the SEM values. Asterisks represent significant differences (P < 0.05) between Inf and Con groups at the same time point.
Lipid synthesis
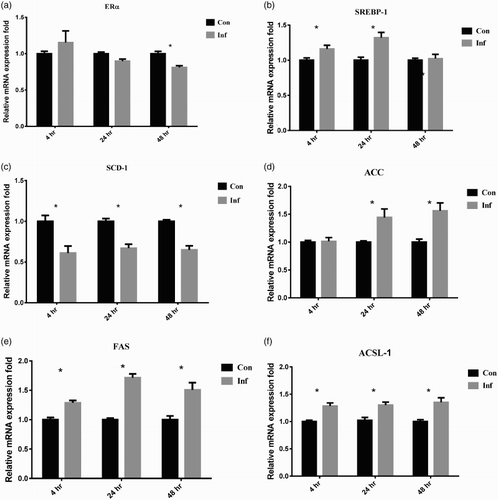
The relative expression fold of ERα mRNA in infection group was slightly up-regulated to 1.15 ± 0.16-fold at 4 hpi, but was down-regulated to 0.9 ± 0.03- and 0.81 ± 0.03-fold at 24 and 48 hpi, respectively ((a)). The upstream transcription factor of liver lipid synthesis, SREBP-1 mRNA, in infection group was significantly up-regulated to 1.16 ± 0.05 and 1.32 ± 0.08-fold at 4 and 24 hpi, respectively and decreased to 1.02 ± 0.06-fold at 48 hpi ((b)). Compared to the control group, the relative expression of SCD-1 mRNA was significantly down-regulated to approximately 0.6-fold at 4, 24, and 48 hpi ((c)). The relative expression of ACC mRNA was significantly up-regulated to 1.44 ± 0.15 and 1.56 ± 0.14-fold at 24 and 48 hpi, respectively ((d)). The relative expression of FAS mRNA was increased (P < 0.05) to 1.29 ± 0.04, 1.71 ± 0.07, and 1.51 ± 0.13-fold at 4, 24, and 48 hpi, respectively ((e)). The relative expression of ACSL-1 mRNA was up-regulated to about 1.3-fold after SE infection throughout the three post-infection time points ((f)).
Figure 2. The mRNA expression of lipid metabolism related genes in LMH-2A cells at 4, 24, and 48 hpi with SE. Comparisons were made between SE-infected (Inf) groups and control groups (Con) of cells co-incubated with β-oestradiol. Error bars indicate SEM values. Asterisks represent significant differences (P < 0.05) between Inf and Con groups at the same time point.
Lipid transportation, VLDL secretion, and ApoB accumulation
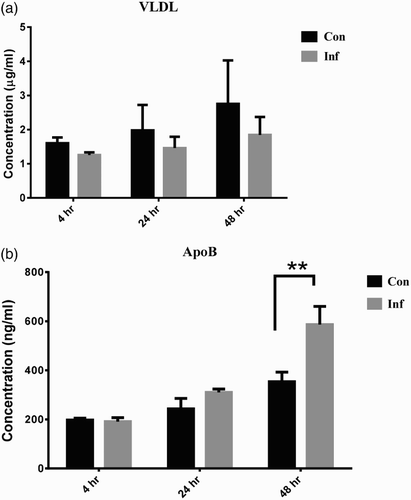
At 4 hpi, the relative expression of PPAR-γ mRNA was significantly up-regulated to 4.48 ± 0.13-fold. It returned to approximately 1.5-fold at 24 and 48 hpi ((a)). After SE infection, VLDL II mRNA expression in infection group was up-regulated significantly to about 3-fold at 4, 24, and 48 hpi compared to the control group ((b)). The relative expression level of ApoB mRNA was down-regulated at 4 hpi, and then returned to normal levels at 24 and 48 hpi ((c)). However, the concentration of VLDL in the supernatant was decreased at 24 and 48 hpi ((a)). The concentration of ApoB inside the LMH-2A was gradually increased and reached to significant difference at 48 hpi ((b)).
Figure 3. The mRNA expression of lipid transportation related genes in LMH-2A cells at 4, 24, and 48 hpi with SE. Comparisons were made between groups of SE-infected (Inf) and control (Con) LMH-2A cells co-incubated with β-oestradiol. Error bars indicate SEM values. Asterisks represent significant differences (P < 0.05) between Inf and Con groups at the same time point.
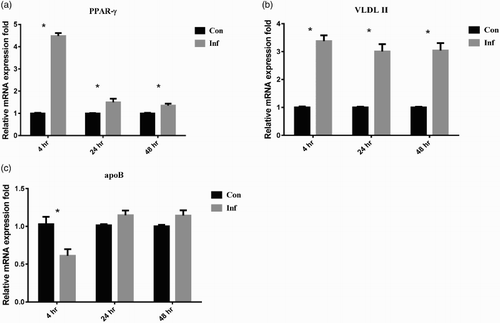
Figure 4. The lipoprotein concentrations of LMH-2A at 4, 24, and 48 hpi with SE. The VLDL secretion of LMH-2A (a) and the accumulation of ApoB in LMH-2A (b) at 4, 24, and 48 hpi with SE. Comparisons were made between SE-infected (Inf) and control (Con) LMH-2A cells co-incubated with β-oestradiol. Error bars indicate SEM values. Two asterisks represent significant differences (P < 0.01) between Inf and Con groups at the same time point.
Discussion
SE infects laying hens and may persist as a chronic clinical infection in the liver for long time period (Gast & Holt, Citation2000). Most infected hens do not present with gross or microscopic lesions in the visceral organs. However, Coble et al. found that infection with SE results in down-regulation of metabolic genes and increased liver inflammation in chicken (Coble et al., Citation2012; Suzuki, Citation1994). The data of our study indicated that SE infection results in disturbed lipid metabolism ((a),(b)) without massive cellular death of LMH-2A cells during the 4–48 h period after SE infection. To the best of our knowledge, this report is the first in-vitro model using chicken hepatoma-derived LMH-2A cell line to elucidate cellular lipid mechanisms affected by SE infection.
During the egg-laying period, the lipogenesis process becomes activated to produce abundant triglycerides in hepatocytes (Hermier, Citation1997). These triglyceride-rich vesicles are temporarily stored in the liver, and then the vesicles are assembled into VLDL by participation of lysosomes and transported to growing oocytes (Mooney & Lane, Citation1981; Schneider et al., Citation1990). When hepatic lipogenesis exceeds VLDL capacity, excessive lipid accumulates in the liver leading fatty liver called steatosis. Liver steatosis reduces egg production and increases mortality in laying hens (Hermier, Citation1997). Our data indicated the secretion of VLDL from SE-infected LMH-2A cells reduced ((a)) and the lipid accumulation in hepatocytes increased ((a),(b)). Lee et al. (Citation2010) indicated that excessive triacylglycerol deposited in the hepatocytes of laying hens reduced egg production. We speculate that SE infection leads to excessive lipid accumulation in the LMH-2A cells and it may then have impact on egg production. However, in vivo study is needed to confirm our hypothesis.
During the onset of egg production in laying hens, oestrogen increases lipogenesis in the liver to supply the rapidly growing oocytes and dramatically induces de novo synthesis of VLDL II and ApoB, which are the main components of VLDLy (Williams, Citation1979). Oestrogen also can shift lipoprotein production from VLDL to VLDLy in laying hens (Walzem et al., Citation1999). Our data indicated ERα mRNA expression was down-regulated by SE infection ((a)), suggesting that SE infected LMH-2A cells may become less sensitive to oestrogen stimulation. Results of our previous study indicated that SE infection of the ovary decreases the production of progesterone that is the upstream hormone to stimulate oestrogen synthesis (Bahr et al., Citation1983; Wang et al., Citation2013a). Since SE affects the ovary and liver of laying hens (Suzuki, Citation1994), a decrease of egg production may be due to less sensitivity of hepatocytes to oestrogen stimulation as well as a decrease in progesterone production in ovary.
SREBP-1 is responsible for the transcription of lipogenic genes, including FAS, ACC, ACSL-1, and SCD-1 (Zhang et al., Citation2003). In human cytomegalovirus infection, activated SREBP-1 induces adipocyte-like lipogenesis and provides high levels of constitutive lipid synthesis (Yu et al., Citation2012). The higher expression levels of FAS, ACC, and ACSL-1 mRNA along with the higher expression level of SREBP-1 mRNA suggested that SE infection stimulated higher levels of cellular lipogenesis. The significantly elevated expressions of FAS, ACC, and ACSL-1 suggested that increased lipogenesis in the LMH-2A cells was associated with SE infection. However, in contrast to the up-regulated FAS, ACC, and ACSL-1 gene expression, the expression of SCD-1 was down-regulated throughout the infection period ((c)). A decrease of SCD-1 expression may be related to liver dysfunction and many inflammatory diseases of mammals as reported by others (Brown et al., Citation2008; Liu et al., Citation2011). It has been shown that mouse macrophages infected with SE, inhibited the expression of SCD-1 and resulted in hindering the immunity driven by toll-like receptor 4 (Abasht et al., Citation2008; Brown et al., Citation2008). The down-regulation that occurred in the LMH-2A cells suggested that SCD-1 not only has a role in lipid homeostasis, but may also be involved in cellular inflammation in chickens.
After the cells were infected with SE, lipid transportation was negatively affected, and abnormal lipid accumulation was observed in this experiment. PPAR-γ not only regulates the transcription of downstream lipogenic genes (e.g. SCD-1, FAS, and ACC); it also promotes hepatic lipid accumulation and lipid storage in adipose tissue (Schadinger et al., Citation2005; Bradbury, Citation2006). These genes greatly increase expression in mice with fatty liver disease (Chao et al., Citation2000). Therefore, the greater expression of above-mentioned downstream genes and PPAR-γ in the infected LMH-2A cells ((a)) indicated that SE infection leads to higher lipid production and promoted lipid accumulation. In laying hens’ liver, lipid transportation is majorly regulated by formation and secretion of VLDLy composed of ApoB and VLDL II (Walzem et al., Citation1999). A higher expression of VLDL II may lead higher production of VLDLy. The SE infection has nearly no effect on ApoB mRNA expression ((c)) but positively leads an obvious accumulation of ApoB protein in the cells ((b)). It has been stated that accumulated ApoB in liver cells attenuated the VLDLy secretion from liver cells as well as the VLDLy deposition in oocytes, since ApoB acts a ligand directing VLDLy to entry into oocyte (Walzem et al., Citation1999). It has also been proved that decline in plasma concentration of VLDL or VLDLy attenuates the yolk deposition and further negatively affects the laying performance (Wang et al., Citation2013b). Conclusively, higher expression of PPAR-γ and lower secretion of VLDLy led to excessive lipid accumulation in the LMH-2A cells and may further attenuate the lipoprotein deposition for egg yolk. This excessive lipid accumulation and retarded oocyte growth may contribute to a clinical decline in egg production.
SE can cause a systemic infection and decrease egg production in laying hens. Whether SE infection of the liver is related to reduced egg production is not fully understood. Clinically, SE infects laying hens as a chronic liver infection for a long period (Gast & Holt, Citation2000). Though the mild alterations of some lipid metabolism related genes were observed in infected cells, and in light of biological relevance, the increased accumulation of lipid and apoprotein in this study suggests the long-term effects of these altered genes in the infected cells. Our study explained that reduced egg production in SE infected laying hens may be associated with the progressive changes in chicken liver lipid metabolism that occurs; however, due to the experiments being conducted with a single liver cell line, other physiological mechanisms may also have impact on the egg production. Therefore, a further in vivo study is needed to support the observation. Our previous studies revealed that SE infection negatively affects steroidogenesis, cell proliferation of granulosa cells from the prehierarchical and hierarchical follicles, and innate immune function (Tsai et al., Citation2010; Wang et al., Citation2013a, Citation2014). The results of this study consistently revealed that SE infection may not only cause abnormal hepatic lipid metabolism, but also diminish lipoprotein deposition.
Additional information
Funding
References
- Abasht, B., Kaiser, M.G. & Lamont, S.J. (2008). Toll-like receptor gene expression in cecum and spleen of advanced intercross line chicks infected with Salmonella enterica serovar Enteritidis. Veterinary Immunology and Immunopathology, 123, 314–323. doi: 10.1016/j.vetimm.2008.02.010
- Bahr, J.M., Wang, S.C., Huang, M.Y. & Calvo, F.O. (1983). Steroid concentrations in isolated theca and granulosa layers of preovulatory follicles during the ovulatory cycle of the domestic hen. Biology of Reproduction, 29, 326–334. doi: 10.1095/biolreprod29.2.326
- Bradbury, M.W. (2006). Lipid metabolism and liver inflammation. I. Hepatic fatty acid uptake: possible role in steatosis. American Journal of Physiology: Gastrointestinal and Liver Physiology, 290, G194–G198.
- Brown, J.M., Chung, S., Sawyer, J.K., Degirolamo, C., Alger, H.M., Nguyen, T., Zhu, X., Duong, M.N., Wibley, A.L., Shah, R., Davis, M.A., Kelley, K., Wilson, M.D., Kent, C., Parks, J.S. & Rudel, L.L. (2008). Inhibition of stearoyl-coenzyme A desaturase 1 dissociates insulin resistance and obesity from atherosclerosis. Circulation, 118, 1467–1475. doi: 10.1161/CIRCULATIONAHA.108.793182
- Bujo, H., Hermann, M., Lindstedt, K.A., Nimpf, J. & Schneider, W.J. (1997). Low density lipoprotein receptor gene family members mediate yolk deposition. The Journal of Nutrition, 127, 801S–804S.
- Burghelle-Mayeur, C., Demarne, Y. & Merat, P. (1989). Influence of the sex-linked dwarfing gene (dw) on the lipid composition of plasma, egg yolk and abdominal fat pad in White Leghorn laying hens: effect of dietary fat. The Journal of Nutrition, 119, 1361–1368.
- Chao, L., Marcus-Samuels, B., Mason, M.M., Moitra, J., Vinson, C., Arioglu, E., Gavrilova, O. & Reitman, M.L. (2000). Adipose tissue is required for the antidiabetic, but not for the hypolipidemic, effect of thiazolidinediones. The Journal of Clinical Investigation, 106, 1221–1228. doi: 10.1172/JCI11245
- Coble, D.J., Sandford, E.E., Ji, T., Abernathy, J., Fleming, D., Zhou, H. & Lamont, S.J. (2012). Impacts of Salmonella Enteritidis infection on liver transcriptome in broilers. Genesis, 51, 357–364. doi: 10.1002/dvg.22351
- Cury-Boaventura, M.F., Pompéia, C. & Curi, R. (2004). Comparative toxicity of oleic acid and linoleic acid on Jurkat cells. Clinical Nutrition, 23, 721–732. doi: 10.1016/j.clnu.2003.12.004
- Freeman, B.M. & Chubb, L.G. (1964). Effect of Salmonella gallinarum on certain Krebs cycle intermediates of domestic fowl. Journal of Bacteriology, 88, 93–95.
- Gast, R.K. & Holt, P.S. (2000). Deposition of phage type 4 and 13a Salmonella Enteritidis strains in the yolk and albumen of eggs laid by experimentally infected hens. Avian Diseases, 44, 706–710. doi: 10.2307/1593116
- Hermann, M., Foisner, R., Schneider, W.J. & Ivessa, N.E. (2003). Regulation by estrogen of synthesis and secretion of apolipoprotein A–I in the chicken hepatoma cell line, LMH-2A. Biochimica et Biophysica Acta (BBA) – Molecular Cell Research, 1641, 25–33. doi: 10.1016/S0167-4889(03)00046-6
- Hermann, M., Seif, F., Schneider, W.J. & Ivessa, N.E. (1997). Estrogen dependence of synthesis and secretion of apolipoprotein B-containing lipoproteins in the chicken hepatoma cell line, LMH-2A. Journal of Lipid Research, 38, 1308–1317.
- Hermier, D. (1997). Lipoprotein metabolism and fattening in poultry. The Journal of Nutrition, 127, 805S–808S.
- Hoop, R.K. & Pospischil, A. (1993). Bacteriological, serological, histological and immunohistochemical findings in laying hens with naturally acquired Salmonella Enteritidis phage type 4 infection. The Veterinary Record, 133, 391–393. doi: 10.1136/vr.133.16.391
- Kaestner, K.H., Ntambi, J.M., Kelly, T.J. Jr. & Lane, M.D. (1989). Differentiation-induced gene expression in 3T3-L1 preadipocytes. A second differentially expressed gene encoding stearoyl-CoA desaturase. The Journal of Biological Chemistry, 264, 14755–14761.
- Lee, B.K., Kim, J.S., Ahn, H.J., Hwang, J.H., Kim, J.M., Lee, H.T., An, B.K. & Kang, C.W. (2010). Changes in hepatic lipid parameters and hepatic messenger ribonucleic acid expression following estradiol administration in laying hens (Gallus domesticus). Poultry Science, 89, 2660–2667. doi: 10.3382/ps.2010-00686
- Li, L.O., Ellis, J.M., Paich, H.A., Wang, S., Gong, N., Altshuller, G., Thresher, R.J., Koves, T.R., Watkins, S.M., Muoio, D.M., Cline, G.W., Shulman, G.I. & Coleman, R.A. (2009). Liver-specific loss of long chain acyl-CoA synthetase-1 decreases triacylglycerol synthesis and beta-oxidation and alters phospholipid fatty acid composition. The Journal of Biological Chemistry, 284, 27816–27826. doi: 10.1074/jbc.M109.022467
- Liu, X., Strable, M.S. & Ntambi, J.M. (2011). Stearoyl CoA desaturase 1: role in cellular inflammation and stress. Advances in Nutrition, 2, 15–22. doi: 10.3945/an.110.000125
- Livak, K. J. & T. D. Schmittgen (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2−△△CT method. Methods, 25, 402–408. doi: 10.1006/meth.2001.1262
- Mooney, R.A. & Lane, M.D. (1981). Formation and turnover of triglyceride-rich vesicles in the chick liver cell. Effects of cAMP and carnitine on triglyceride mobilization and conversion to ketones. The Journal of Biological Chemistry, 256, 11724–11733.
- Prokopowicz, D. & Ananko, J. (1979). Injury of liver in experimental salmonellosis of rabbits infected by Salmonella Agona. Acta Hepato-gastroenterologica, 26, 17–22.
- Schadinger, S.E., Bucher, N.L., Schreiber, B.M. & Farmer, S.R. (2005). PPARgamma2 regulates lipogenesis and lipid accumulation in steatotic hepatocytes. Endocrinology and Metabolism, 288, E1195–E1205.
- Schneider, W.J., Carroll, R., Severson, D.L. & Nimpf, J. (1990). Apolipoprotein VLDL-II inhibits lipolysis of triglyceride-rich lipoproteins in the laying hen. Journal of Lipid Research, 31, 507–513.
- Shah, D.H., Zhou, X., Kim, H.Y., Call, D.R. & Guard, J. (2012). Transposon mutagenesis of Salmonella enterica serovar Enteritidis identifies genes that contribute to invasiveness in human and chicken cells and survival in egg albumen. Infection and Immunity, 80, 4203–4215. doi: 10.1128/IAI.00790-12
- Shivaprasad, H.L., Timoney, J.F., Morales, S., Lucio, B. & Baker, R.C. (1990). Pathogenesis of Salmonella Enteritidis infection in laying chickens. I. Studies on egg transmission, clinical signs, fecal shedding, and serologic responses. Avian Diseases, 34, 548–557. doi: 10.2307/1591243
- Suzuki, S. (1994). Pathogenicity of Salmonella Enteritidis in poultry. International Journal of Food Microbiology, 21, 89–105. doi: 10.1016/0168-1605(94)90203-8
- Tsai, H.J., Chiu, C.H., Wang, C.L. & Chou, C.H. (2010). A time-course study of gene responses of chicken granulosa cells to Salmonella Enteritidis infection. Veterinary Microbiology, 144, 325–333. doi: 10.1016/j.vetmic.2010.01.004
- Walzem, R.L., Hansen, R.J., Williams, D.L. & Hamilton, R.L. (1999). Estrogen induction of VLDLy assembly in egg-laying hens. The Journal of Nutrition, 129, 467S–472S.
- Wang, C.L., Chuang, T.F., Chiu, C.H. & Chou, C.H. (2013a). Mechanism of decreased progesterone synthesis in Salmonella Enteritidis-infected chicken granulosa cells. Taiwan Veterinary Journal, 39, 225–232.
- Wang, C.L., Fan, Y.C., Tseng, C.H., Chiu, C.H., Tsai, H.J. & Chou, C.H. (2014). Salmonella Enteritidis infection slows steroidogenesis and impedes cell growth in hen granulosa cells. Avian Diseases, 58, 511–517. doi: 10.1637/10846-041414-Reg.1
- Wang, X.J., Li, Y., Song, Q.Q., Guo, Y.Y., Jiao, H.C., Song, Z.G. & Lin, H. (2013b). Corticosterone regulation of ovarian follicular development is dependent on the energy status of laying hens. Journal of Lipid Research, 54, 1860–1876. doi: 10.1194/jlr.M036301
- Williams, D.L. (1979) Apoproteins of avian very low density lipoprotein: demonstration of a single high molecular weight apoprotein. Biochemistry 18, 1056–1063. doi: 10.1021/bi00573a019
- Yang, S., Suh, Y., Choi, Y.M., Shin, S., Han, J.Y., Lee, K. (2013) Loss of fat with increased adipose triglyceride lipase-mediated lipolysis in adipose tissue during laying stages in quail. Lipids, 48, 13–21. doi: 10.1007/s11745-012-3742-6
- Yen, C.F., Lin, E.C., Wang, Y.H., Wang, P.H., Lin, H.W., Hsu, J.C., Wu, L.S., Jiang, Y.N. & Ding, S.T. (2009). Abundantly expressed hepatic genes and their differential expression in liver of prelaying and laying geese. Poultry Science, 88, 1955–1962. doi: 10.3382/ps.2008-00473
- Yu, Y., Maguire, T.G. & Alwine, J.C. (2012). Human cytomegalovirus infection induces adipocyte-like lipogenesis through activation of sterol regulatory element binding protein 1. Journal of Virology, 86, 2942–2949. doi: 10.1128/JVI.06467-11
- Zhang, Y., Yin, L. & Hillgartner, F.B. (2003). SREBP-1 integrates the actions of thyroid hormone, insulin, cAMP, and medium-chain fatty acids on ACCalpha transcription in hepatocytes. Journal of Lipid Research, 44, 356–368. doi: 10.1194/jlr.M200283-JLR200
