ABSTRACT
Clostridium perfringens is an opportunistic pathogen that can cause food poisoning in humans and various enterotoxaemias in animal species. Recently, C. perfringens was shown to form biofilms, a structured community of bacterial cells enclosed in a self-produced extracellular matrix. However, very little is known on the subject and no information is available on gene expression in C. perfringens biofilms. To gain insights into the differences between free-living C. perfringens cells and those in biofilms, we used RNA sequencing. In total, 25.7% of genes showed differential expression in the two growth modes; about 12.8% of genes were up-regulated and about 12.9% were down-regulated in biofilms. We show that 772 genes were significantly differentially expressed between biofilms and planktonic cells from the supernatant of biofilms. Genes that were down-regulated in biofilm cells, relative to planktonic cells, included those involved in virulence, energy production, amino acid, nucleotide and carbohydrate metabolism, and in translation and ribosomal structure. Genes up-regulated in biofilm cells were mainly involved in amino acid and carbohydrate metabolism, transcription, inorganic ion metabolism and in defence mechanisms. This study provides new insights into the transcriptomic response of C. perfringens during biofilm formation.
Introduction
Clostridium perfringens is a Gram-positive anaerobic bacterium that causes several important human and animal diseases, primarily as a result of its ability to produce many different toxins (Markey et al., Citation2013). A classification based on the production of four major toxins (alpha, beta, epsilon, and iota) divides the C. perfringens into five toxigenic biotypes (A to E) (Petit et al., Citation1999). In humans, C. perfringens is responsible for gas gangrene, enteritis necroticans, food poisoning, and antibiotic-associated diarrheas (Myers et al., Citation2006). Currently, C. perfringens type A food poisoning is one of the most common causes of foodborne illness in North America (Scallan et al., Citation2011; Thomas et al., Citation2013; Scallan et al., Citation2015). This food poisoning is caused by a small group (∼5%) of type A isolates that produce the enterotoxin CPE (Sarker et al. Citation1999; Songer, Citation2010). In poultry, avian-specific C. perfringens strains cause necrotic enteritis, an economically significant poultry disease that costs the global industry around US$5-6 billion annually in losses and control measures (Wade & Keyburn, Citation2015). In some countries, this disease appears to be on the rise because of removal of antibiotic growth promoters (Stanley et al., Citation2014). Isolates of animal origin constitute a risk for transmission to humans through the food chain.
Recently, we, and others, have described the formation of biofilms in C. perfringens (Varga et al., Citation2008; Charlebois et al., Citation2014). Biofilms are structured communities of bacterial cells enclosed in a self-produced extracellular polysaccharide (EPS) matrix which provides increased resistance to environmental stresses (Costerton et al., Citation1999). It was also shown that type IV pilus-dependent gliding motility and the catabolite control protein, a key regulator of the response to carbohydrate limitation, were needed for maximal biofilm formation (Varga et al., Citation2008). One of our studies demonstrated that the biofilm formed by C. perfringens could protect the cells from an exposure to atmospheric oxygen and to high concentrations of antibiotics and anticoccidial agents (Charlebois et al., Citation2014). The matrix of these biofilms was shown to be composed of beta-1,4-linked polysaccharides, proteins and extracellular DNA (Charlebois et al., Citation2014). Recently, it was shown that temperature regulates C. perfringens biofilm morphology and that a sporulation master regulator was involved at a particular temperature (Obana et al., Citation2014). It was shown that at 37°C, C. perfringens adhered to a surface and formed a flat, thin biofilm referred to as adhered biofilm (Obana et al., Citation2014). This formation required AbrB, a global repressor, and PilA2, a component of type IV pili. However, at 25°C, C. perfringens did not adhere but produced a thread-like extracellular matrix, forming a viscous, thick biofilm, referred to as pellicule biofilm. This formation required the sporulation master regulator, Spo0A, and the toxin regulator, CtrAB, and was shown to be enhanced in the absence of AbrB (Obana et al., Citation2014).
Biofilm phenotypic adaptations were shown to be associated with the expression of particular genes in response to their environments (Bridier et al., Citation2011). During the adhesion step, studies have shown that genes coding flagellar proteins are repressed while other genes coding for EPSs and curli are induced (Prigent-Combaret et al., Citation2000; Sauer & Camper, Citation2001). Following the adhesion, bacteria develop into a biofilm with a three-dimensional structure where cells located at the periphery have access to nutrients and oxygen, while those in internal layers have poorer environments containing more metabolic waste elements (Bridier et al., Citation2011). It has been stated that this chemical heterogeneity is the source of the physiological heterogeneity (Stewart & Franklin, Citation2008) where cells with distinctive metabolic rates are present throughout the biofilm, indicating variety in gene expressions. This induces modifications to membrane composition and in defence mechanism expressions leading to an increase in tolerance of bacteria to environmental stresses (Taylor et al., Citation2000; Sabev et al., Citation2006). Also, it has been reported that a small part of the bacterial population within a biofilm may develop into a highly protected state with drastic resistance and referred to as persisters (Harrison et al., Citation2005; Lewis, Citation2005). They may therefore contribute to protection in the biofilm (Harrison et al., Citation2005; Lewis, Citation2005).
In the past decade, the study of the molecular genetics of biofilms was accelerated by the use of DNA microarrays and, more recently, by the use of high-throughput RNA sequencing (RNA-seq) (Carvalhais et al., Citation2014). RNA-seq has been used for transcriptome analysis as an alternative to other transcriptomic techniques due to advantages such as large dynamic range, high technical reproducibility, and because a reference transcriptome is not a requirement (Carvalhais et al., Citation2014). In general, genetic studies have revealed that biofilm formation apparently requires expression of a distinct set of genes that are different from planktonic cells including those related to chemotaxis, motility, EPS biosynthesis, and stress response (Sauer, Citation2003; Zhang et al., Citation2007; Lo et al., Citation2009). Although C. perfringens is known to form biofilms, the genes differentially expressed during the formation of this structure have not yet been described. This study was undertaken to investigate gene expression in C. perfringens biofilms.
Materials and methods
Bacterial growth conditions and biofilm formation
C. perfringens strain CP4 has been previously described (Thompson et al., Citation2006) and was used in this study. It is of poultry origin and has been isolated from a clinical case of necrotic enteritis. Thawed isolate was grown on Columbia agar with 5% sheep blood (Oxoid, Nepean, ON, Canada) and then incubated in anaerobic conditions at 35°C as recommended by the Clinical and Laboratory Standards Institute (CLSI M11-A8) (CLSI, Citation2012). Biofilm was cultured as described earlier with few modifications (Charlebois et al., Citation2014). Briefly, overnight blood agar cultures of C. perfringens were resuspended in Tryptic soy broth (TSB) (BD, Mississauga, ON, Canada) supplemented with 10 mM final concentration of filter-sterilized glucose (Sigma, Oakville, ON, Canada) to OD600 values between 0.08 and 0.1. Five millilitres of culture were added in six-well polystyrene tissue culture plates (Sarstedt, #83.3920, Montréal, Québec, Canada) and incubated anaerobically at 44°C for 24 h. This temperature was used because it was previously described that the biofilm formation is maximal for this bacterium at 44°C (Charlebois et al., Citation2014). After incubation, medium containing planktonic cells were harvested (planktonic cells from a biofilm). Plates were washed once with MiliQ water and attached cells (biofilm) were resuspended in 2 ml of sterile PBS (1 mM KH2PO4, 10 mM Na2HPO4, 137 mM NaCl, and 2.7 mM KCl). For planktonic cells, 5 ml of TSB supplemented with 10 mM of filter-sterilized glucose was inoculated and incubated anaerobically at 44°C for 24 h. After incubation, planktonic cells were harvested. All growth modes were done in triplicate and three independent experiments were performed.
RNA extraction and library construction
Bacterial total RNA from C. perfringens was extracted with the Trizol Max Bacterial Enhancement kit (Invitrogen, Burlington, ON, Canada) as described by the manufacturer. RNA was further purified and concentrated with an RNeasy mini kit (Qiagen, Toronto, ON, Canada). RNA integrity and purity was verified by Experion (Bio-Rad, Mississauga, ON, Canada) and Nanodrop spectrophotometer, respectively. After removing rRNA by using Ambion MICROBExpress Kit (Invitrogen), mRNA was used to generate the cDNA library according to TruSeq RNA Sample Prep Kit protocol (Illumina, San Diego, CA, USA), which was then sequenced using the HiSeq 2000 system (Illumina).
Transcriptome analysis
Data generated from sequencing were stored in the standard FASTQ format for use as input for subsequent analysis. RNA-Seq analysis of sequence data generated was undertaken using the CLC Genomics Workbench (version 7.0.4, CLC Bio, Boston, MA, USA). Experimental inputs for the analysis included the previously described FASTQ files containing sequenced reads generated for each of three experimental conditions (three biological replicates and three technical replicates for each condition), the Genbank files containing the complete sequences of C. perfringens strain ATCC 13124, and two pathogenicity loci associated with necrotic enteritis (NELoc-1 and NELoc-3) from strain CP4 (Lepp et al., Citation2010). Sequenced reads generated for each of the three experimental conditions (planktonic, planktonic cells from a biofilm and biofilm) were mapped against the reference genomes in order to quantify gene expression levels of the bacterium for each experimental condition. Reads counts were normalized using the median ratio. The differentially expressed gene assessments were conducted based on unique reads counts with DESeq2 v1.10.1 package (Love et al., Citation2014). Both tag-wise and common dispersions were calculated and resulting P-values from the exact tests were corrected using the Benjamini–Hochberg (BH) procedure to derive false discovery rates. All three experimental conditions were compared to each other. A gene was considered as differentially expressed when the P-value was <0.01. Genes were subsequently classified using the Clusters of Orthologous Groups system.
Real-time quantitative RT-PCR
Selected transcriptomic results were verified by real-time quantitative RT-PCR (qRT-PCR), using the SsoFast EvaGreen Supermix RT-PCR Kit (Bio-Rad). Primers (Table S1) were PCR-tested before proceeding to qRT-PCR analysis to ensure that amplification with these primers resulted in a single amplicon of the anticipated size. Ten genes were selected for analysis (Table S1). Reactions were performed with a 16-place Cepheid Smart Cycler® System in a total volume of 25 μl consisting of 10 μl 2× SsoFast EvaGreen PCR Master Mix, 5 pmol of forward and reverse primers, and 2 μl of cDNA. PCR conditions included initial denaturation at 95°C for 2 min, followed by 40 cycles of 30 s at 95°C, 30 s at 56°C, 30 s at 72°C, and final extension of 2 min at 72°C followed by melting curve analysis from 65°C to 95°C. Negative controls containing nuclease-free water instead of RNA were run concomitantly to confirm that the samples were free from contamination. Relative expression of each gene as determined by qRT-PCR was normalized to that of the rpoA gene which showed a stable level of expression throughout the different experiments (data not shown). Quantitative measures were obtained using the 2−ΔΔC T method. Three biological and three technical replicates were performed.
Results
RNA sequencing analysis
Biofilms vs. planktonic cells from a biofilm
RNA sequencing analysis identified 772 genes that were significantly differentially expressed in the two growth modes (, , S2 and S3). Functional group analysis categorized 245 of these genes as function unknown or general functional prediction only. Of all the protein coding genes, 384 were up-regulated ( and S2) and 388 were down-regulated in biofilms ( and S3). Genes differentially regulated were grouped in many functional categories (). Some of the regulated genes are known to be involved in virulence, transport, metabolism, transcription, translation, and inorganic ion acquisition (, , S2 and S3). More specifically, virulence genes encoding alpha-clostripain, collagenase, necrotic enteritis toxin B, perfringolysin O, and phospholipase C were down-regulated in biofilms ( and S3). In contrast, one gene encoding haemolysin was up-regulated in biofilms ( and S2). Many genes involved in oxidative stress resistance such as genes encoding rubredoxin/rubrerythrin, gluthatione peroxidase, and haemoglobin were up-regulated in C. perfringens biofilms ( and S2). Genes encoding for several other enzymes from the general stress response were also up-regulated (hrcA, lexA, and recA) ( and S2). A few genes involved in sporulation were differentially expressed during biofilm formation. CPF_2662, sigG, spo0A, spoIIE, spoVB, and spoVD were up-regulated whereas CPF_2814, CPF_2987, and soj were down-regulated (, , S2 and S3).
Figure 1. Functional classification of genes differentially expressed in biofilms. Genes were classified using the Clusters of Orthologous Groups system. Black and white bars represent up-regulated and down-regulated genes, respectively. (A) RNA processing and modification; (B) chromatin structure and dynamics; (c) energy production and conversion; (D) cell cycle control and mitosis; (E) amino acid metabolism and transport; (F) nucleotide metabolism and transport; (G) carbohydrate metabolism and transport; (H) coenzyme metabolsim and transport; (I) lipid metabolism and transport; (J) translation, ribosomal structure and biogenesis; (K) transcription; (L) replication, recombination and repair; (M) cell wall/membrane/envelope biogenesis; (N) cell motility; (O) postranslational modification, protein turnover, chaperone; (P) inorganic ion metabolism and transport; (Q) secondary metabolites biosynthesis, transport and catabolism; (R) general functional prediction only; (S) function unknown; (T) signal transduction mechanisms; (U) intracellular trafficking, secretion and vesicular transport; (V) defence mechanisms, (W) extracellular structures; (X) mobilome: prophage, transposons; (Y) nuclear structure; and (Z) cytoskeleton.
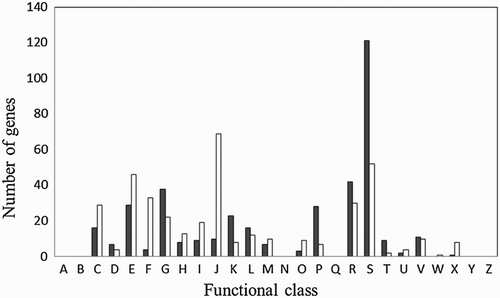
Table 1. Up-regulated genes with the highest fold changes in C. perfringens biofilms compared to planktonic cells from a biofilm.
Table 2. Down-regulated genes with the highest fold changes in C. perfringens biofilms compared to planktonic cells from a biofilm.
Transcriptomic analysis showed that several genes involved in biosynthesis metabolism of carbohydrate, pyruvate and acetate, fatty acids, and phospholipids were up-regulated in biofilm cells ( and S2). Data also showed a down-regulation of genes encoding proteins involved in chromosome replication such as the helicase CPF_0426, single-stranded binding protein Ssb, and the DNA polymerase III beta subunit DnaN ( and S3). Similarly, many genes involved in energy production, translation, and in carbohydrate, nucleotide, and amino acid metabolism were also down-regulated ( and S3). The quorum sensing gene luxS was also down-regulated in biofilms along with prophage genes, genes implicated in cell division and protein excretion ( and S3).
Biofilms vs. planktonic cells from a stationary phase culture
Another set of comparison was performed between biofilms and planktonic cells from a stationary phase culture. RNA sequencing analysis identified 708 genes that were significantly differentially expressed in these two growth modes. Genes were divided into the same functional categories as mentioned above. Of all the protein coding genes, 383 were up-regulated and 325 were down-regulated in biofilms. These results can be found in supplemental materials in Tables S4 and S5.
Planktonic cells from a biofilms vs. planktonic cells from a stationary phase culture
In this set of comparison, 160 genes were found to be differentially expressed between the planktonic cells found in the supernatant of the biofilm and the planktonic cells from a stationary phase culture. Of those, 81 were up-regulated and 79 were down-regulated in biofilms (Tables S6 and S7).
Venn diagram
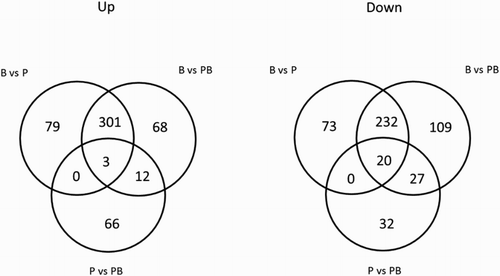
Venn diagram analysis of the comparison groups revealed that 23 differentially expressed genes were shared between all three comparison groups (, intersection of the three circles). Also, many genes were uniquely expressed in each comparison growth mode (). When comparing the biofilms vs. planktonic cells from a biofilm and the biofilms vs. planktonic cells groups, 533 genes were found to be shared by both comparison groups (301 up-regulated and 232 down-regulated) (). Thirty-nine genes were shared between the biofilms vs. planktonic cells from a biofilm group and the planktonic cells from a biofilms vs. planktonic cells group (12 up-regulated and 27 down-regulated). As for the comparison between the biofilms vs. planktonic cells group and the planktonic cells from a biofilm vs. planktonic cells group, no gene was shared between the groups ().
Figure 2. Gene expression under planktonic, planktonic biofilms, and biofilms growth conditions. Venn diagrams showing numbers of significantly up- and down-regulated genes shared between each comparison groups: C. perfringens biofilms (B), planktonic cells (P), and planktonic cells from the biofilm supernatant (PB). The numbers displayed in circles between the Venn diagrams, referred to as intersections, show the number of genes shared between the two overlapping areas.
Validation by quantitative RT-PCR
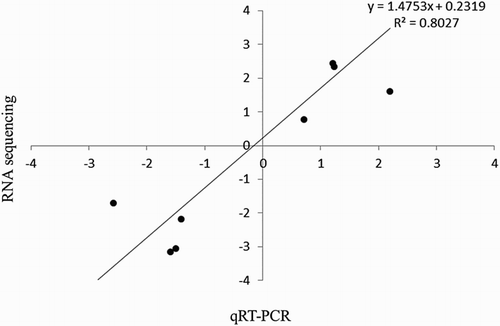
To validate the RNA sequencing results, quantitative RT-PCR was performed on 10 randomly selected differentially expressed genes. Fold changes in gene expressions were calculated after normalization of each gene with the constitutively expressed gene rpoA. There was a good correlation between the expression ratios determined by both methods ().
Figure 3. Validation of transcriptomic results of RNA sequencing by qRT-PCR. Correlation between RNA sequencing and quantitative real-time-PCR gene expression ratios of biofilms and planktonic biofilms. Mean log2 ratios obtained during qRT-PCR experiments are plotted against the mean log2 ratios obtained with the RNA sequencing.
Discussion
In the present study, the biofilm transcriptome of C. perfringens strain CP4, isolated from a clinical case of necrotic enteritis, was analysed for the first time with RNA sequencing. This analysis showed that about 25.7% of genes (n = 772) were significantly differentially expressed in biofilms and planktonic biofilms. Overall, about 12.8% of genes were up-regulated and about 12.9% were down-regulated in biofilms. Our results show that gene expression in biofilm cells demonstrates a number of significant differences to that in free-living cells. In this study, several genes involved in the metabolism of carbohydrate, pyruvate, and acetate were up-regulated in biofilms. The induction of EPS biosynthesis during biofilm formation has been observed in other studies (Zhang et al., Citation2007; Huse et al., Citation2013). The major components of the extracellular polymeric substances are EPSs (Flemming & Wingender, Citation2010). Several EPSs are homopolysaccharides but most of them are heteropolysaccharides that consist of a mixture of neutral and charged sugar residues. They can also contain organic or inorganic substituents like pyruvate, acetate, succinate, or, rarely, sulphate (Esperanza Troyano et al., Citation1996; Flemming & Wingender, Citation2010). EPS has been shown to be required for the initial attachment to a surface, for the structural development of mature biofilms and is considered to be the key component that determines the physico-chemical and biological properties of biofilms (Zhang et al., Citation2007). In Salmonella enterica serovar Typhimurium, EPSs have been shown to be essential for the attachment of the pathogen to HEp-2 cells and chicken intestinal epithelium (Ledeboer & Jones, Citation2005). In C. perfringens, up-regulation of genes involved in EPS biosynthesis could also play a major role in the initial attachment to chicken intestinal cells and could be linked with persistence of the bacteria within the chicken intestines. Indeed, the EPSs of Bifidobacterium spp. were shown to protect these bacteria against the harsh conditions of the gastrointestinal tract, thus improving their persistence in the host (Hidalgo-Cantabrana et al., Citation2014).
Many genes involved in lipid and amino acids biosynthesis, energy production, translation, carbohydrate metabolism, and in chromosome replication were down-regulated in biofilms as well as genes involved in cell division and protein excretions. Only a few genes involved in lipid biosynthesis were up-regulated in biofilms. These results suggest a slowed cell replication and growth rate in biofilms. This is in agreement with other studies on the transcriptomic of biofilms (Yao et al., Citation2005; Zhang et al., Citation2007; Lo et al., Citation2009). The slower growth rate of cells in biofilms has been previously attributed to restricted penetration of nutrients (Lo et al., Citation2009). It has also been involved in reduced susceptibility or tolerance to antibiotics (Hoiby et al., Citation2010) making infections more difficult to treat. For C. perfringens, it has been hypothesized that biofilm formation by this organism in the small intestine could contribute to bacterial persistence through antibiotic treatment (Varga et al., Citation2008).
Several genes involved in oxidative stress resistance and in the general stress response were up-regulated in biofilms. Increased expression of oxidative stress genes has previously been reported in Escherichia coli biofilms (Schembri et al., Citation2003). Moreover, increased protein levels of superoxide dismutase and alkyl hydroperoxide reductase in Pseudomonas aeruginosa biofilm have been observed (Sauer et al., Citation2002). In Neisseria gonorrhoeae, mechanisms for oxidative stress tolerance appear to be required in order to sustain robust biofilms (Falsetta et al., Citation2011). More recently, the oxidative stress response sensor OxyR has been associated with biofilm development in many microorganisms (Honma et al., Citation2009). It has been proposed that the up-regulation of oxidative stress-induced biofilm formation in the host might provide bacteria with a defence strategy (Honma et al., Citation2009) but the associated mechanisms are still not fully understood. Both oxidative stress resistance and general stress response might be important for the development of biofilms in C. perfringens because many genes involved in these processes were up-regulated in this study. Moreover, in previous studies, it was shown that C. perfringens cells in biofilms were more tolerant to atmospheric oxygen and to hydrogen peroxide (Charlebois et al., Citation2014). This up-regulation of oxidative stress resistance genes could be involved in the resistance to reactive oxygen species produced by the immune system or by neighbouring microorganisms of the chicken guts, and in the resistance to reactive oxygen species produced by irradiation and temperature in the environment (Gambino & Cappitelli, Citation2016).
Genes encoding alpha-clostripain, collagenase, necrotic enteritis toxin B, perfringolysin O, and phospholipase C were down-regulated in biofilms. These results are in agreement with what was previously observed in other studies where toxins were down-regulated in biofilms (Resch et al., Citation2005; Sanchez et al., Citation2011). These results suggest that C. perfringens biofilms may not contribute directly to the development of necrotic enteritis lesions but may instead confer a quiescent mode of growth during colonization. The present study also demonstrated that a gene encoding haemolysin was up-regulated in C. perfringens biofilms. Staphylococcus aureus mutants defective in alpha-haemolysin production failed to form biofilms under both static and flow conditions, indicating a role in biofilm formation (Caiazza & O'Toole, Citation2003). This phenotype seems to be associated to an apparent defect in cell-to-cell interactions (Caiazza & O'Toole, Citation2003). Haemolysins of Streptococcus suis and enterococci have also been associated as key elements in biofilms (Tsikrikonis et al., Citation2012; Wang et al., Citation2012). In addition to toxin genes, a few antimicrobial resistance genes were also differentially expressed in this study. A tetracycline resistance gene (CPF-1490), a putative beta-lactamases (CPF_1388), three MATE efflux family proteins (CPF_1409, CPF_2828, CPF_2859), and a multidrug resistance protein (CPF_1028) were upregulated in C. perfringens biofilms. The induction or repression of particular antimicrobial resistance genes in biofilms has not yet been clearly demonstrated and further research is necessary to determine if this phenomenon plays a role in biofilms formation and resistance.
In this study, many genes involved in iron acquisition were up-regulated in biofilms. In P. aeruginosa, it was found that iron serves as a signal in biofilm development (Banin et al., Citation2005). In their study, mutants that were not able to obtain iron through the high-affinity pyoverdine iron acquisition system formed thin biofilms similar to those formed by their parent strain under low iron conditions. Genes involved in iron acquisition were also found to be up-regulated in S. aureus and Acinetobacter baumanii biofilms (Lin et al., Citation2012; Rumbo-Feal et al., Citation2013). Also, Lepp et al. (Citation2013) showed that iron acquisition mechanisms might confer a selective advantage to NE-causing strains (Lepp et al., Citation2013).
The quorum sensing gene luxS was down-regulated in our C. perfringens biofilms. This repression of luxS was associated with a down-regulation of genes encoding toxins. LuxS of C. perfringens has been previously reported as an activator of toxin production (Ohtani et al., Citation2002). In the present study, luxS was more expressed in planktonic biofilms than in biofilms. In Staphylococcus epidermidis, luxS was shown to repress biofilm formation through a cell–cell signalling mechanism based on autoinducer 2 secretion (Xu et al., Citation2006). However, in many studies, luxS has been linked to biofilm formation (Xu et al., Citation2006; Ahmed et al., Citation2009; Vidal et al., Citation2011; Ethapa et al., Citation2013). A Clostridium difficile luxS mutant was found to be unable to form a bacterial monolayer on glass surface, indicating the importance of this gene in the first steps of biofilm formation (Ethapa et al., Citation2013). A study with a C. perfringens luxS mutant could bring more information on the role of this gene in the biofilm formation.
Many genes involved in sporulation (CPF_2662, sigG, spo0A, spoVB, spoVD, and spoIIE) were up-regulated in biofilms. CPF_2662, a spore cortex-lytic enzyme, is implicated in the spore germination process, Spo0A is a master regulator of sporulation in Gram-positive, SpoVB and SpoVD are implicated in the formation of spore cortex, and SpoIIE is involved in asymmetric division, sporulation, and expression of sigma factors σF, σE, and σG (Galperin et al., Citation2012). As for SigG, it regulates gene expression in the forespore (Li & McClane, Citation2010). Previous studies have reported on gene involvement in sporulation in association with biofilm formation. In C. perfringens, Spo0A has been shown to be required for non-adherent pellicle formation at 25°C (Obana et al., Citation2014). In C. difficile, Spo0A was involved in biofilm formation at 37°C (Dawson et al., Citation2012). Similarly, in Bacillus subtilis, a spo0A mutant was impaired for sporulation and biofilm formation (Hamon & Lazazzera, Citation2001). Also, a sleC mutant was shown to disturb the biofilm formation of C. perfringens and C. difficile (Ethapa et al., Citation2013; Pantaléon et al., Citation2014). SleC is a protein involved in the spore germination process, especially in the cortex lysis (Miyata et al., Citation1995; Burns et al., Citation2010). In this study, a sleC homologue (CPF-2662) was found to be up-regulated in C. perfringens biofilms. In Bacillus cereus, more-efficient sporulation was seen in the biofilm phase than in the suspension phase, suggesting that biofilms could function as a centre for sporulation, increasing the spore content in the environment by dispersion (Wijman et al., Citation2007). The involvement of biofilm in these processes warrants investigation in C. perfringens.
In addition to biofilms vs. planktonic biofilm comparison group, two other comparison groups were analysed: biofilm vs. planktonic and planktonic biofilm vs. planktonic. Overall, more genes were differentially expressed between biofilms and planktonic biofilms (n = 772) than between biofilms and planktonic cells (n = 708). It is tempting to speculate that planktonic biofilm cells and biofilm cells are cross-talking, affecting the transcription of many genes, but this hypothesis needs further investigation.
In conclusion, this study provides new insights into the genes differentially expressed during biofilm formation in C. perfringens. Genes involved in virulence, energy production, amino acid, nucleotide and carbohydrate metabolism, and in translation and ribosomal structure were down-regulated whereas genes involved in amino acid and carbohydrate metabolism, transcription, inorganic ion metabolism and in defence mechanisms were up-regulated. Moreover, it was proposed that oxidative stress resistance and the general stress response might be important for the development of biofilms in C. perfringens. Results also suggest that C. perfringens biofilms could be involved in the survival and the persistence of this microorganism in the chicken intestines and in the environment.
Supplemental Data
Download Zip (337.8 KB)Acknowledgements
We thank Dr Brian Boyle for is technical support for the RNA extraction and the RNA sequencing analysis, Dr Jérémy Le Luyer for the DESeq2 analysis and Dr John Prescott for supplying strain CP4.
Notes on contributors
Dr Audrey Chalebois, BSc., MSc., PhD, completed her Ph.D. in 2014 under the supervision of Dr Archambault at the University of Montreal. She has received research excellence student grants from this university. She is now a postdoc fellow in Dr Martine Boulianne's laboratory. Her areas of research include clostridium perfringens and antibiotic resistance.
Dr Marie Archambault, DMV, MSc, PhD, Dipl ACVM, is a Full Professor at the Faculty of Veterinary Medicine of the University of Montreal. She received her Board Certification from the American College of Veterinary Microbiologists in 2002 and was recently awarded the Carl J. Norden teaching excellence award. Her areas of research include clostridium perfringens and antibiotic resistance in veterinary and zoonotic pathogens.
Dr Mario Jacques, BSc., PhD, is a Full Professor at the Faculty of Veterinary Medicine of the University of Montreal. He has won excellence awards in research from this university. He is presently the director of ‘The regroupement de recherche pour un lait de qualité optimale, Op+lait’. His area of research includes the study of virulence factors involved in colonization and in the biofilm formation of bacterial pathogens.
ORCiD
Marie Archambault http://orcid.org/0000-0002-5206-9531
Additional information
Funding
References
- Ahmed, N.A., Petersen, F.C. & Scheie, A.A. (2009). AI-2/LuxS is involved in increased biofilm formation by Streptococcus intermedius in the presence of antibiotics. Antimicrobial Agents and Chemotherapy, 53, 4258–4263. doi: 10.1128/AAC.00546-09
- Banin, E., Vasil, M.L. & Greenberg, E.P. (2005). Iron and Pseudomonas aeruginosa biofilm formation. Proceedings of the National Academy of Sciences, 102, 11076–11081. doi: 10.1073/pnas.0504266102
- Bridier, A., Briandet, R., Thomas, V. & Dubois-Brissonnet, F. (2011). Resistance of bacterial biofilms to disinfectants: a review. Biofouling, 27, 1017–1032. doi: 10.1080/08927014.2011.626899
- Burns, D.A., Heap, J.T. & Minton, N.P. (2010). SleC is essential for germination of Clostridium difficile spores in nutrient-rich medium supplemented with the bile salt taurocholate. Journal of Bacteriology, 192, 657–664. doi: 10.1128/JB.01209-09
- Caiazza, N.C. & O’Toole, G.A. (2003). Alpha-toxin is required for biofilm formation by Staphylococcus aureus. Journal of Bacteriology, 185, 3214–3217. doi: 10.1128/JB.185.10.3214-3217.2003
- Carvalhais, V., Franca, A., Cerca, F., Vitorino, R., Pier, G.B., Vilanova, M. & Cerca, N. (2014). Dormancy within Staphylococcus epidermidis biofilms: a transcriptomic analysis by RNA-seq. Applied Microbiology and Biotechnology, 98, 2585–2596. doi: 10.1007/s00253-014-5548-3
- Charlebois, A., Jacques, M. & Archambault, M. (2014). Biofilm formation of Clostridium perfringens and its exposure to low-dose antimicrobials. Frontiers in Microbiology, 5, 183. doi: 10.3389/fmicb.2014.00183
- CLSI. (2012). Methods for antimicrobial susceptibility testing of anaerobic bacteria; approved standard (8th edn). CLSI document M11-A6. Wayne, PA: Clinical and Laboratory Standards Institute.
- Costerton, J.W., Stewart, P.S. & Greenberg, E.P. (1999). Bacterial biofilms: a common cause of persistent infections. Science, 284, 1318–1322. doi: 10.1126/science.284.5418.1318
- Dawson, L.F., Valiente, E., Faulds-Pain, A., Donahue, E.H. & Wren, B.W. (2012). Characterisation of Clostridium difficile biofilm formation, a role for Spo0A. PLoS One, 7, e50527. doi: 10.1371/journal.pone.0050527
- Esperanza Troyano, S.-P.L., Rha, C.K. & Sinskey, A.J. (1996). Presence of acetate and succinate in the exopolysaccharide produced by Zoogloea ramigera 115SLR. Carbohydrate Polymers, 31, 35–40. doi: 10.1016/S0144-8617(96)00056-2
- Ethapa, T., Leuzzi, R., Ng, Y.K., Baban, S.T., Adamo, R., Kuehne, S.A., Scarselli, M., Minton, N.P., Serruto, D. & Unnikrishnan, M. (2013). Multiple factors modulate biofilm formation by the anaerobic pathogen Clostridium difficile. Journal of Bacteriology, 195, 545–555. doi: 10.1128/JB.01980-12
- Falsetta, M.L., Steichen, C.T., McEwan, A.G., Cho, C., Ketterer, M., Shao, J., Hunt, J., Jennings, M.P. & Apicella, M.A. (2011). The composition and metabolic phenotype of Neisseria gonorrhoeae biofilms. Frontiers in Microbiology, 2, 75. doi: 10.3389/fmicb.2011.00075
- Flemming, H.C. & Wingender, J. (2010). The biofilm matrix. Nature Reviews Microbiology, 8, 623–633.
- Galperin, M.Y., Mekhedov, S.L., Puigbo, P., Smirnov, S., Wolf, Y.I. & Rigden, D.J. (2012). Genomic determinants of sporulation in Bacilli and Clostridia: towards the minimal set of sporulation-specific genes. Environmental Microbiology, 14, 2870–2890. doi: 10.1111/j.1462-2920.2012.02841.x
- Gambino, M. & Cappitelli, F. (2016). Mini-review: biofilm responses to oxidative stress. Biofouling, 32, 167–178. doi: 10.1080/08927014.2015.1134515
- Hamon, M.A. & Lazazzera, B.A. (2001). The sporulation transcription factor Spo0A is required for biofilm development in Bacillus subtilis. Molecular Microbiology, 42, 1199–1209. doi: 10.1046/j.1365-2958.2001.02709.x
- Harrison, J.J., Turner, R.J. & Ceri, H. (2005). Persister cells, the biofilm matrix and tolerance to metal cations in biofilm and planktonic Pseudomonas aeruginosa. Environmental Microbiology, 7, 981–994. doi: 10.1111/j.1462-2920.2005.00777.x
- Hidalgo-Cantabrana, C., Sanchez, B., Milani, C., Ventura, M., Margolles, A. & Ruas-Madiedo, P. (2014). Genomic overview and biological functions of exopolysaccharide biosynthesis in Bifidobacterium spp. Applied and Environmental Microbiology, 80, 9–18. doi: 10.1128/AEM.02977-13
- Hoiby, N., Bjarnsholt, T., Givskov, M., Molin, S. & Ciofu, O. (2010). Antibiotic resistance of bacterial biofilms. International Journal of Antimicrobial Agents, 35, 322–332. doi: 10.1016/j.ijantimicag.2009.12.011
- Honma, K., Mishima, E., Inagaki, S. & Sharma, A. (2009). The OxyR homologue in Tannerella forsythia regulates expression of oxidative stress responses and biofilm formation. Microbiology, 155, 1912–1922. doi: 10.1099/mic.0.027920-0
- Huse, H.K., Kwon, T., Zlosnik, J.E., Speert, D.P., Marcotte, E.M. & Whiteley, M. (2013). Pseudomonas aeruginosa enhances production of a non-alginate exopolysaccharide during long-term colonization of the cystic fibrosis lung. PLoS One, 8, e82621. doi: 10.1371/journal.pone.0082621
- Ledeboer, N.A. & Jones, B.D. (2005). Exopolysaccharide sugars contribute to biofilm formation by Salmonella enterica serovar Typhimurium on HEp-2 cells and chicken intestinal epithelium. Journal of Bacteriology, 187, 3214–3226. doi: 10.1128/JB.187.9.3214-3226.2005
- Lepp, D., Gong, J., Songer, J.G., Boerlin, P., Parreira, V.R. & Prescott, J.F. (2013). Identification of accessory genome regions in poultry Clostridium perfringens isolates carrying the netB plasmid. Journal of Bacteriology, 195, 1152–1166. doi: 10.1128/JB.01032-12
- Lepp, D., Roxas, B., Parreira, V.R., Marri, P.R., Rosey, E.L., Gong, J., Songer, J.G., Vedantam, G. & Prescott, J.F. (2010). Identification of novel pathogenicity loci in Clostridium perfringens strains that cause avian necrotic enteritis. PLoS One, 5, e10795. doi: 10.1371/journal.pone.0010795
- Lewis, K. (2005). Persister cells and the riddle of biofilm survival. Biochemistry (Moscow), 70, 267–274. doi: 10.1007/s10541-005-0111-6
- Li, J. & McClane, B.A. (2010). Evaluating the involvement of alternative sigma factors SigF and SigG in Clostridium perfringens sporulation and enterotoxin synthesis. Infection and Immunity, 78, 4286–4293. doi: 10.1128/IAI.00528-10
- Lin, M.H., Shu, J.C., Huang, H.Y. & Cheng, Y.C. (2012). Involvement of iron in biofilm formation by Staphylococcus aureus. PLoS One, 7, e34388. doi: 10.1371/journal.pone.0034388
- Lo, A.W., Seers, C.A., Boyce, J.D., Dashper, S.G., Slakeski, N., Lissel, J.P. & Reynolds, E.C. (2009). Comparative transcriptomic analysis of Porphyromonas gingivalis biofilm and planktonic cells. BMC Microbiology, 9, 18. doi: 10.1186/1471-2180-9-18
- Love, M.I., Huber, W. & Anders, S. (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biology, 15, 31. doi: 10.1186/s13059-014-0550-8
- Markey, B., Leonard, F., Archambault, M., Cullinane, A. & Maguire, D. (2013). Clinical Veterinary Microbiology (2nd edn., pp. 908). London: Mosby Elsevier.
- Miyata, S., Moriyama, R., Miyahara, N. & Makino, S. (1995). A gene (sleC) encoding a spore-cortex-lytic enzyme from Clostridium perfringens S40 spores; cloning, sequence analysis and molecular characterization. Microbiology, 141, 2643–2650. doi: 10.1099/13500872-141-10-2643
- Myers, G.S., Rasko, D.A., Cheung, J.K., Ravel, J., Seshadri, R., DeBoy, R.T., Ren, Q., Varga, J., Awad, M.M., Brinkac, L.M., Daugherty, S.C., Haft, D.H., Dodson, R.J., Madupu, R., Nelson, W.C., Rosovitz, M.J., Sullivan, S.A., Khouri, H., Dimitrov, G.I., Watkins, K.L., Mulligan, S., Benton, J., Radune, D., Fisher, D.J., Atkins, H.S., Hiscox, T., Jost, B.H., Billington, S.J., Songer, J.G., McClane, B.A., Titball, R.W., Rood, J.I., Melville, S.B. & Paulsen, I.T. (2006). Skewed genomic variability in strains of the toxigenic bacterial pathogen, Clostridium perfringens. Genome Research, 16, 1031–1040. doi: 10.1101/gr.5238106
- Obana, N., Nakamura, K. & Nomura, N. (2014). A sporulation factor is involved in the morphological change of Clostridium perfringens biofilms in response to temperature. Journal of Bacteriology, 196, 1540–1550. doi: 10.1128/JB.01444-13
- Ohtani, K., Hayashi, H. & Shimizu, T. (2002). The luxS gene is involved in cell-cell signalling for toxin production in Clostridium perfringens. Molecular Microbiology, 44, 171–179. doi: 10.1046/j.1365-2958.2002.02863.x
- Pantaléon, V., Bouttier, S., Soavelomandroso, A.P., Janoir, C. & Candela, T. (2014). Biofilms of Clostridium species. Anaerobe, 30, 193–198. doi: 10.1016/j.anaerobe.2014.09.010
- Petit, L., Gibert, M. & Popoff, M.R. (1999). Clostridium perfringens: toxinotype and genotype. Trends in Microbiology, 7, 104–110. doi: 10.1016/S0966-842X(98)01430-9
- Prigent-Combaret, C., Prensier, G., Le Thi, T.T., Vidal, O., Lejeune, P. & Dorel, C. (2000). Developmental pathway for biofilm formation in curli-producing Escherichia coli strains: role of flagella, curli and colanic acid. Environmental Microbiology, 2, 450–464. doi: 10.1046/j.1462-2920.2000.00128.x
- Resch, A., Rosenstein, R., Nerz, C. & Gotz, F. (2005). Differential gene expression profiling of Staphylococcus aureus cultivated under biofilm and planktonic conditions. Applied and Environmental Microbiology, 71, 2663–2676. doi: 10.1128/AEM.71.5.2663-2676.2005
- Rumbo-Feal, S., Gomez, M.J., Gayoso, C., Alvarez-Fraga, L., Cabral, M.P., Aransay, A.M., Rodríguez-Ezpeleta, N., Fullaondo, A., Valle, J., Tomás, M., Bou, G. & Poza, M. (2013). Whole transcriptome analysis of Acinetobacter baumannii assessed by RNA-sequencing reveals different mRNA expression profiles in biofilm compared to planktonic cells. PLoS One, 8, e72968. doi: 10.1371/journal.pone.0072968
- Sabev, H.A., Robson, G.D. & Handley, P.S. (2006). Influence of starvation, surface attachment and biofilm growth on the biocide susceptibility of the biodeteriogenic yeast Aureobasidium pullulans. Journal of Applied Microbiology, 101, 319–330. doi: 10.1111/j.1365-2672.2006.03014.x
- Sanchez, C.J., Kumar, N., Lizcano, A., Shivshankar, P., Dunning Hotopp, J.C., Jorgensen, J.H., Tettelin, H. & Orihuela, C.J. (2011). Streptococcus pneumoniae in biofilms are unable to cause invasive disease due to altered virulence determinant production. PLoS One, 6, e28738. doi: 10.1371/journal.pone.0028738
- Sarker, M.R., Carman, R.J. & McClane, B.A. (1999). Inactivation of the gene (cpe) encoding Clostridium perfringens enterotoxin eliminates the ability of two cpe-positive C. perfringens type A human gastrointestinal disease isolates to affect rabbit ileal loops. Molecular Microbiology, 33, 946–958. doi: 10.1046/j.1365-2958.1999.01534.x
- Sauer, K. (2003). The genomics and proteomics of biofilm formation. Genome Biology, 4, 219. doi: 10.1186/gb-2003-4-6-219
- Sauer, K. & Camper, A.K. (2001). Characterization of phenotypic changes in Pseudomonas putida in response to surface-associated growth. Journal of Bacteriology, 183, 6579–6589. doi: 10.1128/JB.183.22.6579-6589.2001
- Sauer, K., Camper, A.K., Ehrlich, G.D., Costerton, J.W. & Davies, D.G. (2002). Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. Journal of Bacteriology, 184, 1140–1154. doi: 10.1128/jb.184.4.1140-1154.2002
- Scallan, E., Hoekstra, R.M., Angulo, F.J., Tauxe, R.V., Widdowson, M.A., Roy, S.L., Jones, J.L. & Griffin, P.M. (2011). Foodborne illness acquired in the United States—major pathogens. Emerging Infectious Diseases, 17, 7–15. doi: 10.3201/eid1701.P11101
- Scallan, E., Hoekstra, R.M., Mahon, B.E., Jones, T.F. & Griffin, P.M. (2015). An assessment of the human health impact of seven leading foodborne pathogens in the United States using disability adjusted life years. Epidemiology and Infection, 143, 2795–2804. doi: 10.1017/S0950268814003185
- Schembri, M.A., Kjaergaard, K. & Klemm, P. (2003). Global gene expression in Escherichia coli biofilms. Molecular Microbiology, 48, 253–267. doi: 10.1046/j.1365-2958.2003.03432.x
- Songer, J.G. (2010). Clostridia as agents of zoonotic disease. Veterinary Microbiology, 140, 399–404. doi: 10.1016/j.vetmic.2009.07.003
- Stanley, D., Wu, S.B., Rodgers, N., Swick, R.A. & Moore, R.J. (2014). Differential responses of cecal microbiota to fishmeal, Eimeria and Clostridium perfringens in a necrotic enteritis challenge model in chickens. PLoS One, 9, e104739. doi: 10.1371/journal.pone.0104739
- Stewart, P.S. & Franklin, M.J. (2008). Physiological heterogeneity in biofilms. Nature Reviews Microbiology, 6, 199–210. doi: 10.1038/nrmicro1838
- Taylor, R.H., Falkinham, J.O.3rd, Norton, C.D. & LeChevallier, M.W. (2000). Chlorine, chloramine, chlorine dioxide, and ozone susceptibility of Mycobacterium avium. Applied and Environmental Microbiology, 66, 1702–1705. doi: 10.1128/AEM.66.4.1702-1705.2000
- Thomas, M.K., Murray, R., Flockhart, L., Pintar, K., Pollari, F., Fazil, A., Nesbitt, A. & Marshall, B. (2013). Estimates of the burden of foodborne illness in Canada for 30 specified pathogens and unspecified agents, Circa 2006. Foodborne Pathogens and Disease, 10, 639–648.
- Thompson, D.R., Parreira, V.R., Kulkarni, R.R. & Prescott, J.F. (2006). Live attenuated vaccine-based control of necrotic enteritis of broiler chickens. Veterinary Microbiology, 113, 25–34. doi: 10.1016/j.vetmic.2005.10.015
- Tsikrikonis, G., Maniatis, A.N., Labrou, M., Ntokou, E., Michail, G., Daponte, A., Stathopoulos, C., Tsakris, A. & Pournaras S. (2012). Differences in biofilm formation and virulence factors between clinical and fecal enterococcal isolates of human and animal origin. Microbial Pathogenesis, 52, 336–343. doi: 10.1016/j.micpath.2012.03.003
- Varga, J.J., Therit, B. & Melville, S.B. (2008). Type IV pili and the CcpA protein are needed for maximal biofilm formation by the Gram-positive anaerobic pathogen Clostridium perfringens. Infection and Immunity, 76, 4944–4951. doi: 10.1128/IAI.00692-08
- Vidal, J.E., Ludewick, H.P., Kunkel, R.M., Zahner, D. & Klugman, K.P. (2011). The LuxS-dependent quorum-sensing system regulates early biofilm formation by Streptococcus pneumoniae strain D39. Infection and Immunity, 79, 4050–4060. doi: 10.1128/IAI.05186-11
- Wade, B. & Keyburn, A.L. (2015). The true cost of necrotic enteritis. World Poultry.
- Wang, Y., Yi, L., Wu, Z., Shao, J., Liu, G., Fan, H., Zhang, W. & Lu, C. (2012). Comparative proteomic analysis of Streptococcus suis biofilms and planktonic cells that identified biofilm infection-related immunogenic proteins. PLoS One, 7, e33371. doi: 10.1371/journal.pone.0033371
- Wijman, J.G., de Leeuw, P.P., Moezelaar, R., Zwietering, M.H. & Abee, T. (2007). Air-liquid interface biofilms of Bacillus cereus: formation, sporulation, and dispersion. Applied and Environmental Microbiology, 73, 1481–1488. doi: 10.1128/AEM.01781-06
- Xu, L., Li, H., Vuong, C., Vadyvaloo, V., Wang, J., Yao, Y., Otto, M. & Gao, Q.. (2006). Role of the luxS quorum-sensing system in biofilm formation and virulence of Staphylococcus epidermidis. Infection and Immunity, 74, 488–496. doi: 10.1128/IAI.74.1.488-496.2006
- Yao, Y., Sturdevant, D.E. & Otto, M. (2005). Genomewide analysis of gene expression in Staphylococcus epidermidis biofilms: insights into the pathophysiology of S. epidermidis biofilms and the role of phenol-soluble modulins in formation of biofilms. The Journal of Infectious Diseases, 191, 289–298. doi: 10.1086/426945
- Zhang, W., Culley, D.E., Nie, L. & Scholten, J.C. (2007). Comparative transcriptome analysis of Desulfovibrio vulgaris grown in planktonic culture and mature biofilm on a steel surface. Applied Microbiology and Biotechnology, 76, 447–457. doi: 10.1007/s00253-007-1014-9
