ABSTRACT
This case report describes an episode of recurring severe necrotizing and haemorrhagic hepatitis and enteritis experienced in a flock of commercial layer pullets at 12 weeks of age and again at 18 weeks of age in Indiana. Pullets had been vaccinated at 10 weeks old using a trivalent Salmonella Enteritidis (SE)/Newcastle disease/infectious bronchitis oil-emulsion-inactivated vaccine. The pullets were found dead at 12 weeks with firm but friable, enlarged, haemorrhagic livers, enlarged spleens, and necrohaemorrhagic intestines. Histopathologic findings were consistent with a necrotizing and haemorrhagic enteritis and hepatitis. Livers had multiple intra-sinusoidal thrombi, intestines contained Gram-positive bacterial colonies, and spleens had marked lymphoid depletion. The pullets seemed to improve after antibiotic treatment. Pullets were vaccinated with an inactivated SE vaccine at 14 weeks of age. A second spike of mortality occurred at 18 weeks of age. Although clostridial enteritis and hepatitis were highly suspected in the two cases based on macroscopic and microscopic findings, no significant bacterial or viral agents were isolated from the livers and intestines. In summary, lesions in the liver and intestines are speculated to be due to repetitive vaccination, leading to an anamnestic response by the immune system, and resulting in an immune-mediated response. However, much of the pathogenesis is still unclear, and other causes such as unidentified infectious aetiology, transmissible amyloidosis, and hypersensitivity may need further investigation.
Introduction
Pullet hepatopathy syndrome has been described as an emerging concern since the early 2000s as a potential post-vaccination-associated reaction after administration of an inactivated, trivalent Newcastle disease/infectious bronchitis/Salmonella Enteritidis (SE) vaccine injection (Rampin et al., Citation1989; Porter et al., Citation2006). Pullet hepatopathy syndrome, occurring in the early laying period has been observed in a number of flocks vaccinated at a young age by the intramuscular route (Porter et al., Citation2006). There have been two reports in the literature of a similar “necrotic, hemorrhagic, hepatomegalic hepatitis” occurring in layer flocks receiving repeated administration of oil-emulsion vaccines (Tablante et al., Citation1994; Murakami et al., Citation2013). There was also an experimental study to compare endotoxic activities of two different trivalent vaccines from two different vaccine manufacturers (Porter et al., Citation2006). Gross lesions from these reports (Rampin et al., Citation1989; Ritchie & Riddell, Citation1991; Tablante et al., Citation1994; Porter et al., Citation2006; Murakami et al., Citation2013) were characterized by intra-coelomic haemorrhages, enlarged, necrotic livers, and enlarged spleens. No significant bacterial or viral agent was recovered from the lesions. Thus, a prolonged antigenic stimulation due to vaccination, leading to an immune-mediated response, resulting in hepatitis and vasculitis, has been speculated, although hepatitis E virus infection cannot be ruled out in some earlier reports (Tablante et al., Citation1994; Porter et al., Citation2006; Murakami et al., Citation2013).
According to a United States egg layer health 2013 annual survey performed in September/October 2014 by the Association of Veterinarians in Egg Production (AVEP), pullet hepatopathy syndrome possibly related to bacterin-induced hepatitis was an emerging concern with increasing numbers of flocks with similar clinical, pathologic, and operational problems. This syndrome can result in up to 7% mortality starting at two weeks post-administration of SE bacterin (Gingerich, Citation2014).
Materials, methods and results
Case history
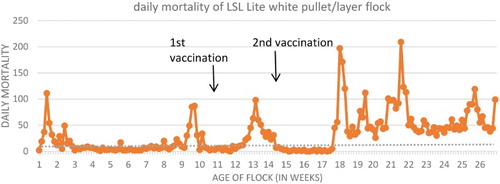
A pullet flock from Indiana experienced an 11-day history of increased mortality between 11–12 weeks of age. The flock was vaccinated at 10 weeks old using a trivalent SE/Newcastle disease/infectious bronchitis oil-emulsion-inactivated vaccine (0.5 ml per bird, subcutaneous route). Cumulative flock morbidity and mortality () from 10 to 12 weeks of age were approximately 5% and 1.5% (normal mortality for this period is <0.05%), respectively, and birds were found dead with firm, enlarged, necrohaemorrhagic livers and necrohaemorrhagic intestines. The pullets were treated with bacitracin methylene disalicylate (BMD, Zoetis®, Florham Park, NJ, USA) at 25 grams/ton in the feed for a week, and the mortality levels in the flock seemed to have improved from losing almost 300 birds per week to approximately 30 birds per week. The flock was vaccinated with an inactivated SE vaccine at 14 weeks of age. The flock experienced a steady increase in mortality since moving to the laying house at 16 weeks. A second mortality spike occurred at 18 weeks of age, with estimated cumulative morbidity of 5–10%, with >5% cumulative mortality (estimated normal mortality from 0 to 18 weeks is 2%).
Gross and histopathologic examination
Livers, intestines, and pancreas from 12-week-old pullets were fixed in 10% neutral-buffered formalin and submitted for histopathologic examination. Fixed tissue samples were then embedded in paraffin, sectioned, and stained with haematoxylin and eosin (H&E) using Armed Forces Institute of Pathology methods (Luna, Citation1968). Upon microscopic examination, there was severe necrotizing and haemorrhagic hepatitis, characterized by multifocal to coalescing areas of haemorrhage and necrosis, as well as vasculitis and vascular thrombosis. A few, small amyloid deposits with poor birefringence were seen in the areas of necrosis. All sections of the intestines had marked mucosal and intraluminal haemorrhage and villous necrosis extending to varying depths, accompanied by a mixed inflammatory cell infiltration and scattered rod-shaped bacterial colonies.
Ten 18-week-old pullets (nine affected birds and one clinically normal bird) from the same flock were submitted for necropsy. Upon post-mortem examination, the majority of the affected birds had clotted blood or serosanguinous fluid in the coelomic cavity. None of the birds were in production. The intestines had sharply demarcated areas of haemorrhage and necrosis, with petechial to ecchymotic serosal and mucosal haemorrhage and frank blood within the intestinal lumen. Affected intestines had well-demarcated necrohaemorrhagic segments with intralesional bacteria, extending from the distal duodenum to the proximal caecum, with individual variation. The liver lesions were characterized by marked hepatomegaly, pale tan to dark red mottling, marked areas of necrosis, and haemorrhage grossly. There were varying degrees of multifocal to coalescing hepatic necrosis, and livers were enlarged and friable, with rounded edges. Some of the livers had evidence of hepatic rupture with subcapsular haemorrhage and haematomas. One of the liver lobes had a sharp area of demarcation consistent with an infarct, and another liver had dull yellow to white, fibrinous material on the hepatic capsule. All affected birds had markedly enlarged spleens with pale white mottling (). A summary of all necropsy findings is provided in .
Figure 2. Gross lesions of 18-week-old pullets affected with necrotizing and haemorrhagic enteritis and hepatitis. (a) The liver was firm but friable with marked hepatomegaly and multifocal hepatic necrosis and haemorrhage. (b) Marked hepatomegaly with diffuse pallor, and a well-demarcated area of infarct. (c) Haemorrhagic and necrotic small intestines, with petechial to ecchymotic mucosal and serosal haemorrhage, and haemorrhage within the intestinal lumen. Note the sharp demarcation of the affected segment versus proximal portions which are unaffected. (d) Markedly enlarged spleen with pale mottling.
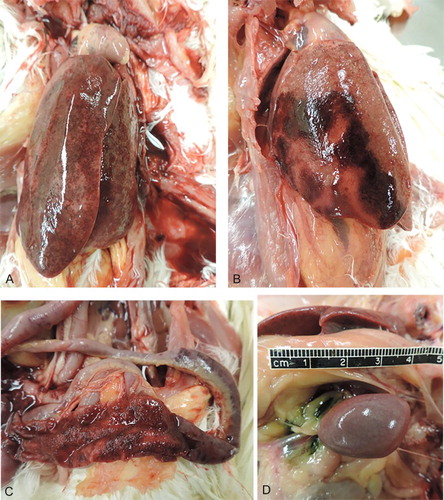
Table 1. Summary of post-mortem findings from ten 18-week-old pullets.
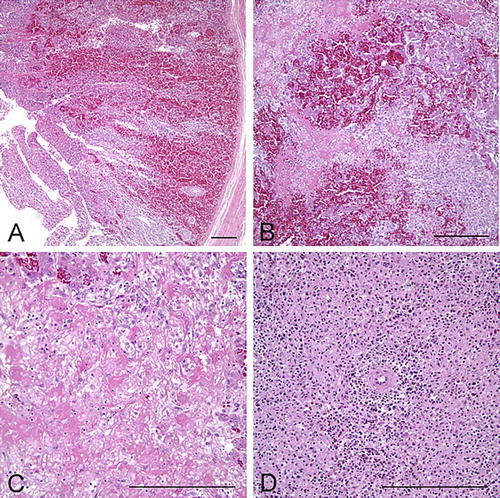
Tissues were collected from the heart, liver, spleen, lungs, kidney, trachea, oesophagus, ventriculus, proventriculus, crop, brain, air sac, skeletal muscle, sciatic nerve, small and large intestines, caecum, thyroid gland, parathyroid gland, and ovaries. Collected samples were fixed in 10% neutral buffered formalin and processed for routine H&E sections (Luna, Citation1968). Microscopically, livers had multifocal to coalescing, or focally extensive necrotizing and haemorrhagic hepatitis with random intra-sinusoidal thrombi (). There was hepatocellular necrosis, degeneration, inflammation, and haemorrhages scattered multifocally, accompanied by multiple, irregular, amorphous, eosinophilic fibrinoid necrosis and thrombi occluding the sinusoids. One section of the liver with fibrinous serositis/airsacculitis on the hepatic capsule upon gross examination contained multifocal granulomas in the hepatic capsule, characterized by necrotic, degenerate cellular debris surrounded by large infiltrates of heterophils and macrophages. The intestinal mucosa had multifocal necrosis with marked haemorrhage and numerous, densely basophilic colonies of Gram-positive, rod-shaped bacteria admixed in degenerate and necrotic enterocytes. There was severe, diffuse lymphoid necrosis and depletion in the spleen.
Figure 3. Microscopic appearance of liver, intestines, and spleen from 18-week-old pullets. (a) Severe haemorrhage and necrosis of the intestinal mucosa, extending deep into the lamina propria; bar = 100 µm, H&E. (b) The liver has massive areas of haemorrhage and hepatocellular necrosis; bar = 100 µm, H&E. (c) Higher magnification of the liver. Random areas of multifocal to coalescing fibrinoid necrosis with amorphous, eosinophilic thrombi that are occluding the sinusoids and replacing normal hepatic architecture; bar = 100 µm, H&E. (d) The spleen has marked lymphoid depletion, with diffuse lymphoid necrosis and loss of white pulp; bar = 100 µm, H&E.
Gram stains (Brown & Brenn and Goodpasture histochemical stains) were used to demonstrate bacterial colonies in the intestinal lesions (Luna, Citation1968). Colonies of bacteria in the intestinal lesions stained positive as Gram-positive rods in both stains. No bacterial colonies were detected within lesions in the liver or spleen.
Additional tests
Tissue pools of liver and spleen with observed gross lesions (in groups of 4–5), as well as small and large intestines with necrosis and haemorrhage were submitted for routine bacteriologic culture using blood and MacConkey agar (ThermoFisher Scientific®, Waltham, MA, USA) for 24 h aerobic culturing at 37°C. An anaerobic blood agar culture at 37°C for 24–48 h was also performed to rule out Clostridium sp., as well as pre-enriched culture using tetrathionate enrichment broth (ThermoFisher Scientific®) at 42°C for 24 h then plated onto XLT4 and BGN (ThermoFisher Scientific®) at 37°C for 24–48 h to look for Salmonella spp. There was no significant growth on aerobic and anaerobic cultures of the liver and spleen pool, and it was negative for Salmonella culture. The small intestine pool was positive for Escherichia coli on aerobic culture, but it was negative for Salmonella and anaerobic culture. A multiplex polymerase chain reaction (mPCR) was performed for E. coli toxin typing (Casey & Bosworth, Citation2009), which was negative for virulence factors which includes fimbria (F18, 987P, F41) and toxins {Labile toxin (LT), Stable toxin b(STb), STaP, STx2e, and intimin (Eae)}. The distal small intestine and large intestine pool was negative on aerobic and anaerobic culture, but was positive for Salmonella on culture. The Salmonella was further speciated as Salmonella Kentucky at the National Veterinary Services Laboratory (Ames, IA, USA).
Virus isolation was performed on tissue suspensions from the liver, spleen, and caecal tonsils inoculated into 11-day-old embryonating chicken eggs via the allantoic sac route. No virus was isolated. A pool of five fresh liver samples with salient gross lesions of necrohaemorrhagic hepatitis was sent to the Iowa State Veterinary Diagnostic Laboratory in Ames, Iowa, for PCR to test for avian hepatitis E virus. Liver samples were negative for avian hepatitis E virus.
Rare Eimeria oocysts were detected in a qualitative faecal flotation test. No Eimeria oocysts or schizonts were seen in the intestines microscopically.
Toxicology tests including heavy metal testing, mycotoxins, and gas chromatography/mass-spectrometry were also performed to rule out possible toxicologic aetiologies. There was no lead detected in the blood samples, and the liver lead was 0.02 (normal reference range for chicken liver lead levels: 0.1–0.5 ppm). Iron, zinc, and copper levels in the liver were within reference range for chickens.
Discussion
Based on gross and histopathologic findings, as well as the results from ancillary tests, a definitive aetiologic diagnosis could not be determined and established in the present case. However, differential diagnoses included clostridial enteritis/hepatitis, SE bacterin-induced hepatitis, toxicosis (heavy metals and mycotoxicosis), and hepatitis-splenomegaly syndrome (Rampin et al., Citation1989; Ritchie & Riddell, Citation1991; Read et al., Citation1994; Tablante et al., Citation1994; Porter et al., Citation2006).
Although clostridial enteritis and hepatitis secondary to toxaemia were highly suspected based on the gross and histopathologic findings (Swayne et al., 2013), clostridial organisms were not cultured from the liver and intestine with lesions. However, necrotizing and haemorrhagic enteritis and hepatitis caused by clostridial toxin and toxaemia cannot be completely ruled out. Hepatitis-splenomegaly syndrome caused by avian hepatitis E virus infection was suspected (Rampin et al., Citation1989; Ritchie & Riddell, Citation1991; Read et al., Citation1994; Tablante et al., Citation1994; Porter et al., Citation2006; Meng & Shivaprasad, Citation2013) because there were similar gross and histopathologic presentations of hepatitis, splenomegaly, and amyloid deposition in affected organs (Billam et al., Citation2005; Gingerich & Porter, Citation2005; Morrow et al., Citation2008). However, the liver specimen was negative for avian hepatitis E virus PCR. In addition, mucosal and intraluminal haemorrhages observed grossly cannot be explained by avian hepatitis E infection. Toxicoses, including mycotoxicosis and heavy metal toxicosis, were considered due to massive hepatic necrosis and haemorrhage observed grossly and histologically. Although a mycotoxin screen was not performed, gross and histopathologic lesions did not represent typical lesions of mycotoxicosis. As far as heavy metal toxicosis is concerned, iron, copper, and zinc levels in the liver were within normal reference ranges for chickens.
On considering histopathologic findings, Salmonella Kentucky isolated from the intestines and its role in contributing to the pathogenesis of the liver and intestinal lesions was most likely equivocal.
Layer hepatopathy is an ongoing problem that is occasionally reported in the layer industry. Recently, cases have been documented in broiler breeders as well. Associated mortality usually ranges from 0.2% to 5%, with a mode of 1–2%. Typical clinical duration is 7–10 days, which often develops 1–2 weeks post-vaccination with a second set of Salmonella vaccines. The incidence is very erratic, although the prevalence seems to be influenced by layer strain genetics, number of vaccinations, and injection dosage (Personal communication, Elanco Layer hepatopathy meeting, 2015).
Based on an 1993 investigation of layer hepatitis syndrome by DEKALB Poultry Research Inc. (Dekalb, IL, USA), there seemed to be a geographic predilection of the disease, with most cases being reported in the midwestern states including Ohio, Michigan, Wisconsin, Indiana, and also in Kansas, California, Colorado, British Columbia, Alberta, Ontario, Quebec, and Jalisco, Mexico, with sparing of the eastern, western, and southern states of America (E. Gingerich, Dekalb report, 1993). There have also been reports on certain layer strains being more or less predisposed to the syndrome (Gingerich, Citation2014; E. Gingerich, personal communication, 2015).
Other differentials considered include Campylobacter jejuni infection, as mentioned in some reports of miliary hepatitis or “spotty liver disease” (Forsyth et al., Citation2005). The 1993 report by DEKALB Poultry Research Incorporation mentioned that approximately 20% of the cases cultured C. jejuni from the bile, but experimental infections of chickens with C. jejuni (Forsyth et al., Citation2005) failed to reproduce liver lesions consistent with miliary hepatitis. Nevertheless, C. jejuni was not isolated from the present case, and the lesions are not similar to those reported as miliary hepatitis caused by C. jejuni.
Vaccine-induced or vaccine-associated hepatitis in young layer flocks (Porter et al., Citation2006; Gingerich, 2013; Murakami et al., Citation2013) has been described in the literature, with speculation of an immune-mediated pathogenesis triggered by bacterin administration contributing to chronic antigenic stimulation and inflammation. A study of the immune response following vaccination of layers against SE using commercial bacterins (Layermune and Maine Biological Laboratories SE 4C) revealed significantly high levels of IgA in the intestine and oviduct, which may possibly lead to a hypersensitive response by deposition of immune complexes (Tran et al., Citation2010). Outbreaks of systemic amyloidosis in layer flocks in Japan are speculated to be induced by repetitive inflammatory stimulation from multiple oil-emulsified bacterin vaccinations, although the pathogenesis is still unclear as amyloid deposits were also found in apparently healthy birds (Murakami et al., Citation2013). A number of cases similar to the present case have been reported from the Wisconsin Veterinary Diagnostic Laboratory (2004), University of Pennsylvania (2005), and the California Animal Health and Food Safety Laboratory (2013), with similar clinical and post-mortem findings, although to the author’s knowledge, this is the first case where the lesions are found after subcutaneous vaccine injections. However, a confirmed aetiology or aetiopathogenesis was not concluded in these cases. With the new discovery of a viral agent responsible for equine serum hepatitis (Chandrani et al., Citation2013), novel viruses, such as a picornavirus which was found upon electron microscopic examination of liver lesions in a 1993 case in southern California, could be a possible cause of massive hepatic necrosis.
The emergence of pullet hepatopathy syndrome has been an ongoing concern in the poultry industry. Although there have been several theories on the aetiology of necrotizing and haemorrhagic enteritis and hepatitis, much of the aetiopathogenesis is still unclear.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Billam, P., Huang, F.F., Sun, Z.F., Pierson, F.W., Duncan, R.B., Elvinger, F., Guenette, D.K., Toth, T.E. & Meng, X.J. (2005). Systematic pathogenesis and replication of avian hepatitis E virus in specific-pathogen-free adult chickens. Journal of Virology, 79, 3429–3437. doi: 10.1128/JVI.79.6.3429-3437.2005
- Casey, T.A. & Bosworth, B.T. (2009). Design and evaluation of a multiplex polymerase chain reaction assay for the simultaneous identification of genes for nine different virulence factors associated with Escherichia coli that cause diarrhea and edema disease in swine. Journal of Veterinary Diagnostic Investigation, 21, 25–30. doi: 10.1177/104063870902100104
- Chandrani, S., Skewes-Cox, P., Zhong, W., Ganem, D.E., Divers, T.J., Van Blaricum, A.J., Tennant, B.C. & Tennant, A.L. (2013). Identification of a previously undescribed divergent virus from the Flaviviridae family in an outbreak of equine serum hepatitis. Proceedings of the National Academy of Sciences of the United States of America, 110, E1407–E1415. doi: 10.1073/pnas.1219217110
- Forsyth, W.M., Hodgeman, R. & Oyay, B.S. (2005). Investigation of the cause of miliary hepatitis in laying chickens – a model of the disease. Rural Industries Research and Development Corporation. RIRDC Publication No 05, project no DAV-226J. 2005.
- Gingerich, E.N. (2014). 2014 USAHA US Layer health report [PowerPoint slides]. Retrieved from http://www.usaha.org/Portals/6/Committees/poultry-avian/presentations/2014-Gingerich-Layer.pdf.
- Gingerich, E.N. & Porter, R. (2005). Pullet hepatopathy syndrome. Presentation from the American Veterinary Medical Association meeting.
- Luna, L.G. (1968). Manual of Histological Staining Methods of the Armed Forces Institute of Pathology, 3rd edn (p. 206). New York: McGraw Hill.
- Meng, X.J. & Shivaprasad, H.L. (2013). Avian hepatitis E virus infections. In D.E. Swayne, J.R. Glisson, L.R. McDougald, L.K. Nolan, D.L. Suarez & V. Nair (Eds.), Diseases of Poultry 13th edn (pp. 494–500). Hoboken, NJ: Wiley-Blackwell.
- Morrow, C.J., Samu, G., Matrai, E., Klausz, A., Wood, A.M., Richter, S., Richter, S., Jaskulska, B. & Hess, M. (2008). Avian hepatitis E virus infection and possible associated clinical disease in broiler breeder flocks in Hungary. Avian Pathology, 37, 527–535. doi: 10.1080/03079450802356946
- Murakami, T., Inoshima, Y., Sakamoto, E., Fukushi, H., Sakai, H., Yanai, T. & Ishiguro, N. (2013). AA amyloidosis in vaccinated growing chickens. Journal of Comparative Pathology, 149, 291–297. doi: 10.1016/j.jcpa.2013.02.002
- Porter, R., Gingerich, E., Toohey-Kurth, K., Radi, C. & Zoromski, D. (2006). The effect of maternal dietary vitamin D3 supplementation on performance and tibial dyschondroplasia of broiler chicks. Poultry Science, 85, 39–47. Abstract retrieved from Abstracts in Poultry Science August 2006 Supplement, EBSCO Host database (Accession No. 22186863). doi: 10.1093/ps/85.1.39
- Rampin, T., Sironi, G. & Gallazi, D. (1989). Episodes of amyloidosis in young hens after repeated use of antibacterial and emulsion vaccines. Deutsche Tierarzliche Wochenschrift, 96, 168–172.
- Read, D.H., Daft, B.M., Barton, J.T., Woolcock, P.R., Cutler, G. & Galey, F. (1994). Necrotic-hemorrhagic hepatitis-splenomegaly syndrome: an unsolved sudden death syndrome in layer leghorn chickens. Proceedings of the 43rd Annual Meeting of the Western Poultry Disease Conference (pp. 8–9). Sacramento, CA, USA.
- Ritchie, S.J. & Riddell, C. (1991). “Hepatitis-splenomegaly” syndrome in commercial egg layer hens. Canadian Veterinary Journal, 32, 500–501.
- Tablante, N.L., Valliancourt, J.-P. & Julian, R.J. (1994). Necrotic, haemorrhagic, hepatomegalic hepatitis associated with vasculitis and amyloidosis in commercial laying hens. Avian Pathology, 23, 725–732. doi: 10.1080/03079459408419041
- Tran, T.Q.L., Quessy, S., Letellier, A., Desrosiers, A. & Boulianne, M. (2010). Immune response following vaccination against Salmonella Enteritidis using 2 commercial bacterins in laying hens. Canadian Journal of Veterinary Research, 74, 185–192.