ABSTRACT
Backyard poultry are regaining popularity in Europe and increased interest in the health and management of non-commercial farms has resulted. Furthermore, commercial poultry farm owners have become concerned about the risk represented by contagious avian diseases that nearby backyard poultry could transmit. Fifty-one voluntary backyard chicken farms were visited between October 2012 and January 2013. Blood samples and individual cloacal swabs were collected from 457 chickens. In 44 farms (86%), one or more of the tested chickens had antibodies against avian encephalomyelitis and chicken infectious anaemia viruses, 24 farms (47%) had chickens seropositive for infectious bronchitis virus, 10 farms (20%) had chickens seropositive for infectious bursal disease virus, six farms (12%) had chickens seropositive for infectious laryngotracheitis virus and two farms (5.4%) had chickens seropositive for avian influenza virus. No farms had chickens seropositive for Newcastle disease virus. Of the 51 farms, five (10%) had chickens positive for coronavirus reverse transcription polymerase chain reaction. A phylogenetic analysis showed that all backyard chicken coronaviruses collected were QX type infectious bronchitis viruses. All chickens tested for avian influenza and Newcastle disease viruses using real time reverse transcription polymerase chain reaction were negative. To our knowledge, there is no evidence to date to suggest that these diseases would have been transmitted between commercial and non-commercial flocks.
Introduction
Backyard poultry are regaining popularity in Europe and increased interest in the health and management of non-commercial farms has resulted (Wunderwald & Hoop, Citation2002; De Wit et al., Citation2004; Zheng et al., Citation2010; Burns et al., Citation2011; Karabozhilova et al., Citation2012; Smith et al., Citation2012; Haesendonck et al., Citation2014; Pohjola et al., Citation2015; Pohjola et al., Citation2016). Commercial poultry farm owners are often worried about the risk represented by contagious diseases that nearby backyard poultry could transmit. Backyard chickens are more susceptible to contagious diseases as they usually have access to outdoors and live in close contact with wild birds (Karabozhilova et al., Citation2012; Smith et al., Citation2012; Pohjola et al., Citation2015). On the other hand, the population densities among backyard flocks are commonly low, which decreases the risk of transmission. On occasion, novel infectious diseases are known to emerge first in backyard flocks but the epidemiological studies have indicated their role to be only marginal (Capua et al., Citation2002; Bavinck et al., Citation2009).
Infectious bursal disease (IBD), chicken infectious anaemia (CIA) and avian encephalomyelitis (AE) are contagious viral diseases that can cause clinical disease in young chickens. CIA and AE are both vertically and horizontally transmitted, but IBD is only reported to transmit horizontally (Saif, Citation2008). In addition, IBD and CIA can cause variable degrees of immunosuppression, rendering the chickens susceptible to secondary infections. Commercial poultry are routinely vaccinated against these diseases but vaccinations are rare in Finnish backyard flocks (Pohjola et al., Citation2015).
Infectious bronchitis (IB), infectious laryngotracheitis (ILT), Newcastle disease (ND) and avian influenza (AI) are viral diseases typically characterized by respiratory signs, depression and reduced feed intake, and also, ultimately, mortality. In egg laying birds, there is often a decline in egg production. The viruses are transmitted horizontally and are very easily spread (Saif, Citation2008). Vaccination of commercial poultry against ND and AI viruses is not allowed in Finland, and the emergence of any of these diseases in hobby flocks could represent a risk to nearby commercial flocks. Naturally, commercial farm owners’ own activities and biosecurity measures play a significant role in keeping these diseases out of the chicken house.
We performed a serological study among Finnish backyard chickens for a selected group of viral pathogens to obtain more information on their spread. In addition, the swab samples were subjected to AIV (avian influenza virus), NDV (Newcastle disease virus), and coronavirus reverse transcription polymerase chain reactions (RT-PCRs) and sequencing. A subsequent phylogenetic analysis was carried out to establish the infection routes.
Materials and methods
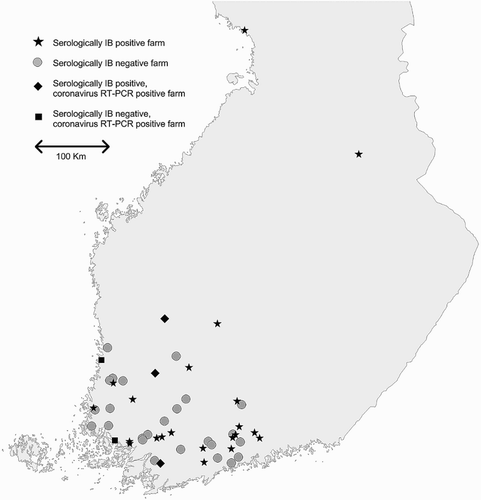
Fifty-one voluntary multi-age backyard chicken farms were visited between October 2012 and January 2013 (). The farms were located mainly (78%) in the southwest of Finland, a region of high poultry density. Backyard poultry were defined as flocks where the birds were kept for egg production or other products consumed mainly by the owners, and for which the overall number of birds on the farm was fewer than 500. Most (94%) of the sampled farms had fewer than 50 birds. These farms were previously studied using a questionnaire (Pohjola et al., Citation2015). Blood samples and individual cloacal swabs were collected from 457 chickens. In small farms (<20 chickens), 10 of the chickens were sampled (or less if the number of chickens was less). In large farms (>20 chickens), 20 chickens were tested. The serum samples were tested for AE, CIA, IBD, IB and ILT antibodies, and swabs for coronaviruses by RT-PCR. In addition, 37 farm owners (representing 298 chickens) gave permission to test for ND and AI antibodies and viruses. Permission was asked because in Finland, AI and ND are notifiable diseases and a positive finding of highly pathogenic viruses would result in compulsory eradication of the affected flock. The total number of the chickens in the 51 farms studied was 1121, indicating that 41% of the chickens were sampled.
The blood samples were sent to the Finnish Food Safety Authority Evira veterinary virology laboratory, and the serum samples were stored at −20°C until further analysis. The samples were tested for IBV, ILTV, CIAV, IBDV and AEV antibodies with enzyme-linked immunosorbent assays (ELISA): Infectious Bronchitis Virus Antibody Test Kit, Chicken Anaemia Virus Antibody Test Kit, Infectious Bursal Disease Virus Antibody Test Kit, Avian Encephalomyelitis Virus Antibody Test Kit by IDEXX (IDEXX Corporation, Westbrook, ME, USA) and Fowl Laryngotracheitis Virus Antibody Test Kit by Synbiotics (Synbiotics Corporation, San Diego, CA, USA). The kits were used according to the manufacturers’ instructions. ND virus antibodies were tested using a haemagglutination inhibition test according to the Council Directive 92/66/EEC (Community Measures for the Control of Newcastle Disease, Citation1992). AI virus antibodies were tested first with ELISA (ID Screen Influenza A Antibody Competition Multi-species ID Vet, Grabels, France) and positive samples were then tested for H5 and H7 antibodies (haemagglutination inhibition test) to exclude infection with H5 and H7 AI.
For virus detection, the RNA was extracted from sample suspension supernatants by using the QIAamp Viral Mini Kit (QIAGEN, Hilden, Germany) according to the instructions of the manufacturer. The OneStep RT-PCR Kit (QIAGEN) was used in all RT-PCR reactions. The ND L-gene real time RT-PCR was carried out according to the protocol published by Fuller et al. (Citation2010) and AI m-gene real time RT-PCR according to the EU Diagnostic Manual for Avian Influenza (Commission Decision Citation2006/Citation437/EC, Citation2006). The primary coronavirus detection was carried out with RdRp gene primers published by Muradratsoli et al. (Citation2010). For phylogenetic analysis the partial spike gene was amplified with primers qx3 (5′tgcactgttggtgttattaagg3′) and qx4 (5′tcgcgcaaaactacatcttg3′). The following thermal profile was used in both PCRs: a single cycle of reverse transcription for 30 min at 50°C, 15 min at 95°C for reverse transcriptase inactivation and DNA polymerase activation followed by 40 amplification cycles of 30 s at 95°C, 30 s at 50°C and 30 s at 72°C. After agarose gel electrophoresis, the positive bands were cut from the gel and DNAs were extracted with a Qiaquick Gel Extraction Kit (QIAGEN).
The sequencing was done with the primers used in the spike gene RT-PCR, BigDye Terminator Cycle Sequencing Kit v3.1 and ABI3130 automatic sequencer (Applied Biosystems, Foster City, CA, USA). The sequences were edited and the nucleotide identities calculated using the EMBOSS package (Rice et al., Citation2000). The sequences chosen for the phylogenetic analysis were aligned with the ClustalW program (Thompson et al., Citation2002) and the neighbouring joining phylogenetic tree was created with the MEGA 6 program (Tamura et al., Citation2013). The data were bootstrapped 1000 times and only values higher than 85% were shown.
Results
Of the sampled farms, in 44 (86%), one or more of the tested chickens had antibodies against AE and CIA viruses, 24 (47%) were seropositive for IB viruses, 10 (20%) were seropositive for IBD viruses, six (12%) were seropositive for ILT viruses and two (5.4%) were seropositive for AI viruses (). All the farms were negative for ND viruses. Of the 457 chickens studied, 53% were seropositive for CIA viruses, 34% were seropositive for AE viruses, 21% were seropositive for IB viruses, 4% were seropositive for ILT viruses and 3% were seropositive for IBD viruses; of the 298 chickens studied, 0.7% were seropositive for AI viruses. AI seropositive samples were tested for H5 and H7 antibodies and found to be negative. The seroprevalence within each flock is shown in .
Table 1. Occurrence of antibodies against avian viral pathogens in backyard chickens in Finland.
Table 2. Occurrence of antibodies against avian viral pathogens within studied backyard flocks.
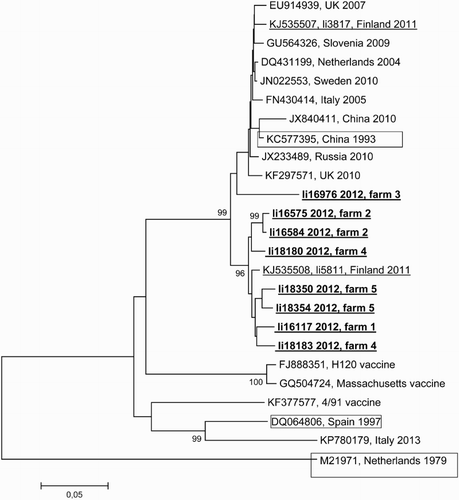
Five of the 51 farms (10%) had chickens that tested positive for coronaviruses by RT-PCR. In total, coronavirus was detected in nine birds with the primary RT-PCR. The phylogenetic analysis based on 436 nucleotides of the spike gene () shows that all coronaviruses from backyard chickens collected in this study were QX type IB viruses grouping together with GI-19 lineage (Valastro et al., Citation2016). The Finnish backyard chicken 2012 viruses shared 86% nucleotide identity, at a minimum. One of the farm number three strains could not be completely sequenced for phylogenetic analysis. The Finnish strains li5811/2011 (KJ535508) and li3817/2011 (KJ535507) grouped tightly with the Finnish backyard poultry 2012 strains, as did for example the Swedish strain isolated in 2010 (Abro et al., Citation2012) and the Italian isolate from 2005 (Ducatez et al., Citation2009). All chickens tested for AI and ND viruses using real time RT-PCR were negative.
Figure 2. Phylogenetic analysis of Finnish backyard poultry coronavirus strains based on 436 nucleotides of the spike gene. Only bootstrap values higher than 85% are shown. The Finnish 2012 backyard poultry virus strains are in bold and underlined and the Finnish 2011 strains are underlined. The prototype strains of lineages GI-19, GI-21 and GII-1 according to Valastro et al. (Citation2016) are boxed. The GenBank accession numbers for Finnish backyard poultry coronavirus 2012 strains are KX398036 – KX398043.
Discussion
AE and CIA viruses are ubiquitous in poultry-producing countries. In this study, the majority of farms were seropositive for CIA and AE viruses, which is consistent with previous studies (McBride et al., Citation1991; Wunderwald & Hoop, Citation2002; Hernandez-Divers et al., Citation2006). Surprisingly, the occurrence of antibodies against IBD viruses in the backyard chickens was very low; in total, only 13 of the 457 sampled chickens had IBD virus antibodies. Of the 10 (20%) positive farms, only one farm had several (four) seropositive chickens with high antibody titres. The remaining farms had only one seropositive chicken each; for three of those, the antibody titre was low (). Although the positive samples were retested, this could indicate false positive results as ELISA tests are known to give false positive results due to non-specific reactions.
It is also possible that at least in some of the cases, the IBD virus seropositive findings are from birds that are originally from other backyard poultry farms where they had got the infection. From the questionnaire we know that these birds are actively exchanged between flocks (Pohjola et al., Citation2015). In Switzerland, 65% of fancy breed chickens and 93% of the flocks were seropositive for IBD virus (Wunderwald & Hoop, Citation2002). In Ecuador, 100% of tested backyard chickens were seropositive for IBD virus and in California, 23% of farms had seropositive chickens (McBride et al., Citation1991; Hernandez-Divers et al., Citation2006). At the time of sampling in Finland, no clinical cases were reported in commercial poultry. In the twentieth century, clinical cases of IBD have not been recorded in Finnish backyard chickens submitted for postmortem examination to Evira (Pohjola et al., Citation2015). This indicates that the environmental burden of the disease was low in Finland at the time of this study. None of the farmers had vaccinated their chickens; so the positive results were due to a natural infection (Pohjola et al., Citation2015).
Respiratory infections were rare compared with reports from other studies: 21% of the chickens and 47% of the flocks were seropositive for IBV. In Belgium, 76% of the chickens and 91% of the flocks were positive for IBV (Haesendonck et al., Citation2014). In the Netherlands, 95% and in Switzerland, 93% of fancy breed flocks had antibodies against IBV (Wunderwald & Hoop, Citation2002; De Wit et al., Citation2004). In Ecuador, 85% and in California, 22% of tested backyard chickens were seropositive for IBV (McBride et al., Citation1991; Hernandez-Diver et al., Citation2006). Finland was free of clinical IBV cases for almost three decades and only since 2011 have outbreaks involving genotypes QX, D274-like and 4/91-like occurred (Pohjola et al., Citation2014). Interestingly, two of the coronavirus-positive farms (2 and 5) were serologically negative for IBV and the farmers reported no signs of recent infection (). As the coronaviruses isolated from these farms were QX-like, it would be likely that they would cause clear signs for the susceptible birds. It is possible though unlikely that the birds had just acquired the infection and they did not yet have any signs and antibody levels were yet to rise or that the ELISA test that we used could not detect the antibodies against the virus.
The occurrence of ILTV antibodies was low (4%). Only one ILTV-positive farm, with high antibody levels, was detected and the owner also reported a recent incidence of severe respiratory signs and mortality. In each of five other farms there was only a single seropositive sample, which could indicate a false positive result. The use of live-attenuated vaccines has been associated with adverse effects such as spreading of vaccine to non-vaccinated chickens, insufficient attenuation, development of latent carriers and even gaining virulence and resulting in outbreaks of vaccinal laryngotracheitis (Guy & Garcia, Citation2008). In Finland, ILT vaccines are not used and this may have an impact to the low numbers of infected farms. In previous studies, in Belgium, 30% of the birds and 64% of the flocks were seropositive for ILTV. In the Dutch and Swiss studies, 65% and 64% of the fancy breed flocks, respectively, were seropositive for ILTV, and 28% of the Swiss birds were seropositive (Wunderwald & Hoop, Citation2002; De Wit et al., Citation2004; Haesendonck et al., Citation2014). A study of Maryland backyard flocks reported that 77% of the studied flocks were seropositive for ILTV (Madsen et al., Citation2013). Knowing the high incidence of trading among chicken hobbyists in Finland, it is clear that respiratory disease viruses continue their rapid spread among these farms (Pohjola et al., Citation2015).
The samples were taken during winter time (October to January) when most of the chickens are housed and the contacts with wild birds are rare. It is possible, especially among older birds, that antibody levels of previous infection have already lowered under the detection level. But it is probably safe to assume that infections that occurred the previous summer would still be detectable. Antibodies after AI virus infection were detectable by ELISA in chickens at least between days 11 and 157 (Meulemans et al., Citation1987).
Though the occurrence of coronaviruses in this study was rare, the QX-like IB viruses seem to be circulating in Finnish backyard poultry farms. The first detections were made in 2011 when two different QX-subtypes were found, one in a commercial layer flock in southeast Finland (li3817/2011, KJ535507) and the second in a backyard poultry flock in western Finland (li5811/11, KJ535508) (Pohjola et al., Citation2014). The backyard poultry IB viruses seem to have been spread between farms. IBV is currently relatively rare event in commercial poultry farms even though live vaccinations are not used and the QX type has not been found from commercial farms since the initial outbreak in a layer farm. The serotype currently found from commercial farms is a D274-like IB virus (Pohjola et al., Citation2014).
This study shows that backyard poultry flocks in Finland frequently carry AE and CIA virus antibodies but only sporadically IBD virus antibodies. IB and ILT virus antibodies are rare occurrences compared with studies from other countries. IB-causing coronaviruses are rare findings but QX type seems to be circulating among them. As commercial poultry flocks in Finland are not vaccinated against IB and ILT viruses with live vaccines, backyard chickens can represent a threat to commercial chickens but as the disease seroprevalence is low, the risk is quite low. The risk can be diminished by good commercial farm management and employment of strict biosecurity measures. To date, there is no evidence that these diseases have been transmitted between commercial and non-commercial flocks.
Acknowledgements
We wish to thank Merja Hautala, Marika Karlsson, Essi Kuitunen and other personnel at Evira for excellent technical assistance. The authors also thank Pia Vilen for providing the map.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abro, S.H., Renstrom, L.H., Ullman, K., Isaksson, M., Zohari, S., Jansson, D.S., Belak, S. & Baule, C. (2012). Emergence of novel strains of avian infectious bronchitis virus in Sweden. Veterinary Microbiology, 155, 237–246. doi: 10.1016/j.vetmic.2011.09.022
- Bavinck, V.A., Bouma, A., van Boven, M., Bos, M.E., Stassen, E. & Stegeman, J.A. (2009). The role of backyard poultry flocks in the epidemic of highly pathogenic avian influenza virus (H7N7) in the Netherlands in 2003. Preventive Veterinary Medicine, 88, 247–254. doi: 10.1016/j.prevetmed.2008.10.007
- Burns, T.E., Kelton, D., Ribble, C. & Stephen, C. (2011). Preliminary investigation of bird and human movements and disease-management practices in noncommercial poultry flocks in southwestern British Columbia. Avian Diseases, 55, 350–357. doi: 10.1637/9646-010411-Reg.1
- Capua, I., Dalla, P.M., Mutinelli, F., Marangon, S. & Terregino, C. (2002). Newcastle disease outbreaks in Italy during 2000. The Veterinary Record, 150, 565–568. doi: 10.1136/vr.150.18.565
- Commission Decision Approving a Diagnostic Manual for Avian Influenza. (2006). Commission Decision 2006/437/EC. http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32006D0437
- Community Measures for the Control of Newcastle Disease. (1992). Council Directive 92/66/EEC. http://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1449572784086&uri=CELEX:01992L0066-20080903
- De Wit, J. J., van Eck, J. H. H., Crooijmans, R. P. M. A. & Pijpers, A. (2004). A serological survey for pathogens in old fancy chicken breeds in central and eastern part of the Netherlands. Tijdschr Diergeneeskd, 129, 324–327.
- Ducatez, M.F., Martin, A.M., Owoade, A.A., Olatoye, I.O., Alkali, B.R., Maikano, I., Snoeck, C.J., Sausy, A., Cordioli, P. & Muller, C.P. (2009). Characterization of a new genotype and serotype of infectious bronchitis virus in Western Africa. Journal of General Virology, 90, 2679–2685. doi: 10.1099/vir.0.012476-0
- Fuller, C.M., Brodd, L., Irvine, R.M., Alexander, D.J. & Aldous, E.W. (2010). Development of an L gene real-time reverse-transcription PCR assay for the detection of avian paramyxovirus type 1 RNA in clinical samples. Archives of Virology, 155, 817–823. doi: 10.1007/s00705-010-0632-1
- Guy, J.S. & Garcia, M. (2008). Laryngotracheitis. In Y.M. Saif, A.M. Fadly, J.R. Glisson, L.R. McDougald, L.K. Nolan, & D.E. Swayne, Diseases of Poultry, 12th edn (pp. 137–152). Arres, IA: Blackwell Publishing.
- Haesendonck, R., Verlinden, M., Devos, G., Michiels, T., Butaye, P., Haesebrouck, F., Pasmans, F. & Martel, A. (2014). High seroprevalence of respiratory pathogens in hobby poultry. Avian Diseases, 58, 623–627. doi: 10.1637/10870-052314-ResNote.1
- Hernandez-Divers, S.M., Villegas, P., Prieto, F., Unda, J.C., Stedman, N., Ritchie, B., Carroll, R. & Hernandez-Divers, S.J. (2006). A survey of selected avian pathogens of backyard poultry in northwestern Ecuador. Journal of Avian Medicine and Surgery, 20, 147–158. doi: 10.1647/2005-015R.1
- Karabozhilova, I., Wieland, B., Alonso, S., Salonen, L. & Häsler, B. (2012). Backyard chicken keeping in the Greater London Urban Area: welfare status, biosecurity and disease control issues. British Poultry Science, 53, 421–430. doi: 10.1080/00071668.2012.707309
- Madsen, J.M., Zimmermann, N.G., Timmons, J. & Tablante, N.L. (2013). Prevalence and differentiation of diseases in Maryland backyard flocks. Avian Diseases, 57, 587–594. doi: 10.1637/10423-101612-Reg.1
- McBride, M.D., Hird, D.W., Carpenter, T.E., Snipes, K.P., Danaye-Elmi, C. & Utterbac, W.W. (1991). Health survey of backyard poultry and other avian species located within one mile of commercial California meat-turkey flocks. Avian Diseases, 35, 403–407. doi: 10.2307/1591198
- Meulemans, G., Carlier, M.C., Gonze, M. & Petit, P. (1987). Comparison of hemagglutination-inhibition, agar gel precipitin, and enzyme-linked immunosorbent assay for measuring antibodies against influenza viruses in chickens. Avian Diseases, 31, 560–563. doi: 10.2307/1590740
- Muradrasoli, S., Bálint, A., Wahlgre, J., Waldenström, J., Belák, S., Blomberg, J. & Olsen, B. (2010). Prevalence and phylogeny of coronaviruses in wild birds from the Bering Strait area (Beringia). PLoS One, 5, e13640. doi: 10.1371/journal.pone.0013640
- Pohjola, L.K., Ek-Kommonen, S.C., Tammiranta, N.E., Kaukonen, E.S., Rossow, L.M. & Huovilainen, T.A. (2014). Emergence of avian infectious bronchitis in a non-vaccinating country. Avian Pathology, 43, 244–248. doi: 10.1080/03079457.2014.913770
- Pohjola, L., Nykäsenoja, S., Kivistö, R., Soveri, T., Huovilainen, A., Hänninen, M.L. & Fredriksson-Ahomaa, M. (2016). Zoonotic public health hazards in backyard chickens. Zoonoses and Public Health, 63, 420–430. doi: 10.1111/zph.12247
- Pohjola, L., Rossow, L., Huovilainen, A., Soveri, T., Hänninen, M.L. & Fredriksson-Ahomaa, M. (2015). Questionnaire study and postmortem findings in backyard chicken flocks in Finland. Acta Veterinaria Scandinavica, 57, 3. doi:10.1186/s13028-015-0095-1
- Rice, P., Longden, I. & Bleasby, A. (2000). EMBOSS: the European Molecular Biology Open Software Suite. Trends in Genetics, 16, 276–277. doi: 10.1016/S0168-9525(00)02024-2
- Saif, Y.M. (2008). Diseases of Poultry. 12th ed. Ames, IA: Blackwell.
- Smith, E.I., Reif, J.S., Hill, A.E., Slota, K.E., Miller, R.S., Bjork, K.E. & Pabilonia, K.L. (2012). Epidemiologic characterization of Colorado backyard bird flocks. Avian Diseases, 56, 263–271. doi: 10.1637/9865-072811-Reg.1
- Tamura, K., Stecher, G., Peterson, D., Filipski, A. & Kumar, A. (2013). MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution, 30, 2725–2729. doi: 10.1093/molbev/mst197
- Thompson, J.D., Gibson, T.J. & Higgins, D.G. (2002). Multiple Sequence Alignment Using ClustalW and ClustalX. In A.D. Baxevanis (Ed.). Current Protocols in Bioinformatics (Chapter 2, Unit 2.3). Hoboken, NJ: John Wiley.
- Valastro, V., Holmes, E.C., Britton, P., Fusaro, A., Jackwood, M.W., Cattoli, G. & Monne, I. (2016). S1 gene-based phylogeny of infectious bronchitis virus: an attempt to harmonize virus classification. Infections, Genetics and Evolution, 39, 349–364. doi: 10.1016/j.meegid.2016.02.015
- Wunderwald, C., & Hoop, R.K. (2002). Serological monitoring of 40 Swiss fancy breed poultry flocks. Avian Pathology, 31, 157–162. doi: 10.1080/03079450120118649
- Zheng, T., Adlam, B., Rawdon, T.G., Stanislawek, W.L., Cork, S.C., Hope, V., Buddle, B.M., Grimwood, K., Baker, M.G., O’Keefe, J.S. & Huang, Q.S. (2010). A cross-sectional survey of influenza A infection, and management practices in small rural backyard poultry flocks in two regions of New Zealand. New Zealand Veterinary Journal, 58, 74–80. doi: 10.1080/00480169.2010.65086