ABSTRACT
Pulmonary hypertension (PH) is a major disease in the broiler breeding industry. During PH, the pulmonary artery undergoes remodelling, which is caused by pulmonary vascular smooth muscle cell proliferation. CyclinD1 regulates cell proliferation. This study investigated the role of cyclinD1 in the development of PH in broilers, and which bioactivators and signalling pathway are involved in the pathological process. The PH group contained 3–4-week-old broilers with clinical PH, and the healthy group broilers from the same flock without PH. Histopathology indicated pulmonary arterial walls were thicker in the PH group compared with the healthy group. Target gene expressions of macrophage migration inhibitory factor (MIF), extracellular signal-regulated kinase (ERK), and cyclinD1 detected by quantitative real-time PCR were upregulated in the PH group compared with the healthy group. Immunohistochemistry showed MIF, phosphorylated ERK (p-ERK) and cyclinD1 were present on pulmonary vascular walls; MIF was present in the cytoplasm of arterial endothelial cells and smooth muscle cells; p-ERK and cyclinD1 were present in smooth muscle cell cytoplasm. Western blotting demonstrated that MIF, p-ERKand cyclinD1 levels were significantly higher (P < 0.01) in the PH group compared with the healthy group. In summary, increased MIF in PH broiler pulmonary arteries upregulated cyclinD1 via the ERK signalling pathway to induce pulmonary vascular smooth muscle cell proliferation, causing pulmonary artery remodelling and hypertension.
Introduction
Pulmonary hypertension (PH) in broilers, which is termed as the ascites syndrome, is characterized by pulmonary artery remodelling, right heart failure and increased yellow fluid accumulation in the abdomen. PH in broilers is a major disease that influences the broiler breeding industry, and is receiving increased attention from scholars and experts. Many factors are involved in PH pathogenesis including heredity, environmental hypoxia, nutritional imbalance, stress, improper feeding and management. Among those factors, hypoxia-induced pulmonary arterial remodelling plays a critical role in the development of PH in broilers. Pulmonary arterial remodelling is characterized by the proliferation of endothelial cells, the migration and proliferation of smooth muscle cells, the growth of fibroblasts and macrophage accumulation (Humbert et al., Citation2004; Zhang et al., Citation2012). A previous study indicated that the proliferation of smooth muscle cells was the most critical pathway in the development of pulmonary arterial remodelling (Shimoda & Laurie, Citation2013; Wideman et al., Citation2013).
Many previous studies of pulmonary vascular smooth muscle cells (PVSMCs) reported that their proliferation was stimulated by cyclinD1, which promoted the transition of dividing cells from the G1 phase to the S phase. In addition, cyclinD1 expression during the G2 phase accelerated the G2 phase, thus causing the cell cycle to become shortened (Hitomi & Stacey, Citation1999, Citation2001). However, whether cyclinD1 is involved in the development of PH in broilers has not been reported previously. Therefore, we focussed on cyclinD1, a key factor in the proliferation of PVSMCs, to investigate the pathogenesis of PH in broilers. CyclinD1 is regulated by several upstream signalling pathways, of which the mitogen-activated protein kinase (MAPK) pathway plays a crucial role. An important pathway of the MAPK signalling pathway is mediated by extracellular signal-regulated kinase (ERK), which is closely related to cellular proliferation (Fournier et al., Citation2012; Sun et al., Citation2015). ERK can be activated by many cytokines, especially macrophage migration inhibitory factor (MIF). Taken together, the aim of this study was to investigate the role of MIF, phosphorylated ERK (p-ERK)/ERK and cyclinD1 in the pathogenesis of PH in broilers.
Materials and methods
The selection of PH broilers
This experiment was planned taking into account all of the national legislations concerning the protection of animal welfare and following the strict guidelines and approval of the Institution Animal Care and Use Committee of Shanxi Agricultural University Taigu, China.
Broilers were determined as PH when they developed signs including depression, dullness, open beak breathing, distended abdomen, cyanosis, more than 10 ml ascetic fluid in the peritoneum, generalized venous congestion (Hakim, Citation1988), and right ventricular weight to total ventricular weight ratio ≥0.30 (Zhou et al., Citation2008).
Experimental groups and sample collection
The PH group was 3–4-week-old commercial Arbor Acres broilers with clinical PH (n = 10), and the healthy group (control group) was obtained from the same flock (n = 10). Broilers were provided by the Daxiang Group (Shanxi Province, China). The Daxiang Taigu farm is located at 37.42°N latitude, 112.53°E longitude and 791 metres elevation. The overall incidence of clinical PH in the flock was around 3.3% in 2014 winter. During the winter, the average temperature was around −11°C at night, and the lowest record was −17°C. Therefore, the ventilation and heating were contradictory in winter. The hen house temperature dropped intermittently with automatic ventilating. The poor air and intermittent low temperature could be the main suspected triggering mechanism of PH in chicken. Samples used for paraffin blocks were collected from the middle part of the left lung and fixed in Bouin’s fixative. The rest of the lung was stored in 1.5 ml vials in liquid nitrogen for quantitative real-time (RT-q)PCR and Western blotting.
Haematoxylin and eosin (HE) staining
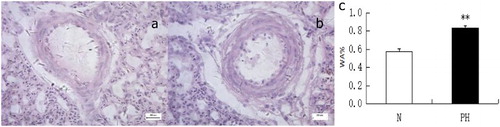
Lungs were fixed in Bouin’s fixative for 3 days at room temperature, and running water was used to rinse the residual fixative from the tissues overnight. Then the tissues were dehydrated in graded ethanol solutions, cleared in xylene and embedded in paraffin wax. Sections were cut at 4-μm thickness, spread in a 42°C water bath, then placed on slides treated with polylysine. The cytoblast and cytoplasm were stained by HE, respectively. The small and medium size pulmonary arteries (n = 40) in each broiler from each group were selected to determine the cross-sectional area (CSA) and lumen area by Image Pro Plus. The wall area percent (WA%) was compared between two groups. The WA% = (CSA−lumen area)/CSA × 100%.
Immunohistochemistry
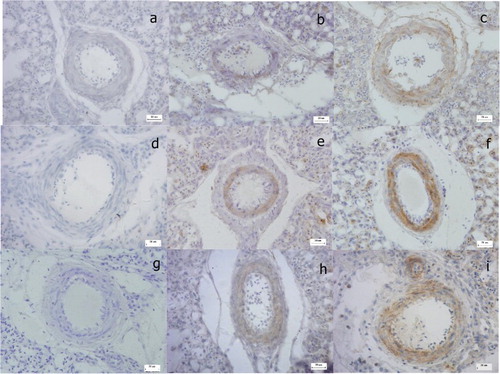
For immunohistochemistry (IHC) analysis, an IHC kit (Boster, Wuhan, China) was used. P-ERK and cylinD1 proteins were detected and localized with rabbit antibodies to cyclinD1 (Bioss, Beijing, China) or p-ERK (Sangon Biotech, Shanghai, China) both at dilutions of 1:200. Rabbit anti-chicken MIF antibodies were generated in our laboratory and used at a dilution of 1:150. All the antibodies were incubated with tissues at 4°C overnight, and negative controls consisted of phosphate buffer solution. Then, 3% peroxide was used to quench endogenous peroxidase activity for 10 min, and sodium citrate solution was used for antigen retrieval by water-bath heating.
RT-qPCR
Lungs were placed in liquid nitrogen and then ground with liquid nitrogen in a mortar. Total RNA was extracted by the conventional Trizol method, and was reverse transcribed into cDNA using a reverse transcription kit (TaKaRa, Dalian, China) following the manufacturer’s protocol: 42°C for 15 min and 85°C for 5 s. RT-qPCR was performed using a TaKaRa kit and the following conditions were used: one cycle at 95°C for 30 s, 40 cycles at 95°C for 5 s, 55°C for 30 s and 72°C for 30 s. The primers were based on published Gallus gallus sequences of MIF, ERK, cyclinD1 and β-actin ().
Table 1. Primers used for RT-PCR analysis.
Western blotting
Approximately 100 mg of frozen lung was ground with liquid nitrogen in a mortar, and then radio-immunoprecipitation assay (RIPA) was used as an ice cold lysis buffer for 30 min. Then the supernatant was collected after centrifugation at 4°C (12,000 x g) for 10 min. The concentration was determined by the BCA assay (Boster). The samples were denatured by adding loading buffer in a boiling water bath for 8 min, then they were separated in a 12% polyacrylamide gel, transferred to a nitrocellulose filter membrane, and treated with 5% skim milk at room temperature for 2 h. The protein expressions of MIF, p-ERK/ERK, cyclinD1 and β-actin were detected by anti-chicken MIF (our laboratory) and rabbit antibodies to β-actin (Bioss), p-ERK and ERK (Sangon Biotech), and mouse antibodies to cyclinD1 (Proteintech, Wuhan, China). The dilutions were 1:300 (MIF), 1:500 (p-ERK), 1:500 (ERK), 1:500 (cyclinD1) and 1:2000 (β-actin). Samples were incubated at 4°C overnight. The dilution of specific secondary antibodies was 1:3000, incubated at room temperature for 1 h. The protein band was observed under X-ray film with enhanced chemiluminescence (ECL) substrate (Boster).
Results
Morphological comparison of lungs between the PH and healthy groups
The results of HE staining showed that the structure of the lung in the healthy group was normal and without inflammatory cell infiltration. Compared with the normal group, the PH group showed arterial wall thickening, luminal narrowing and increased pulmonary inflammatory cell infiltration around the pulmonary vessels (). The occurrence of pulmonary arterial remodelling is considered as a sign of PH, therefore the WA% reflects the differences of pulmonary artery walls between the PH group and healthy group. There was an obvious trend for the PH group to have a larger WA% compared with the healthy group.
Location of MIF, p-ERK and cyclinD1 proteins in broiler lungs
The locations of MIF, p-ERK and cyclinD1 proteins were determined by IHC. Positive reactions for MIF, p-ERK and cyclinD1 proteins were observed in the pulmonary vascular walls (). The expression of MIF was located in the cytoplasm of pulmonary vascular endothelial cells (PVECs) and PVSMCs, whereas p-ERK and cyclinD1 were located in the cytoplasm of PVSMCs alone. The immunoreactivity of all proteins was markedly stronger in the PH group than in the healthy group.
Expression of MIF, p-ERK/ERK and cyclinD1 in broiler lungs
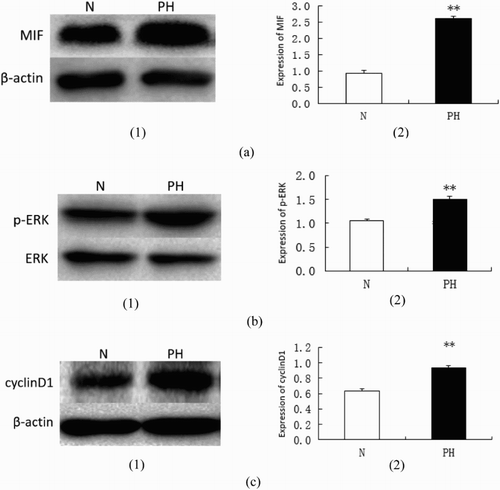
RT-qPCR analysis of MIF, ERK and cyclin D1 mRNA in broiler lungs () showed that their expression was significantly upregulated in the PH group compared with the healthy group (P < 0.01). Results of Western blotting () showed the significantly increased expression of MIF, cyclinD1 and p-ERK in the PH group (all P < 0.01) compared with the normal group, which indicated that the ERK pathway was highly activated.
Figure 3. RT-qPCR analysis of MIF (a), ERK (b) and cyclinD1 (c) in broiler lungs (**P < 0.01). Expression levels were normalized to the levels of the geometric mean of β-actin gene expression.
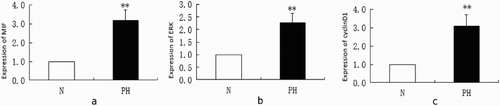
Figure 4. Immunoblot analysis of MIF, p-ERK and cyclinD1 in broiler lungs (**P < 0.01). (a) Tissue lysates were subjected to immunoblotting analysis using antibodies against MIF and β-actin (1). Bar graph showing MIF protein levels obtained from quantitative densitometry analysis (2). (b) Tissue lysates were subjected to immunoblotting analysis using antibodies against phospho-ERK (p-ERK) and total ERK (1). Bar graph showing ERK protein levels obtained from quantitative densitometry analysis (2). (c) Tissue lysates were subjected to immunoblotting analysis using antibodies against cyclinD1 and β-actin (1). Bar graph showing cyclinD1 protein levels obtained from quantitative densitometry analysis (2).
Discussion
PH is a major disease in the broiler breeding industry. PH is induced by many factors, especially hypoxia. Many factors can reduce the environmental oxygen partial pressure during the modernized farming of broilers; however, functions of the heart and lung are relatively weaker compared with their fast growth properties, which further increases their sensitivity to hypoxia (Akşit et al., Citation2008). Under the influence of these two factors, broilers are susceptible to hypoxia (Gupta, Citation2011).
Increasing evidence has indicated that pulmonary vascular remodelling, mainly caused by the proliferation of PVSMCs, is common and important during the development of PH. A previous study demonstrated that hypoxia directly stimulated the proliferation of PVSMCs (Penumatsa et al., Citation2014). Furthermore, hypoxia stimulated vascular endothelial cells to secrete MIF, which induced the proliferation of vascular smooth muscle cells (Zhang et al., Citation2010). Our HE staining analysed 40 small pulmonary arteries, selected randomly from each group, by optical microscopy, and the WA% was analysed using Image Pro Plus software. The small pulmonary arterial wall in the PH group was markedly thicker compared with the healthy group, and was mainly manifested in the muscular layer, whose cellular components are mostly PVSMCs.
Cell proliferation is regulated by a variety of cell cycle regulatory factors, especially cyclinD1, which is an active subunit of cyclin/CDK complexes. CyclinD1 induces the phosphorylation of retinoblastoma (Rb) protein by binding to and activating cyclin-dependent kinase (CDK4 or CDK6). After being phosphorylated, the Rb protein is deprived of the inhibition to transcription factor E2F, which regulates the activation of genes to accelerate the G1–S phase of the cell cycle. Currently, the role of cyclinD1 in PVSMC proliferation has been studied using plasmid-based shRNAs to suppress cyclinD1 expression in the vessels of rats. After cyclinD1 gene silencing, monocrotaline was used to induce PH in rats, and the proliferation of pulmonary artery smooth muscle cells in the cyclinD1-silenced group was reported to significantly decrease compared with the control group (Zeng et al., Citation2013). Connective tissue growth factors in rats promote PVSMC proliferation by upregulating cyclinD1 expression (Li et al., Citation2014). The IHC analysis in the current study demonstrated positive cyclinD1 reactions in the cytoplasm of PVSMCs, and the immunoreactivity was greater in the PH group compared with the healthy group. The expression of the cyclinD1 gene and its protein was detected by RT-qPCR and Western blotting, and the results confirmed that both the gene and protein were significantly upregulated in the lungs of PH broilers (P < 0.01), compared with healthy broilers. This suggests that cyclinD1 is an important factor for the promotion of PVSMC proliferation during the development of PH in broilers.
The expression of cyclinD1, a critical factor of cell cycle regulation, is affected by several upstream signal pathways including PI3 K/Akt (Hong et al., Citation2012), protein kinase C (Korulu et al., Citation2013) and MAPK, which is involved in basic life activities, especially cell growth, differentiation and division. There are three parallel MAPK pathways: the c-Jun N-terminal kinase (JNK) pathway is involved in stress responses to radiation, osmotic pressure and temperature changes; p38 mediates inflammation and apoptosis; and cell growth and differentiation are mainly affected by ERK. Therefore, the ERK pathway was selected for investigation in our study. IHC analysis indicated that p-ERK was located in the cytoplasm of PVSMCs in broiler lungs. Gene expression and protein activation, detected by RT-qPCR and Western blotting, respectively, demonstrated that the ERK gene was upregulated and that p-ERK protein was increased significantly (P < 0.01) in the PH group compared with the healthy group. This indicates that the ERK signalling pathway is highly activated in the development of PH in broilers. Treatment of vascular smooth muscle cells with tumour necrosis factor-α induced their proliferation via the activation of ERK, confirming a previous study demonstrating the role of the ERK signalling pathway in vascular smooth muscle cell proliferation (Freise & Querfeld, Citation2015).
Many cytokines signal via the ERK pathway including the vascular endothelial cell growth factor, tumour necrosis factor-α and nerve growth factor (Loeb et al., Citation1992; Xiao et al., Citation2007; Freise & Querfeld, Citation2015). MIF upregulated the expression of pro-inflammatory prostaglandin and leukotrienes, and promoted cell proliferation by binding to the CD74 receptor to activate the ERK pathway (Schevzov et al., Citation2015). In a rat model of PH induced by hypoxia, MIF directly stimulated the proliferation of PVSMCs by activating ERK signalling (Zhang et al., Citation2012), indicating that the biosignal transduction of MIF relies on ERK signalling. MIF also participates in other pathological processes, including the proliferation of fibroblasts (Calandra et al., Citation1994), endothelial cells (Onodera et al., Citation2004) and smooth muscle cells (Chen et al., Citation2015), which constitute the main components of vessel walls. Both MIF mRNA and protein expressions were upregulated in a rat model of PH, indicating that MIF plays a crucial role in the proliferation of PVSMCs (Fu et al., Citation2010; Zhang et al., Citation2012). In our study, MIF was located in the cytoplasm of both PVECs and PVSMCs. MIF mRNA and protein expression were upregulated significantly (P < 0.01) in the PH group compared with the healthy group. A previous study reported that vascular endothelial cells and vascular smooth muscle cells released MIF under the stressors of inflammation and tissue damage (Asare et al., Citation2013), suggesting PVECs express stress receptors, and that stress stimulation signalling activates them to produce and secrete MIF that induces the proliferation of PVSMCs.
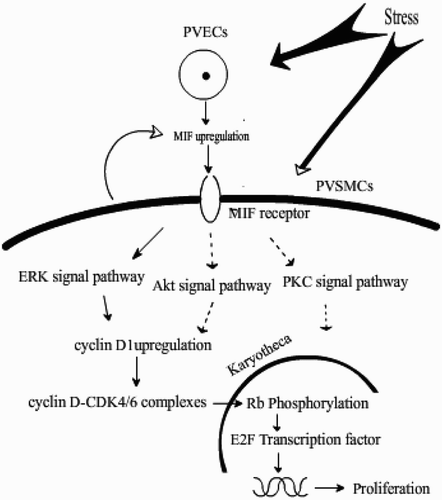
In conclusion, the present study demonstrated that the PH broilers developed the following characteristics – thickened pulmonary arterial wall, upregulated MIF expression in PVECs, and upregulated MIF and cylinD1 expression in PVSMCs compared with healthy broilers. It is reasonable to presume that MIF can induce PVSMC proliferation by the upregulation of cyclinD1 expression via the ERK signalling pathway, whereupon cyclinD1 phosphorylates Rb protein, further activating the E2F transcription factor, and subsequently inducing arterial remodelling in the development of PH in broilers ().
This study reports the role of MIF, ERK and cyclinD1 in the development of PH in broilers; however further studies are required to determine the specific mechanisms involved.
Acknowledgements
The author is very thankful to Hongmei Ning, Junping He, Xiuju Yu, Liying Qiao, Institute of Animal Science and Technology, for their kind comments and valuable advices to improve this manuscript and all kinds of help during experimentation.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Wenkui Wang http://orcid.org/0000-0001-7248-4321
Additional information
Funding
References
- Akşit, M., Altan, Ö., Karul, A.B., Balkaya, M., & Özdemir, D. (2008). Effects of cold temperature and vitamin E supplementation on oxidative stress, troponin-T level, and other ascites-related traits in broilers. Archiv für Geflügelkunde, 72, 221–230.
- Asare, Y., Schmitt, M., & Bernhagen, J. (2013). The vascular biology of macrophage migration inhibitory factor (MIF). Expression and effects in inflammation, atherogenesis and angiogenesis. Thrombosis and Haemostasis, 109, 391–398. doi: 10.1160/TH12-11-0831
- Calandra, T., Bernhagen, J., Mitchell, R.A., & Bucala, R. (1994). The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. Journal of Experimental Medicine, 179, 1895–1902. doi: 10.1084/jem.179.6.1895
- Chen, D., Xia, M., Hayford, C., Tham el-L., Semik, V., Hurst, S., Chen, Y., Tam, H.H., Pan, J., Wang, Y., Tan, X., Lan, H.Y., Shen, H., Kakkar, V.V., Xu, Q., McVey, J.H., & Dorling, A. (2015). Expression of human tissue factor pathway inhibitor on vascular smooth muscle cells inhibits secretion of macrophage migration inhibitory factor and attenuates atherosclerosis in apoe-/- mice. Circulation, 131, 1350–1360. doi: 10.1161/CIRCULATIONAHA.114.013423
- Fournier, N.M., Lee, B., Banasr, M., Elsayed, M., & Duman, R.S. (2012). Vascular endothelial growth factor regulates adult hippocampal cell proliferation through MEK/ERK- and PI3K/Akt-dependent signaling. Neuropharmacology, 63, 642–652. doi: 10.1016/j.neuropharm.2012.04.033
- Freise, C., & Querfeld, U. (2015). The lignan (+)-episesamin interferes with TNF-α-induced activation of VSMC via diminished activation of NF-ĸB, ERK1/2 and Akt and decreased activity of gelatinases. Acta Physiologica, 213, 642–652. doi: 10.1111/apha.12400
- Fu, H., Luo, F., Li, Y., Wu, W., & Liu, X. (2010). Hypoxia stimulates the expression of macrophage migration inhibitory factor in human vascular smooth muscle cells via hif-1α dependent pathway. BMC Cell Biology, 11, 1–13. doi: 10.1186/1471-2121-11-66
- Gupta, A.R. (2011). Ascites syndrome in poultry: a review. World’s Poultry Science Journal, 67, 457–468. doi: 10.1017/S004393391100050X
- Hakim, T.S. (1988). Erythrocyte deformability and segmental pulmonary vascular resistance: osmolarity and heat treatment. Journal of Applied Physiology, 65, 1634–1641.
- Hitomi, M., & Stacey, D. (1999). Cyclin D1 production in cycling cells depends on RAS in a cell-cycle-specific manner. Current Biology, 9, 1075–1084. doi: 10.1016/S0960-9822(99)80476-X
- Hitomi, M., & Stacey, D.W. (2001). RAS-dependent cell cycle commitment during G2 phase. FEBS Letters, 490, 123–131. doi: 10.1016/S0014-5793(01)02115-9
- Hong, J., Qian, T., Le, Q., Sun, X., Wu, J., Chen, J., Yu, X., & Xu, J. (2012). NGF promotes cell cycle progression by regulating D-type cyclins via PI3K/Akt and MAPK/Erk activation in human corneal epithelial cells. Molecular Vision, 18, 758–764.
- Humbert, M., Morrell, N.W., Archer, S.L., Stenmark, K.R., Maclean, M.R., Lang, I.M., Christman, B.W., Weir, E.K., Eickelberg, O., Voelkel, N.F., & Rabinovitch, M. (2004). Cellular and molecular pathobiology of pulmonary arterial hypertension. Journal of the American College of Cardiology, 43, S13–S24. doi: 10.1016/j.jacc.2004.02.029
- Korulu, S., Yildiz-Unal, A., Yuksel, M., & Karabay, A. (2013). Protein kinase C activation causes neurite retraction via cyclinD1 and p60-katanin increase in rat hippocampal neurons. European Journal of Neuroscience, 37, 1610–1619. doi: 10.1111/ejn.12185
- Li, T., Song, T., Ni, L., Yang, G., Song, X., Wu, L., Liu, B., & Liu, C. (2014). The p-ERK–p-c-Jun–cyclinD1 pathway is involved in proliferation of smooth muscle cells after exposure to cigarette smoke extract. Biochemical and Biophysical Research Communications, 453, 316–320. doi: 10.1016/j.bbrc.2014.09.062
- Loeb, D.M., Tsao, H., Cobb, M.H., & Greene, L.A. (1992). NGF and other growth factors induce an association between ERK1 and the NGF receptor, gp140prototrk. Neuron, 9, 1053–1065. doi: 10.1016/0896-6273(92)90065-L
- Onodera, S., Nishihira, J., Koyama, Y., Majima, T., Aoki, Y., Ichiyama, H., Ishibashi, T., & Minami, A.. (2004). Macrophage migration inhibitory factor up-regulates the expression of interleukin-8 messenger RNA in synovial fibroblasts of rheumatoid arthritis patients: common transcriptional regulatory mechanism between interleukin-8 and interleukin-1β. Arthritis & Rheumatism, 50, 1437–1447. doi: 10.1002/art.20190
- Penumatsa, K.C., Toksoz, D., Warburton, R.R., Hilmer, A. J., Liu, T., Khosla, C., Comhair, S.A.A., & Fanburg, B.L. (2014). Role of hypoxia-induced transglutaminase 2 in pulmonary artery smooth muscle cell proliferation. AJP: Lung Cellular and Molecular Physiology, 307, L576–L585.
- Schevzov, G., Kee, A.J., Wang, B., Sequeira, V.B., Hook, J., Coombes, J.D., Lucas, C.A., Stehn, J.R., Musgrove, E.A., Cretu, A., Assoian, R., Fath, T., Hanoch, T., Seger, R., Pleines, I., Kile, B.T., Hardeman, E.C., & Gunning, P.W. (2015). Regulation of cell proliferation by ERK and signal-dependent nuclear translocation of ERK is dependent on Tm5NM1-containing actin filaments. Molecular Biology of the Cell, 26, 2475–2490. doi: 10.1091/mbc.E14-10-1453
- Shimoda, L.A., & Laurie, S.S. (2013). Vascular remodeling in pulmonary hypertension. Journal of Molecular Medicine, 91, 297–309. doi: 10.1007/s00109-013-0998-0
- Sun, Y., Liu, W.Z., Liu, T., Feng, X., Yang, N., & Zhou, H.F. (2015). Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. Journal of Receptor & Signal Transduction Research, 35, 600–604. doi: 10.3109/10799893.2015.1030412
- Wideman, R.F., Rhoads, D.D., Erf, G.F., & Anthony, N.B. (2013). Pulmonary arterial hypertension (ascites syndrome) in broilers: a review. Poultry Science, 92, 64–83. doi: 10.3382/ps.2012-02745
- Xiao, Z., Kong, Y., Yang, S., Li, M., Wen, J., & Li, L. (2007). Upregulation of FLK-1 by BFGF via the ERK pathway is essential for VEGF-mediated promotion of neural stem cell proliferation. Cell Research, 17, 73–79. doi: 10.1038/sj.cr.7310126
- Zeng, D.X., Xu, G.P., Lei, W., Wang, R., Wang, C.G., & Huang, J.A. (2013). Suppression of cyclin D1 by plasmid-based short hairpin RNA ameliorated experimental pulmonary vascular remodeling. Microvascular Research, 90, 144–149. doi: 10.1016/j.mvr.2013.07.012
- Zhang, B., Shen, M., Xu, M., Liu, L.L., Luo, Y., Xu, D.Q., Wang, Y.X., Liu, M.L., Liu, Y., Dong, H.Y., Zhao, P.T., & Li, Z.C. (2012). Role of macrophage migration inhibitory factor in the proliferation of smooth muscle cell in pulmonary hypertension. Mediators of Inflammation, 2012, Article ID 840737, 1–10.
- Zhang, Y., Koga, K., Linge, H.M., Lin, K., Talwar, A., Miller, E. J. (2010). Increased lung MIF expression in a hypoxia induced pulmonary hypertension mice model. Abstracts of the American Thoracic Society 2010 International Conference. Animal Models for Pulmonary Arterial Hypertension: New Ideas and Insights (p. 6328). New Orleans, USA.
- Zhou, D.H., Wu, J., Yang, S.J., Cheng, D.C., & Guo, D.Z. (2008). Intravenous endothelin-1 triggers pulmonary hypertension syndrome (ascites) in broilers. Veterinární Medicína, 53, 381–391.