ABSTRACT
We investigated an epidemic mortality cluster of yellow-eyed penguins (Megadyptes antipodes) that involved 67 moribund or dead birds found on various beaches of the Otago Peninsula, New Zealand, between 21 January and 20 March 2013. Twenty-four carcases were examined post-mortem. Histological lesions of pulmonary, hepatic and splenic erythrophagocytosis and haemosiderosis were found in 23 of 24 birds. Fifteen birds also had haemoglobin-like protein droplets within renal tubular epithelial cells. Despite consistent histological lesions, a cause of death could not be established. Virology, bacteriology and molecular tests for avian influenza, avian paramyxovirus-1, avipoxvirus, Chlamydia psittaci, Plasmodium spp., Babesia spp., Leucocytozoon spp. and Toxoplasma gondii were negative. Tissue concentrations of a range of heavy metals (n = 4 birds) were consistent with low level exposure, while examination of proventricular contents and mucus failed to detect any marine biotoxins or Clostridium botulinum toxin. Hepatic concentrations of total polycyclic aromatic hydrocarbons (PAHs) (n = 5 birds) were similar to background concentrations of polycyclic aromatic hydrocarbons previously found in yellow-eyed penguins from the South Island of New Zealand, but there were significantly higher concentrations of 1-methylnapthelene and 2-methylnapthelene in the birds found dead in this mortality cluster. The biological significance of this finding is unclear. A temporal investigation of the epidemic did not indicate either a common source or propagative epidemic pattern. Although our investigation did not definitively implicate a toxic or infectious agent, we could not rule out causes such as toxic marine organisms or mycoplasmosis. Further investigations should therefore by carried out in the event of future mortality clusters.
Introduction
There are frequent mass mortality events of both adult and juvenile sub-Antarctic penguins in which the contributing factors leading to the mortality are often poorly documented. Published reports have suggested a range of causal factors, including: increases in the numbers of predators (Crawford et al., Citation2006, Citation2009); infectious disease outbreaks (Graczyk et al., Citation1995; Crawford et al., Citation2006); shifts in prey availability (Crawford et al., Citation2009); marine biotoxins (Gill & Darby, Citation1993) and environmental change, specifically the El Niño Southern Oscillation (Vargas et al., Citation2006, Citation2007).
The yellow-eyed penguin (Megadyptes antipodes), or hōiho, is the only extant member of its genus and is listed as an endangered species on the IUCN RedList, and Nationally Vulnerable by the New Zealand Department of Conservation (Robertson et al., Citation2013), with an estimated wild population of less than 7000 mature individuals, making it one of the rarest of the world’s 18 species of penguin. Yellow-eyed penguins are endemic to New Zealand and their breeding distribution comprises the mainland population, from the southeast coast of the South Island to Stewart and Codfish Islands as well as the sub-Antarctic region where they breed on Auckland and Campbell Islands (Boessenkool et al., Citation2009; Seddon et al., Citation2013). The mainland population of yellow-eyed penguins has been extensively monitored and full nest counts are made in most areas on an annual basis resulting in reliable population data (McKinlay, Citation2001; Seddon et al., Citation2013). The same level of monitoring has not been undertaken in the sub-Antarctic and very little population data are available or the information is dated (Moore, Citation1992; Moore et al., Citation2001; Seddon et al., Citation2013).
The yellow-eyed penguin population on the mainland of New Zealand has undergone several declines in recent years. The most dramatic decline occurred during the austral summer of 1989–1990 when around 150 of the estimated 500 adult birds present died on the Otago Peninsula (Gill & Darby, Citation1993). An unidentified toxin was suggested as a cause of these mortalities (Gill & Darby, Citation1993) and serological studies raised the possibility of avian malaria (Graczyk et al., Citation1995). In other years, however, food shortages or availability of quality prey have been implicated as a cause of high adult and chick mortality (van Heezik, Citation1990). During the 2011/2012 breeding season, total counts of yellow-eyed penguins numbered 184 nests and an estimated 440 adults on the Otago Peninsula (Ellenberg & Mattern, Citation2012).
During the first three months of 2013, an unusual increase in the number of adult yellow-eyed penguin mortalities occurred around the Otago Peninsula, involving birds either found dead in good body condition, or alive in good body condition and exhibiting a short period (<24 hours) of ataxia and seizures prior to death. The purpose of this study was to investigate the possible causes of this mortality event using pathological, toxicological and epidemiological methods.
Materials and methods
Pathology
Sixty-seven moribund or dead yellow-eyed penguins were found on various beaches of the Otago Peninsula, New Zealand, between 21 January and 20 March 2013. Yellow-eyed penguins were selected for post-mortem examination based on the state of decomposition on recovery. Some were excluded because of post-mortem scavenging or decomposition, and others simply to reduce the logistical costs of the investigation. Birds that were not examined by post-mortem were stored frozen for retrospective analysis if required. To ensure that representative samples were taken throughout the period of the investigation up to three bodies were submitted for each day that dead birds were found. The birds selected for post-mortem examination were representative of all locations and times involved in the mortality cluster.
A total of 24 (21 adults and three juveniles) penguins were necropsied from 24 January to 3 March, at Wildbase, Massey University. Case data included band number, date submitted, species, age, sex, weight, location and history. After gross post-mortem examination, samples of various tissues, including the lungs, heart, liver, spleen, kidney, brain, skeletal muscle and where suitable, gastrointestinal tract were placed in 10% buffered formalin for histopathology. Sections of these tissues were processed routinely, embedded in paraffin and then stained with haematoxylin and eosin. Selected tissues were also stained with Gram, Giemsa and Perls’ Prussian Blue.
Toxicology
Marine biotoxin screening
Proventricular contents, when present, as well as scrapings of proventriculus mucus were collected from four birds and frozen prior to submission to the Cawthron Institute, Nelson for screening for marine biotoxins by using liquid chromatography–mass spectrometry (MacKenzie et al., Citation2005) and pre-column oxidation screening (AOAC 2005.06) (Harwood et al., Citation2013). These methods are able to detect a range of shellfish toxins () including: amnesic shellfish poisoning (ASP) toxins; diarrhetic shellfish poisoning (DSP) toxins; neurotoxic shellfish poisoning (NSP) toxins and paralytic shellfish poisoning (PSP) toxins.
Table 1. The results of marine biotoxin screening by liquid chromatography–mass spectrometry (LCMS) and pre-column oxidation screen of the proventricular content from four dead yellow-eyed penguins (M. antipodes).
Mouse toxicity of proventriculus contents
Freeze-dried samples of proventriculus contents from nine affected yellow-eyed penguins were sent to AgResearch, Hamilton for further toxin testing. They were kept frozen until extraction. Small sub-samples of proventriculus content from three penguins were extracted separately. Due to low sample volume two samples were obtained by pooling the proventricular contents from three individual penguins before extraction. This gave a total of five extracted samples for analysis. Methanol (30 ml) was added to each sample and the mixture was homogenized first using a Polytron homogenizer and then a glass-Teflon Potter-Elvehjem homogenizer. The homogenate was centrifuged and the supernate removed. The residue was extracted four more times with methanol, and the supernates combined. The supernates were evaporated at 30°C on a rotary evaporator to a volume of 20 ml. Water (1.6 ml) was added and the pH of the extract was adjusted to 6.0 with 0.3 M HCl. This solution was extracted three times with an equal volume of petroleum ether (boiling point 40–60°C). The methanol and petroleum ether extracts were evaporated to dryness on the rotary evaporator, and the residue transferred to weighed glass sample tubes. The last traces of solvent were removed on a freeze drier.
Female Swiss mice, Mus musculus initial body weight 18–22 g, bred at AgResearch Ruakura, Hamilton, were used. The test groups were identified by means of tail-marking. The mice were individually housed in plastic cages with wire mesh tops, and softwood chips were provided as bedding. Tap water and food (Rat and Mouse Cubes, Speciality Feeds Ltd, Glen Forrest, Western Australia) were available at all times.
The body weights of the mice were recorded immediately before dosing, and the required dose of the extracts was calculated on the basis of grams/kg body weight. The methanol extracts were dissolved in 1% Tween 60 in saline. Five mice were injected intraperitoneally with this solution at a dose of 2 g/kg, in a total volume of 1 ml. A second group of five mice were dosed by gavage at a dose of 10 g/kg in a total volume of 200 μl. The petroleum ether extracts were suspended in 1% Tween 60 in saline and injected intraperitoneally at a dose of 1 g/kg, in a total volume of 1 ml into a third group of five mice. The mice were examined intensively during the day of dosing. They were subjected to clinical examination each day for 14 days after dosing, and their body weights were recorded. On the 15th day after dosing, the animals were killed by carbon dioxide inhalation and examined post-mortem. The weights of liver, kidneys, spleen, heart and lungs were recorded at necropsy, and relative organ weights were calculated as a percentage of terminal body weight. Organ to body weight ratios was used as a trigger for histological examination. Histology is not usually prescribed in acute toxicity tests unless there is macroscopic evidence of tissue damage. In retrospect, a repeat-dosing study, looking for evidence of haemolysis would have been a good idea, but we did not follow up on this.
Ethical statement
All animal manipulations were approved by the Ruakura Animal Ethics Committee established under the Animal Protection (code of ethical conduct) Regulations Act, 1987 (New Zealand).
Heavy metal assays
Fresh/frozen liver samples from four birds were sent to AsureQuality Laboratories (Wellington, New Zealand) for lead, zinc, cadmium, arsenic and mercury analysis using International Accreditation New Zealand (IANZ) accredited methods. Briefly, the liver samples were prepared using wet oxidation for cadmium, arsenic and lead analysis, and acid digestion for mercury and zinc. The heavy metal assays were then performed using Inductively Coupled Plasma Mass Spectrometry, and the results are reported in milligrams per kilogram wet weight (mg/kg).
Polycyclic aromatic hydrocarbons
Fresh/frozen liver samples of at least 6 g wet weight were collected from five birds and stored frozen (approx. −20°C) in tinfoil until being sent to AsureQuality Laboratories for polyaromatic hydrocarbon (PAH) analysis. Liver samples were homogenized with anhydrous sodium sulphate at a ratio of approximately 4:1 (sodium sulphate:liver) to provide a dry, free flowing sample. Test portions equivalent to ∼2 g of liver tissue were spiked with internal standards (13C labelled analogues) and Soxhlet extracted with a 1:1 mixture of dichloromethane:hexane for 16 h. A portion of the extract (20%) was taken for gravimetric determination of lipid content. The remaining portion of the extract was taken for PAH analysis. The majority of lipids were removed from the PAH extract using freezing lipid precipitation (Yoon et al., Citation2015).
The extract was then further cleaned using silica/potassium silicate column chromatography. Clean extracts were spiked with recovery standards (deuterated PAHs), and concentrated to a final volume of 0.5 ml (iso-octane). Sample extracts were analysed by Gas Chromatography-High-Resolution Mass Spectrometry using an Agilent 6890/7890 gas chromatograph coupled with Waters Ultima/Premier high-resolution (≥8000) mass spectrometer. The individual analyte concentrations were calculated from their relative response to an internal standard against the slope of a multi-point calibration curve. Each sample was tested for the 22 PAH compounds, naphthalene, 2-methylnaphthalene, 1-methylnaphthalene, acenaphthylene, acenaphthene, fluorene, phenanthrene, 2-methylphenanthrene, 1-methylphenanthrene, anthracene, fluoranthene, pyrene, benz[a]anthracene, chrysene, benzo[b]fluoranthene, benzo[k]fluoranthene, benzo[e]pyrene, benzo[a]pyrene, perylene, indeno[1,2,3-c,d]pyrene, dibenz[a,h]anthracene and benzo[g,h,i]perylene.
Method blanks for each batch were also analysed to determine laboratory background levels and a blank correction procedure was applied. Blank correction involved subtracting the total amount of analyte detected in the laboratory blank from the total amount of analyte detected in the samples. Where a negative number was obtained, or if the number was below the limit of detection, then the result was reported as not detected. The results were then corrected for sample weights and reported as nanograms per gram (ng/g) wet weight, to three significant figures.
The lipid content of each sample was determined by evaporating the sample extract to constant weight. The residue was determined gravimetrically on a calibrated balance. The lipid content is expressed as a percentage of wet weight (% w/w) of the total sample to two significant figures. Analytical results were reported on a wet weight basis, but were also converted to ng/g lipid for comparison with other research.
The results of PAH analysis from yellow-eyed penguins were compared to background levels of PAHs previously surveyed in yellow-eyed penguins from the Otago region (McConnell et al., Citation2015). The samples from yellow-eyed penguins in this previous survey were obtained from sporadic mortality that occurred due to a variety of causes including trauma, starvation and infectious disease. Data were analysed using Kruskal Wallis Univariate Analysis of Variance (SPSS 21, IBM).
Microbiology
Aerobic and anaerobic cultures of a variety of tissues including intestine, liver, lung, heart and kidneys were performed by New Zealand Veterinary Pathology (Palmerston North, New Zealand) and the Ministry for Primary Industries (MPI) (Wellington, New Zealand) using routine methodology.
Molecular diagnostics
Formalin-fixed, paraffin-embedded sections (10 µm) of lung, liver and spleen from five yellow-eyed penguins were used for DNA extraction using a Qiagen DNeasy Kit (Qiagen, Valencia, CA, USA) as per manufacturer’s instructions. DNA was stored at −20°C until needed for polymerase chain reaction (PCR). A nested PCR protocol was used to determine the presence of the cytochrome b gene of Plasmodium, Haemoproteus and Leucocytozoon spp. as described by Hellogren et al. (Citation2004). The presence of Toxoplasma gondii DNA was detected using a nested PCR protocol for the amplification of the Pppk- dhps gene as described by Roe et al. (Citation2013). PCR assays to detect avipoxvirus 4b core protein gene and the 18S gene of Babesia and Theileria spp. were carried out as described by Ha et al. (Citation2011) and Hodge et al. (Citation2015), respectively. To determine successful amplification of DNA in all PCR protocols, 1.5 µl of PCR products were run on 1.5% agarose gel (UltraPure Agarose, Invitrogen, Carlsbad, CA, USA) containing ethidium bromide and visualized under UV light.
Samples of liver from five birds were submitted for Chlamydia psittaci PCR (as described in Gartrell et al. Citation2013). MPI also conducted PCR testing for avian influenza and avian paramyxovirus (APMV-1) on tissue and gut samples from five birds.
Gut samples from four birds were examined by multiplex PCR assay for Clostridium perfringens toxins. Five sets of forward and reverse primers targeting genes coding for alpha (cpa: cpa F and cpa R), beta (cpb: cpb F and cpb R), epsilon (etx: etx F and etx R), iota (iA: iA F and iA R) and enterotoxin (cpe: cpe F and cpe R) from C. perfringens described by Heikinheimo and Korkeala (Citation2005) were used. The PCR reaction consisted of 12.5 µl Kapa2GTM Fast Ready Mix (2×) (Kapa Biosystems, Wilmington, MA, USA), 0.5 µM each for cpa F and cpa R, 0.36 µM each for cpb F and cpb R, 0.46 µM each for etx F and etx R, 0.52 µM each for iA F and iA R, 0.34 µM each for cpe F and cpe R and 2.5 µl DNA. The amount of water was adjusted to make total volumes of 25 μl. The thermal cycling conditions began with an initial denaturation at 95°C for 1 min, then 35 cycles of denaturation for 15 s at 95°C, annealing for 15 s at 53°C and extension for 1 s at 72°C. DNA extracted from C. perfringens type A (NCTC8237), type B (NCTC8533), type C (NCTC8081), type D (NCTC8504) and type E (NCTC8084) were used as positive controls. All PCR products were visualized by electrophoresis on a 1.5% (w/v) ultra-pure agarose gel (Invitrogen) containing GelRed (Biotium, Hayward, CA, USA).
Virology
MPI conducted virology cultures on tissue and gut samples from five birds using two cell lines, chicken embryo fibroblast (CEF) and quail fibroblast cell line, designated QT-35 as described in Moresco et al. (Citation2010).
Epidemiology
The temporal and spatial–temporal patterns of the outbreak were examined to characterize the outbreak type (common source or propagative) and identify significant clustering in time or space, or time and space. Temporal clustering was examined using the scan test for temporal clustering (Naus, Citation1965) and SPSTAT v.4.7 software (University of California, Davis, CA, USA). Spatial–temporal clustering was examined at the local level, using the spatial–temporal permutation test (Kulldorff et al., Citation2005) and SATSCAN software v.9.3 (Kulldorff & Nagarwalla, Citation1995) and globally using the Knox test (Knox, Citation1964) and SPSTAT. The Knox test requires the specification of a critical time and space distance separation, within which it is thought disease transmission from an infectious to susceptible host may occur. As these critical values were unknown, the Knox test was used to generate hypotheses rather than to test them. Therefore, multiple (40) tests were performed, examining clustering within critical times of 3–14 days and 1–8 km. A local spatial–temporal test is able to detect if, when and where there is clustering; however, a global spatial–temporal test is able to detect if there is clustering in the population at risk but not the specific location(s) or time period(s) that contribute to the cluster.
Results
Pathology
Twenty-four penguins were necropsied. Two birds were found alive but died within 24 h of capture. One bird showed only laboured breathing before death and the other showed ataxia and disorientation prior to the development of open mouth breathing and death. No ante-mortem diagnostics were performed as all birds died before veterinary attention was sought. In total, 46 carcasses were collected.
All 24 necropsied birds showed similar gross findings and were in good body condition, weighing 4.5 kg or heavier (normal range), with no obvious external injuries. The overwhelming majority were adults (n = 21) and three were juvenile birds. There was no apparent sex predilection (M:F = 13:10). Sex could not be determined in one bird that had its gonads removed by scavenging prior to post-mortem examination. Most birds had no ingesta within their proventriculus/ventriculus, only mucus, which was occasionally bile stained. No other obvious gross abnormalities were noted.
The histopathological lesions were similar in most birds, despite a varying degree of autolysis and freeze–thaw artefact. There was marked congestion of most internal organs with evidence of pulmonary, hepatic and splenic erythrophagocytosis (n = 22) and mild haemosiderosis (n = 22) ((a)–(c)). Most birds (n = 22) also showed mild lipid accumulation within hepatocytes, consistent with peripheral lipid mobilization. Where the kidney could be examined histologically (n = 19), most (15/19) of the birds showed varying degrees of swelling/vacuolation of the proximal and distal renal tubular epithelial cells, associated with intracytoplasmic eosinophilic droplets resembling haemoglobin within the epithelial cells, consistent with intravascular haemolysis and haemoglobinuric nephropathy ((d)). In these 15 birds, occasional proximal tubules were necrotic and showed luminal accumulation of dense proteinaceous material. Respiratory system changes included marked congestion of the lungs and varying degrees of pulmonary oedema. The pulmonary interstitium (n = 22) was hypercellular due to the accumulation of moderate numbers of mononuclear cells, macrophages and a few heterophils. Many of the macrophages contained finely granular to coarser golden-brown intracytoplasmic pigment. A Perls’ Prussion Blue stain on sections of lung (six out of six birds tested) stained this intracytoplasmic pigment blue, indicating the presence of iron. Similar pigment was present within hepatic macrophages, which were moderately increased in number. Two birds also had marked proliferation of Gram-positive rod-shaped bacteria within sections of intestine that were relatively well preserved, but these changes were absent in all other birds. One of these birds had an erosive typhlitis associated with these bacteria.
Figure 1. Histological appearance of issues from a yellow-eyed penguin (M. antipodes) that died with typical lesions of pulmonary, hepatic and splenic erythrophagocytosis and mild haemosiderosis. (a) The lung shows marked congestion with varying degrees of pulmonary oedema, and an accumulation of macrophages that contain finely granular to clumpier golden-brown intracytoplasmic pigment/material (black arrow). (b) The liver demonstrates increased numbers of Kupffer cells, with many containing similar pigment/material to that observed within the lung (black arrow) which was confirmed as iron with Perls’ Prussian Blue stain. (c) The spleen also shows increased numbers of histiocytic-like cells, many of which contained pigment/material similar to that seen in the lung and liver. In addition, in several of the better preserved sections of spleen, these cells appeared to contain swollen erythrocytes (black arrows). (d) The histological appearance of the kidney showing typical pathology of swelling and vacuolation of the proximal and distal renal tubular epithelial cells, associated with intracytoplasmic eosinophilic droplets resembling haemoglobin within the epithelial cells (grey arrows). Haematoxylin and eosin stain. Scale bars represent 50 μm.
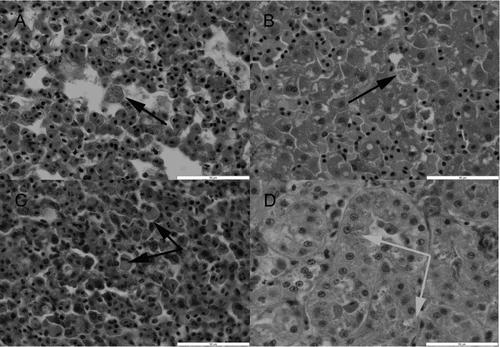
Within the spleen, there was also evidence of increased phagocytic activity and around many sheathed arterioles there were increased numbers of macrophages, many of which contained pigmented material that occasionally stained positive with a Perls’ Prussion Blue stain. Occasional macrophages contained swollen erythrocytes.
No infectious agents were identified on Gram and Giemsa stained sections of all tissues other than the intestinal bacilli noted above.
Toxicology
Marine biotoxin screening
Proventriculus contents from all four birds sampled were tested for the presence of common marine biotoxins () and the results were all below the minimum detectable limit of the assays.
Mouse toxicity of proventriculus contents
None of the extracts caused significant toxic effects in mice. Mice dosed with the methanol extracts by intraperitoneal injection at 2 g/kg became lethargic, with abdominal breathing, within 10 min after administration. They remained in this state for 1.0–2.2 h, after which their appearance and behaviour became normal, and remained normal throughout the remainder of the day. The mice lost 0.5–1.7 g in body weight by the following day, but this was subsequently regained. All the animals appeared entirely normal subsequently throughout the observation period. No abnormalities were detected at necropsy, and the relative weights of liver, kidneys, spleen, heart and lungs were within the normal range. Normal organ to body weight ratios do not necessarily indicate confirmation in mice of no effect of the extract. It was one of the parameters examined, and a change in such ratios may have been a trigger for histological examination, particularly if the spleen had been enlarged, since splenic enlargement is commonly seen in rodents after induction of haemolysis.
Mice dosed with the petroleum spirit extracts by intraperitoneal injection at 1 g/kg also became lethargic, with abdominal breathing, within 10 min after administration. They remained in this state for up to 4.5 h, after which their appearance and behaviour became normal. The appearance and behaviour of the mice remained normal throughout the remainder of the day of dosing. The mice lost 0.5–2.0 g in body weight by the following day, but this was subsequently regained. All the animals appeared entirely normal throughout the remainder of the observation period. Adhesions between the liver, stomach and spleen were observed in two of two mice injected with extracts of one individual and in two of six mice injected with two pooled samples. The relative weights of liver, kidneys, spleen, heart and lungs of all animals were within the normal range.
No effects were recorded in mice dosed by gavage with the methanol extract at 10 g/kg at any time. They lost no body weight after dosing, and relative organ weights were within the normal range.
Heavy metal assays
Assays of the livers from four affected penguins showed low concentrations of arsenic, cadmium, mercury and zinc () and tissue concentrations of lead were below the minimum detectable level of the assay (<0.01 mg/kg). Comparison of these tissue concentrations with previously reported studies in seabirds suggests that these represent levels of exposure to heavy metals below the reported thresholds for lethality in other species of birds. In retrospect, copper would have been a useful addition to this heavy metal screening panel.
Table 2. Hepatic concentrations of heavy metals (arsenic, cadmium, lead, mercury and zinc) from four yellow-eyed penguins (M. antipodes) that died during an outbreak of mortality in the South Island of New Zealand between January and March 2013.
Polycyclic aromatic hydrocarbons
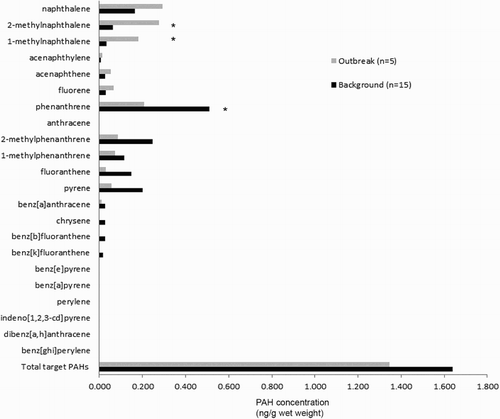
The results of assays for PAHs in the liver of dead yellow-eyed penguins overall showed low concentrations (). The total target PAH concentrations of penguins that died in this outbreak were not significantly different from previously reported background concentrations of PAHs in yellow-eyed penguins (McConnell et al., Citation2015) when compared by either concentration of PAH per g of liver wet weight (H = 0.02, 1 d.f., P = 0.965) or per g of liver lipid (H = 1.830, 1 d.f., P = 0.176). There was, however, a significantly higher concentration of 1-methylnaphthalene (0.182 ng/g wet weight; H = 9.631, 1 d.f., P = 0.002) and 2-methylnaphthalene (0.278 ng/g wet weight; H = 8.091, 1 d.f., P = 0.004) in the outbreak birds compared to the background concentrations of 1-methylnaphthalene (0.034 ng/g wet weight) or 2-methylnaphthalene (0.063 ng/g wet weight) found by McConnell et al. (Citation2015) (). The concentration of phenanthrene was significantly lower (H = 5.35, 1 d.f., P = 0.021) in penguins that died in this outbreak (0.206 ng/g wet weight) than background concentrations (0.509 ng/g wet weight).
Figure 2. The hepatic concentrations of PAHs from yellow-eyed penguins (M. antipodes) that died in the 2013 outbreak compared to background levels of PAHs in yellow-eyed penguins previously reported from the Otago region (McConnell et al., Citation2015). Significant differences are indicated by *.
Microbiology
Cultures of the liver performed on eight birds grew a mix of organisms in each case. The organisms cultured included: Escherichia coli (n = 8); alpha-haemolytic Streptococcus sp. (n = 5); non-haemolytic Streptococci sp. (n = 3); Bacillus sp. (n = 1); Edwardsiella tarda (n = 1); Enterococcus sp. (n = 1); Enterobacter sp. (n = 1); Morganella morganii (n = 1); Providencia sp. (n = 1); coagulase negative Staphylococcus sp. (n = 1) and Staphylococcus intermedius (n = 1).
Two homogenates consisting of pooled samples of lung from five birds, and a second sample of liver from the same five birds were cultured by the MPI IDC laboratory. These grew a mixed culture of bacteria in which Moellerella wisconsensis and E. coli predominated in both samples.
Molecular diagnostics
PCR assays for avian influenza and avian paramyxovirus-1, avipoxvirus, C. psittaci, Plasmodium spp., Babesia spp., Leucocytozoon spp., and T. gondii were negative.
C. perfringens PCR was positive on the gut samples from one of four birds tested and was consistent with type A.
Virology
Viral cultures of liver homogenates from five yellow-eyed penguins were negative.
Epidemiology
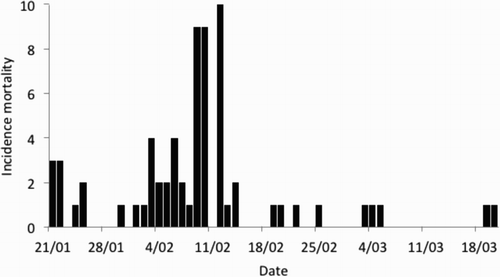
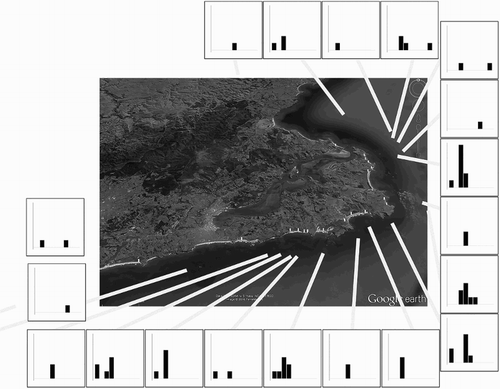
The epidemic curve of the 67 reported cases is shown in . The maximum number of cases (10) was reported on 12 February at six of the 19 sites and the most statistically significant (P < 0.001) temporal cluster of 28 cases occurred over a 4-day period (9–12 February) at 12 sites. No statistically significant (P ≥ 0.08) local spatial–temporal cluster was found at any time during the outbreak. At the global level, the temporal and spatial relationship between 2211 case pairs showed a mean time between cases of 9.51 days and a mean distance of 7.77 km. No statistically significant (P ≥ 0.55) spatial–temporal clustering was found at the global scale after examining 32 putative critical time and space distances, ranging from 3 to 10 days and 1–8 km, respectively (). The mortality cluster was limited to the Otago Peninsula, a small geographic area compared to the known distribution of the yellow-eyed penguins.
Discussion
Despite intensive investigation, the primary cause of the mortality cluster in adult yellow-eyed penguins seen in the period of January to March 2013 has not been identified. We were able to document the temporal and geographical distribution of the mortalities and common histological features of pulmonary, hepatic and splenic erythrophagocytosis and haemosiderosis and a suspected haemoglobinuric nephropathy. Bacterial and viral cultures revealed no primary pathogenic organisms from the dead birds and targeted DNA probes for specific pathogens were negative. Overall, these results are most likely to indicate a toxic cause of the mortality cluster, but in our investigation we were unable to identify a specific toxic agent.
The pathological evidence of haemolysis was a consistent feature in the majority of birds examined and the differential diagnoses considered for this included: exposure to PAHs, lead toxicosis, zinc toxicosis, infection with avian Plasmodium species (and other haemoparasites such as Babesia), avian chlamydiosis, salmonellosis, colibacillosis, clostridiosis, and aflatoxicosis. Mycoplasmosis was considered but not investigated.
Other causes of rapid death of relatively large numbers of seabirds in good body condition that were considered included organic toxins (including marine biotoxins), inorganic toxins, bacterial toxins such as from Clostridium botulinum, and viruses such as highly pathogenic avian influenza as well as moderately to highly virulent strains of Newcastle disease. However, the lack of spatial–temporal clustering within sites does not support the local infectious disease hypothesis.
Polyaromatic hydrocarbon analysis from the liver of affected birds showed increased exposure to 1- and 2- methylnaphthalenes, but not an overall increase in petrogenic PAHs compared to baseline samples for yellow-eyed penguins reported in McConnell et al. (Citation2015). This suggests that the change in PAH exposure is not related to petrogenic sources. Naphthalenes are associated with central nervous system (CNS) effects and haemolytic anaemia in people (especially infants with naphthalene treated nappies), but this condition cannot be replicated in laboratory animals (rats, mice, rabbits) (Sudakin et al., Citation2011). This suggests that the biological effects of PAH exposure may depend on species variability in metabolism. The concentration of PAHs found in the penguins, while higher than background levels, was not consistent with toxicity. However, in mammals and people, naphthalenes are metabolized and eliminated in 24–48 h after a single dose (Sudakin et al., Citation2011).
While the role of PAH exposure in this mortality was not determined, three main hypotheses were formulated. Firstly, that the evidence of increased methylnaphthalene exposure was random and of no biological significance. Secondly, that the evidence of increased methylnaphthalene exposure was not directly associated with pathological effects but indicates exposure to an unrecognized source of anthropogenic chemicals. Finally, that the increased methylnaphthalene exposure may have resulted in pathological effects, but the tissue concentrations were reduced by metabolism and elimination prior to death. Further study is required to differentiate between these hypotheses.
Concentrations of heavy metals were generally low and well below reported toxicity thresholds associated with lethality. The concentrations of mercury were in a range that has been suggested to cause measurable biological effects in reproduction in Arctic seabirds (Scheuhammer et al., Citation2015), however, only tissue levels in birds >15 mg/kg are reported to cause overt signs of intoxication and death (Wiener et al., Citation2003).
The rapid onset of death, lack of major gross pathological changes, constrained geographic area and limited time course of the mortality are all consistent with acute exposure to a toxic agent. Although there was an epidemic peak, which lasted 4 days, cases were distributed over several sites, with no indication of spatial clustering, which might have indicated either an infectious disease agent of common exposure to individual or neighbouring site populations. On the other hand, evidence of clustering over a period of 10–14 days, and a wide range of distance, 1–8 km, support the hypothesis that the birds may have been exposed to a common source, for example, when feeding, and then returned to their sites with limited or no additional spread. The histological findings of haemoglobinuric nephrosis and erythrophagocytosis are also consistent with a toxic aetiology. However, the lack of toxicity of extracted stomach contents from yellow-eyed penguins inoculated into mice, absence of external contamination of plumage, lack of evidence of similar deaths in chicks and the lack of other species affected are inconsistent with a widespread environmental toxin such a marine algal bloom. We have definitively ruled out domoic acid, brevetoxins and other common marine biotoxins in stomach contents in this investigation.
Nutritional sources of the putative toxin are a possibility, particularly as yellow-eyed penguins are almost exclusively benthic feeders, unlike most other penguins which are pelagic foragers (Moore, Citation1999; Mattern et al., Citation2007, Citation2013). Other penguins are thus better able to adapt to seasonal and other major changes in the marine environment which can sometimes cause unfavourable prey dynamics. Around the Otago Peninsula, where this mortality cluster occurred, the yellow-eyed penguins are known to stay close to their breeding sites throughout the year, feed primarily on fish species on the seafloor and the birds consistently make short trips (c. 25 km) often to the same localities (Mattern et al., Citation2007, Citation2013). Toxins accumulating in local benthic fish species could therefore be a possible cause, although it is also possible that the mortality cluster occurred due to a prey switch to a toxic food source, although the good body condition of the birds makes this unlikely. One group of marine organisms known to cause haemolysis in humans and fish are the jellyfish (Phylum Cnidaria, Class Scyphozoa) that contain protein toxins in their tentacles and nematocysts (Bailey et al., Citation2005; Nie et al., Citation2008; Brinkman & Burnell, Citation2009). These toxins should be investigated as part of any similar mortality cluster in yellow-eyed penguins.
This mortality cluster has many similarities to previous mortality events in yellow-eyed penguins. A mortality cluster of approximately 150 adult yellow-eyed penguins was reported in 1990 by Gill and Darby (Citation1993). The signalment of birds affected, the clinical signs of birds found alive and the post-mortem findings in the dead birds are all comparable to the 2013 event. The time series of that event (mid-December to mid-March) is also comparable. Toxicology tests performed at the time ruled out poisoning by copper, zinc, iron, lead, arsenic, selenium, cadmium, mercury, organophosphates, organochlorines, polychlorinated biphenyls, hexachlorobenzene or the toxins of C. perfringens, C. botulinum and dinoflagellates. However, the authors concluded that an unidentified toxin was the most likely cause of the earlier mortality (Gill & Darby, Citation1993). It is worth noting that any toxin involved in both the 1990 and current mortality outbreaks would need to be an agent that yellow-eyed penguins alone are exposed to during the summer months. It could perhaps be a prey item in the penguins’ diet that becomes seasonally toxic under certain environmental conditions.
A review of the Massey University post-mortem database records identified two yellow-eyed penguins from the Otago Coast that had died suddenly in January 2012 in good body condition, and these showed histological changes of erythrophagocytosis and early haemosiderosis similar to the current outbreak. This lends credence to the possibility that the cause may be a toxic prey species that is only used irregularly by the yellow-eyed penguins. Investigation of this hypothesis is hindered by the minimal stomach contents present in the birds at the time of death. We hypothesize that the putative toxin may induce vomiting before death, explaining this common feature of the post-mortem examinations.
Environmental toxins were recently suspected as a cause of neonatal deformities affecting yellow-eyed penguins. In 2009, there was an unusual cluster of brachygnathia in yellow-eyed penguin chicks in the same area as the current cases (Buckle et al., Citation2014). Extensive analyses for teratogenic toxins, including assays for polychlorinated hydrocarbons and organochloride pesticides in the affected chicks, were also negative but this event highlights the possibility of unidentified toxic elements on the seafloor around this locality.
The key features of the mortality cluster that we were able to identify are as follows: only one wildlife species (yellow-eyed penguins) was affected; the majority of birds affected were adults and all showed similar histopathological changes suggestive of haemolysis; the birds died acutely and in good body condition; two birds that were found alive died within hours of capture; the onset of illness appears to have occurred at sea; the mortalities occurred in a localized geographic area around the Otago Peninsula and the time course of the mortalities was restricted to a period of 59 days over January to March 2013.
Recommendations for conservation managers include the development of protocols for response to future mass mortality events, including those actions that will facilitate epidemiological research. Continued monitoring and marking of yellow-eyed penguins will significantly aid the response and future studies to determine the cause of such catastrophic mortality events in this endangered species.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Bailey, P.M., Bakker, A.J., Seymour, J.E. & Wilce, J.A. (2005). A functional comparison of the venom of three Australian jellyfish: Chironex fleckeri, Chiropsalmus sp., and Carybdea xaymacana – on cytosolic Ca2+, haemolysis and Artemia sp. lethality. Toxicon, 45, 233–242. doi: 10.1016/j.toxicon.2004.10.013
- Boessenkool, S., Star, B., Waters, J.M. & Seddon P.J. (2009). Multilocus assignment analyses reveal multiple units and rare migration events in the recently expanded yellow-eyed penguin (Megadyptes antipodes). Molecular Ecology, 18, 2390–2400. doi: 10.1111/j.1365-294X.2009.04203.x
- Brinkman, D.L. & Burnell, J.N. (2009). Biochemical and molecular characterisation of cubozoan protein toxins. Toxicon, 54, 1162–1173. doi: 10.1016/j.toxicon.2009.02.006
- Buckle, K.N., Young, M.J. & Alley, M.R. (2014). Investigation of an outbreak of craniofacial deformity in yellow-eyed penguin (Megadyptes antipodes) chicks. New Zealand Veterinary Journal, 62, 250–257. doi: 10.1080/00480169.2014.906332
- Cain, B.W., Sileo, L., Franson, J.C. & Moore, J. (1983). Effects of dietary cadmium on mallard ducklings. Environmental Research, 32, 286–297.
- Choong, B., Allinson, G., Salzman, S. & Overeem, R. (2007). Trace metal concentrations in the Little Penguin (Eudyptula minor) from Southern Victoria, Australia. Bulletin of Environmental Contamination and Toxicology, 78, 53–57.
- Crawford, R., Dyer, B., Cooper, J. & Underhill, L. (2006). Breeding numbers and success of Eudyptes penguins at Marion Island, and the influence of mass and time of arrival of adults. CCAMLR Science, 13, 175–190.
- Crawford, R., Whittington, P., Upfold, L., Ryan, P., Petersen, S., Dyer, B. & Cooper, J. (2009). Recent trends in numbers of four species of penguins at the Prince Edward Islands. African Journal of Marine Science, 31, 419–426.
- De Francisco, N., Ruiz Troya, J.D. & Agüera, E.I. (2003). Lead and lead toxicity in domestic and free living birds. Avian Pathology, 32, 3–13.
- Ellenberg, U. & Mattern, T. (2012). Yellow-eyed penguin – review of population information (Contract 4350 OPP2011-08). Report to the Department of Conservation, 144pp.
- Gartrell, B.D., French, N.P., Howe, L., Nelson, N.J., Houston, M., Burrows, E.A., Russell, J.C. & Anderson, S.H. (2013). First detection of Chlamydia psittaci from a wild native passerine bird in New Zealand. New Zealand Veterinary Journal, 61, 174–176. doi: 10.1080/00480169.2012.740656
- Gill, J.M. & Darby, J.T. (1993). Deaths in yellow-eyed penguins (Megadyptes antipodes) on the Otago Peninsula during the summer of 1990. New Zealand Veterinary Journal, 41, 39–42. doi: 10.1080/00480169.1993.35733
- Graczyk, T.K., Cockrem, J.F., Cranfield, M.R., Darby, J.T. & Moore, P. (1995). Avian malaria seroprevalence in wild New Zealand penguins. Parasite-Journal De La Societe Francaise De Parasitologie, 2, 401–405.
- Ha, H.J., Howe, L., Alley, M.R. & Gartrell, B. (2011). The phylogenetic analysis of avipoxvirus in New Zealand. Veterinary Microbiology, 150, 80–87. doi: 10.1016/j.vetmic.2011.01.011
- Harwood, D.T., Boundy, M., Selwood, A.I., van Ginkel, R., MacKenzie, L. & McNabb, P.S. (2013). Refinement and implementation of the Lawrence method (AOAC 2005.06) in a commercial laboratory: assay performance during an Alexandrium catenella bloom event. Harmful Algae, 24, 20–31. doi: 10.1016/j.hal.2013.01.003
- van Heezik, Y. (1990). Diets of yellow-eyed, fiordland crested, and little blue penguins breeding sympatrically on Codfish Island, New Zealand. New Zealand Journal of Zoology, 17, 543–548. doi: 10.1080/03014223.1990.10422952
- Heikinheimo, A. & Korkeala, H. (2005). Multiplex PCR assay for toxinotyping Clostridium perfringens isolates obtained from Finnish broiler chickens. Letters in Applied Microbiology, 40, 407–411. doi: 10.1111/j.1472-765X.2005.01702.x
- Hellogren, O., Waldenstrom, J. & Bensch, S. (2004). A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium, and Haemoproteus from avian blood. Journal of Parasitology, 90, 797–802. doi: 10.1645/GE-184R1
- Hodge, J., Howe, L. & Ezenwa, V.O. (2015). Identification of novel Theileria genotypes from Grant’s gazelle. International Journal for Parasitology: Parasites and Wildlife, 4, 239–243.
- Knox, G. (1964). The detection of space-time interactions. Applied Statistics, 13, 25–29. doi: 10.2307/2985220
- Kulldorff, M., Heffernan, R., Hartman, J., Assunção, R.M. & Mostashari, F. (2005). A space–time permutation scan statistic for disease outbreak detection. PLoS Medicine, 2, 216–224. doi: 10.1371/journal.pmed.0020059
- Kulldorff, M. & Nagarwalla, N. (1995). Spatial disease clusters: detection and inference. Statistics in Medicine, 14, 799–810. doi: 10.1002/sim.4780140809
- Lock, J.W., Thompson, D.R., Furness, R.W. & Bartle, J.A. (1992). Metal concentrations in seabirds of the New Zealand region. Environmental Pollution, 75, 289–300. doi: 10.1016/0269-7491(92)90129-X
- MacKenzie, L., Beuzenberg, V., Holland, P., McNabb, P., Suzuki, T. & Selwood, A. (2005). Pectenotoxin and okadaic acid-based toxin profiles in Dinophysis acuta and Dinophysis acuminata from New Zealand. Harmful Algae, 4, 75–85. doi: 10.1016/j.hal.2003.12.001
- Mattern, T., Ellenberg, U., Houston, D.M. & Davis, L.S. (2007). Consistent foraging routes and benthic foraging behaviour in yellow-eyed penguins. Marine Ecology Progress Series, 343, 295–306. doi: 10.3354/meps06954
- Mattern, T., Ellenberg, U., Houston, D.M., Lamare, M., Davis, L.S., van Heezik, Y. & Seddon, P.J. (2013). Straight line foraging in yellow-eyed penguins: new insights into cascading fisheries effects and orientation capabilities of marine predators. PLoS One, 8, e84381. doi: 10.1371/journal.pone.0084381
- McConnell, H.M., Gartrell, B.D., Chilvers, B.L., Finlayson, S.T., Bridgen, P.C.E. & Morgan, K.J. (2015). Baseline hydrocarbon levels in New Zealand coastal and marine avifauna. Marine Pollution Bulletin, 94, 290–298. doi: 10.1016/j.marpolbul.2015.02.001
- McKinlay, B. (2001). Hoiho (Megadyptes Antipodes) Recovery Plan 2000–2025 (35). Department of Conservation, Wellington, New Zealand.
- Moore, P.J. (1992). Breeding biology of the yellow-eyed penguin Megadyptes antipodes on Campbell Island. Emu, 92, 157–162. doi: 10.1071/MU9920157
- Moore, P.J. (1999). Foraging range of the yellow-eyed penguin Megadyptes antipodes. Marine Ornithology, 27, 56–58.
- Moore, P.J., Fletcher, D. & Amey, J. (2001). Population estimates of yellow-eyed penguins, Megadyptes antipodes, on Campbell Island, 1987–98. Emu, 101, 225–235. doi: 10.1071/MU00037
- Moresco, K.A., Stallknecht, D.E. & Swayne, D.E. (2010). Evaluation and attempted optimization of avian embryos and cell culture methods for efficient isolation and propagation of low pathogenicity avian influenza viruses. Avian Diseases, 54, 622–626. doi: 10.1637/8837-040309-Reg.1
- Naus, J., (1965). The distribution of the size of the maximum cluster of points on a line. Journal of the American Statistical Association, 60, 532–538. doi: 10.1080/01621459.1965.10480810
- Nie, F., Xiao, L., Zhang, J., He, Q., Fan, J.-W., Li, Y. & Zhang, L.-M. (2008). Comparison of haemolytic activities of venom separations from jellyfish Cyanea capillata and their influencing factors. Academic Journal of Second Military Medical University, 28, 83–86. doi: 10.3724/SP.J.1008.2008.00083
- Puschner, B., St. Leger, J. & Galey, F.D. (1999). Normal and toxic zinc concentrations in serum/plasma and liver of psittacines with respect to genus differences. Journal of Veterinary Diagnostic Investigation, 11, 522–527.
- Robertson, H.A., Dowding, J.E., Elliott, G.P., Hitchmough, R.A., Miskelly, C.M., O’Donnell, C.F.J., Powlesland, R.G., Sagar, P.M., Scofield, R.P. & Taylor, G.A. (2013). Conservation status of New Zealand Birds, 2012. New Zealand Threat Classification Series 4 (p. 22). Wellington: Department of Conservation.
- Roe, W.D., Howe, L., Baker, E.J., Burrows, L. & Hunter, S.A. (2013). An atypical genotype of Toxoplasma gondii as a cause of mortality in Hector’s Dolphins (Cephalorhynchus hectori). Veterinary Parasitology, 192, 67–74. doi: 10.1016/j.vetpar.2012.11.001
- Scheuhammer, A., Braune, B., Chan, H.M., Frouin, H., Krey, A., Letcher, R., Loseto, L., Noël, M., Ostertag, S., Ross, P. & Wayland, M. (2015). Recent progress on our understanding of the biological effects of mercury in fish and wildlife in the Canadian Arctic. Science of the Total Environment, 509–510, 91–103. doi: 10.1016/j.scitotenv.2014.05.142
- Seddon, P.J., van Heezik, Y. & Ellenberg, U. (2013). Yellow-eyed penguin. In P.G. Borboroglu & P.D. Boersma (Eds.), Penguins: Natural History and Conservation (pp. 91–100). Seattle: University of Washington Press.
- Sudakin, D.L., Stone, D.L. & Power, L. (2011). Naphthalene mothballs: emerging and recurring issues and their relevance to environmental health. Current Topics in Toxicology, 7, 13–19.
- Vargas, F.H., Harrison, S., Rea, S. & Macdonald, D.W. (2006). Biological effects of El Nino on the Galapagos penguin. Biological Conservation, 127, 107–114.
- Vargas, F.H., Lacy, R.C., Johnson, P.J., Steinfurth, A., Crawford, R.J.M., Boersma, P.D. & Macdonald, D.W. (2007). Modelling the effect of El Nino on the persistence of small populations: The Galapagos penguin as a case study. Biological Conservation, 137, 138–148.
- Wiener, J.G., Krabbenhoft, D.P., Heinz, G.H. & Scheuhammer, A.M. (2003). Ecotoxicology of mercury. In D.J. Hoffman, B.A. Rattner, G.A. Burton & J. Cairns (Eds.), Handbook of Ecotoxicology 2nd edn (pp. 409–463). Boca Raton, FL: CRC Press.
- Yoon, S.H., Kim, M.-S., Kim, S.H., Park, H.M., Pyo, H., Lee, Y.M., Lee, K.-T. & Hong, J. (2015). Effective application of freezing lipid precipitation and SCX-SPE for determination of pyrrolizidine alkaloids in high lipid foodstuffs by LC-ESI-MS/MS. Journal of Chromatography B, 992, 56–66. doi: 10.1016/j.jchromb.2015.04.007