ABSTRACT
Infectious bursal disease is a severe acute viral disease of young chickens, affecting mainly the B-lymphocytes in the bursa of Fabricius, leading to severe immunosuppression as a result of the death of lymphoid cells. In the bursa infected with infectious bursal disease virus, viral replication is associated with apoptosis of lymphoid cells, inflammatory change and atrophy. Vaccination has appeared to be a crucial factor for control, with live attenuated vaccines being the most used. However, the apoptotic effect of these vaccines on the bursa has not been tested. We determined the apoptotic effect caused by the most used vaccines in local production on the bursa of Fabricius cells and the correlation with histological changes. In this study, it was demonstrated that apoptosis levels in the vaccinated groups were higher than those observed in the non-vaccinated birds leading to the conclusion that the action of the live virus vaccine strains modifies the boundary of the bursa and shapes processes of cell death by apoptosis. In contrast to other studies, the vaccine strains used did not show the phenomenon of bursal atrophy during the experimental period.
Introduction
Infectious bursal disease (IBD), infectious bursitis or Gumboro disease, is an acute and highly contagious viral disease in young chickens, occurring between the third and sixth weeks of life. Infection with IBD virus from the genus Avibirnavirus within the family Birnaviridae, leads to severe immunosuppression (Van den Berg, Citation2000; Mahgoub, Citation2012). Although the B-lymphocytes die partly due to mechanisms involving necrosis, findings indicate that immunosuppression could be the result of the apoptotic death of lymphoid cells (Jungmann et al., Citation2001; Rodríguez-Lecompte et al., Citation2005; Wei et al., Citation2011). The use of live vaccines for disease control and their regular application have been of great help; nevertheless, the apoptotic effects of these strains on the bursa have not yet been clearly determined or described (Lucio & Hitchner, Citation1979; Perozo et al., Citation2008; Müller et al., Citation2012). Vaccines against IBD are commercialized with both live and inactivated viruses. Live virus vaccines are classified according to their range of pathogenicity from mild, intermediate and intermediate plus/hot strains. The aim of this study was to determine the degree of apoptosis in the bursa after vaccination with different types of commercial vaccines. The selected vaccines were considered to be those most used in local production against IBD.
Materials and methods
Chickens
One-day-old broiler chickens of the type Cobb 500, with no sex classification, were divided into four groups of 55 birds each. They were reared with a heat source, bedding, and water and food supply “ad libitum”. The birds were fed on pellets according to age.
Two groups were inoculated with two different vaccines (ST-14 and Lukert Groups). A third group was taken as a control group and was inoculated just with the diluent of one of the vaccines (Diluent Group). The fourth group became the control group without procedures (Control). Ten birds from each group were euthanized at the 3rd, 7th, 14th, 21st and 28th day after inoculation by cervical dislocation as recommended by the Institutional Committee for Use and Care of Experimental Animals, Facultad Ciencias Veterinarias, Universidad de Buenos Aires (CICUAL).
Vaccines
Two different types of vaccine were used according to the most commercialized in Argentinian poultry production (Servicio Nacional de Sanidad Animal – SENASA).
These were strain ST-14: mild intermediate- live virus vaccine, frozen (Merial Laboratories) and Lukert intermediate strain: live virus modified vaccine, produced in SPF chicken embryos and COFALs negative (complement fixation test for avian leucosis) lyophilized (Fort Dodge Laboratories).
These vaccines were administered in only one dose according to specifications for each.
Strain ST-14 was diluted in the supplied diluent and inoculation was under the skin in the neck fold. The Lukert intermediate vaccine was administered in the drinking water.
Evaluation of bursa weight
The birds were weighed and the bursa was removed from each at necropsy. Each bursa was weighed before processing and the bursa to body weight ratio was calculated for each of the inoculated groups and their controls in order to determine the degree of size increase or atrophy of the bursa for each of these groups. The ratio was established as follows: weight of bursa of Fabricius (g) × 100/body weight (g).
Histopathological study of the bursa
Histological sections were prepared from bursae after standard formalin fixation and paraffin embedding procedures and the sections were stained with Mayer haematoxylin–eosin (H&E). Sections from three birds for each experimental group were evaluated under a microscope on the 3rd, 7th, 14th, 21st and 28th days post inoculation. An average of the degree of bursal injury was determined according to ordinal scale or bursa lesion score (BLS) normally used in bursa analysis, ranging from 0 for a normal bursa to 5 for a severely affected bursa (Henry et al., Citation1980).
The degree of inflammatory response (IR) due to the existence of typical cell infiltrates was also included.
Morphological characterization of apoptosis
Haematoxylin and eosin staining
After routine histological staining with Mayer H&E morphological changes were recorded in the affected cells, such as chromatin condensation, nuclear membrane disintegration, the formation of apoptotic bodies, etc. Ten birds per group were observed for each period of time (3rd, 7th, 14th, 21st and 28th days after inoculation). Eighteen medullar fields in each bird were randomly evaluated to obtain an apoptotic score. The score was expressed as the number of cells with positive morphological changes per field. The score of each group was averaged.
TUNEL assay
TUNEL (dUTP nick end labelling) assays were performed by In Situ Cell Detection kit, POD (Roche©, Mannheim, Germany) on three birds per group for each period of time (3rd, 7th, 14th, 21st and 28th days after inoculation). Eighteen medullar fields in each bird were evaluated to obtain an apoptotic score. The score was expressed as the number of TUNEL-positive cells per field. The score of each group was averaged. A light green stain was used as a cytoplasmatic contrast.
Biochemical characterization of the apoptosis
Bax–Bcl 2 as apoptotic mediators
Bursae of both the control and the inoculated birds underwent immunohistochemistry (IHC) assay against Bax–Bcl 2 to determine the implication of the different pathways involved in the apoptotic process. IHC was carried out in the sample groups studied on the 3rd, 14th and 28th days after inoculation. The antibodies used were: anti Bax Clon 6A7 (Sigma-Aldrich Co.©, St.Louis, MO, USA) monoclonal (in mouse) used in a dilution of 1:400 and polyclonal (in rabbit) anti-Bcl-2(C21) SC-783 (Santa Cruz Biotechnology, Inc.©, St.Louis, MO, USA) at a dilution of 1:400. The assay was developed with a DakoCytomation LSAB 2 System-HRP© kit.
Detection of activated caspase-3
Determination through immunohistochemistry of the active caspase 3 (cleaved) as an executive protein within the apoptotic process was carried out on the bursae of the experimental groups on the 3rd, 14th and 28th days post vaccination. An anti-caspase 3 (fraction of IgG) polyclonal antibody of rabbit origin (Sigma-Aldrich Co.©) was used at a dilution of 1:600, and developed through DakoCytomation LSAB 2 System-HRP© kit.
Statistical analysis
The results obtained were statistically analysed through analysis of variance (ANOVA) using InfoStat v.2008/L software. In all cases, normality was verified with the Shapiro test. The level of significance was taken as P < 0.05.
Results
Bursa/body weight ratio
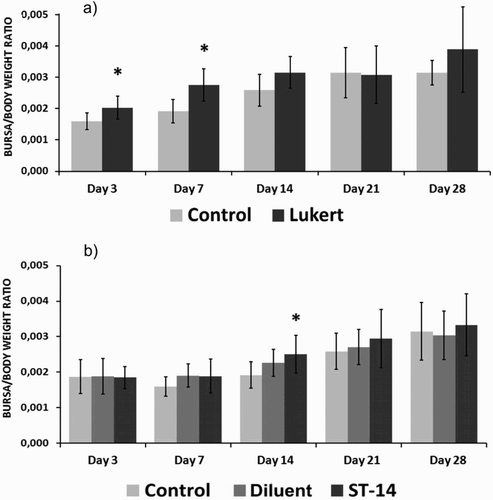
The birds vaccinated with the ST-14 strain and Lukert strain showed slightly higher values of weight ratio than their respective control groups during the sampling days. The separate analysis of each vaccine group and their controls, day by day, revealed that the intermediate Lukert strain showed significant differences on the 3rd and 7th days after inoculation (P < 0.01). As for the mild intermediate ST-14 strain, significant values were revealed only on the 14th day after inoculation (P < 0.04) ().
Histopathology of the bursae
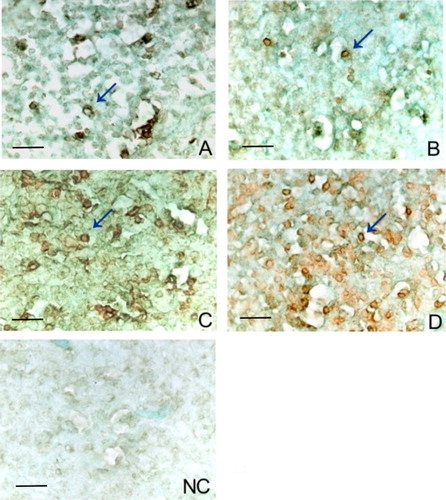
BLS and IR analyses of the bursa for each of the experimental groups revealed a gradual increase in their values after vaccination. Even though the lesions of the vaccinated birds did not exceed grade 3 with severe lymphoid depletion in more than 50% of the follicles, it was in the birds vaccinated with the Lukert intermediate strain where damage appeared to be clearer (). However, the IR proved to be increasing similarly in both vaccinated groups in relation to their controls. From the 14th day post inoculation, the rates became higher, remaining constant up to the 28th day in the case of the ST-14 strain but decreasing at the end of the observation period in the case of the Lukert strain ().
Figure 2. Photomicrographs of tissue lesions in the bursae of birds vaccinated against IBDV (H&E stain). Plicae showing lymphocyte depletion areas (arrow) after vaccination with ST-14 (A) and Lukert (B) strains at 21st day P.I. (Scale bars = 200 µm). IR with infiltrates of heterophils. (arrows) ST-14 (C) and Lukert (D) strains at 28th day P.I. (Scale bars = 50 µm). (E).Control at 21st day P.I. (Scale bar = 200 µm).
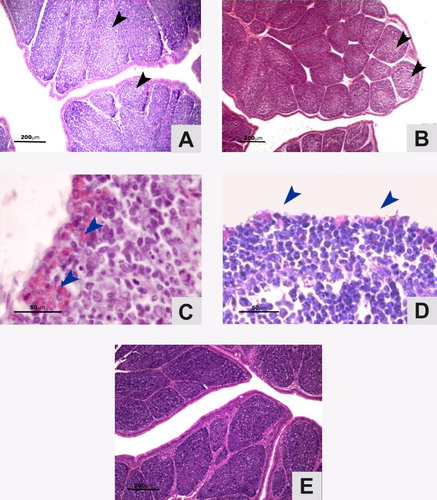
Table 1. Average values for the BLS and IR for each vaccinated group inoculated with ST-14 or Lukert strain and their controls in each of the periods post vaccination.
Haematoxylin and eosin staining
Apoptotic images were observed in each medullar field of all the birds included in the experiment.
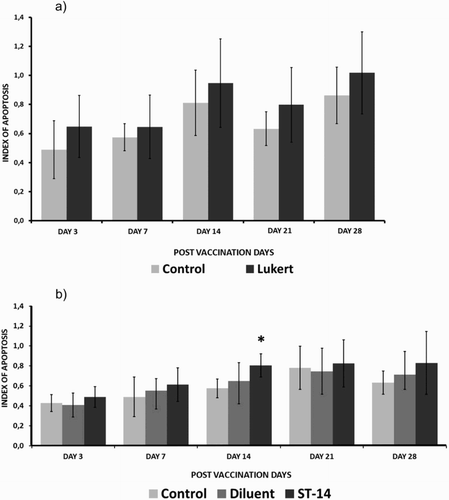
Birds in the groups inoculated with either the mild intermediate strain ST-14 or intermediate Lukert strain showed an increase in the number of apoptotic cells in the samples taken on the 3rd, 7th, 14th, 21st and 28th days after vaccination compared to the non-inoculated controls or those inoculated only with the diluent. Apoptotic cells had a preferential distribution in the medullar area of the bursa follicles.
On all the days of evaluation the apoptotic rate in the vaccinated groups was higher than the controls, reaching the highest values as from day 14 post inoculation. Although the intermediate Lukert strain revealed gradual kinetics growth with a little decline on day 21, results were not significant in comparison to the controls for the same period. Birds vaccinated with the mild intermediate strain ST 14 also showed a gradual increase in the number of apoptotic cells with a rise from day 14, but unlike the Lukert strain on day 14, the comparative rates with the control and the diluent group were statistically significant ().
TUNEL assay
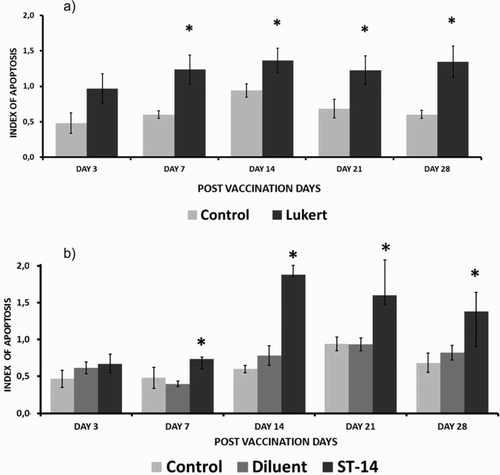
The TUNEL assay results revealed apoptotic cells in each of the four groups analysed (). When the vaccinated groups were compared to the controls (Negative control and Diluent) on the sampling days, they revealed higher rates of apoptosis than those analysed by bright field microscopy after H and E staining.
Figure 4. Marking of apoptotic cell with TUNEL assay at day 14 post inoculation (arrow) (A). Control (B). Diluent (C). Lukert strain (D). ST-14 strain negative control (NC) of the assay. A light green stain was used as a cytoplasmatic contrast. Scale bars = 20 µm.
Both vaccinated groups showed a statistically significant increase in the number of apoptotic cells compared to the non-vaccinated group. Results of the study on the 3rd, 7th, 14th, 21st and 28th days post inoculation showed a gradual increase in the number of apoptotic cells reaching a peak on about the 14th day for both vaccinated groups with highly significant differences (P < 0.001). The intermediate Lukert strain apoptotic index on the 3rd, 7th, 14th, 21st and 28th days post vaccination had a significant increase compared to those of their respective control, while the strain ST-14 3 days post inoculation did not show significant change ().
Biochemical characterization of the apoptosis
Expression of Bax–Bcl-2
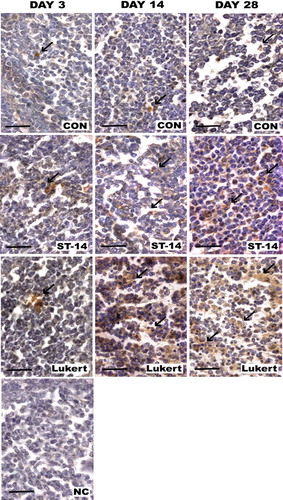
The number of positive markings in the medulla of the vaccinated groups for Bax was far more numerous and intense compared to those of the control group and for each of the sampling days, showing an increase in the marking not only throughout time but also in comparison to the control bursae ().
Figure 6. Bax expression in bursa: Photomicrographs of medullar fields with IHC for Bax comparing days 3, 14 and 28 post vaccination between vaccine strains (ST-14 and Lukert) and the Control group. Haematoxylin staining was used as nuclear contrast. NC, negative control of the assay. Scale bars = 50 µm.
The expression of Bcl-2 was poor or null for all the groups alike, not showing markings either in the inoculated birds or the ones of the control group (data not shown).
Presence of active caspase 3
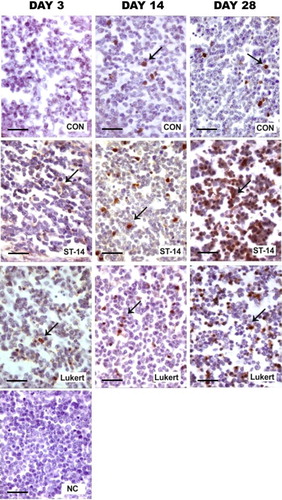
As expected, the presence of active caspase 3 was evidenced in the medulla of all the studied bursae. The number of positive markings in the vaccinated groups showed stronger signal than control ().
Figure 7. Activity of caspase 3: Photomicrographs of medullar fields with IHC for active caspase 3 comparing days 3, 14 and 28 post vaccination between vaccine strains ST-14 and Lukert and the Control group. Haematoxylin staining was used as nuclear contrast. NC, negative control of the assay. Scale bars = 50 µm.
Discussion
This study was initiated to determine whether there was induction of apoptosis in the bursa of Fabricius after vaccination with either of two different vaccine strains. It was shown that apoptotic levels in the vaccinated groups were higher than those observed in the non-vaccinated birds. As the bursa is an organ of maturation of B-lymphocytes, the selection process of the B population is carried out by apoptosis (Lam, Citation1997; Sayegh & Ratcliffe, Citation2000). In the present study the apoptotic basal levels registered in the bursa of Fabricius of the control birds confirm earlier suggestions by others (Nieper et al., Citation1999; Sayegh & Ratcliffe, Citation2000). Although there was apoptosis in each of the bursae of the observed groups, it was in those vaccinated groups where we observed a higher rise in the number of affected cells with significant differences. The live virus Lukert intermediate strain as well as the mild intermediate ST-14 produced a significant increase in the level of apoptosis recorded in the bursae of the inoculated birds.
The presence of the live virus strain used in the formulation of these vaccines produced changes inside the bursa that are similar to the action of the pathogenic virus that leads to the death of lymphocytes through the apoptotic phenomenon. The study confirms observations from earlier studies for cells in culture (Rodríguez-Lecomple et al., Citation2005). Although the presence and replication of the pathogenic virus as well as the vaccine virus are quickly detected inside the bursa (Rautenschlein et al., Citation2007; Schat & Skinner, Citation2008), we have seen that the highest rates of apoptosis (number of apoptotic cells over a total of cells per field) appear gradually. The highest levels were reached at about the 14th day after inoculation, which reveals that it is not only the direct action of the virus in the bursa of Fabricius but (as other studies have observed) also the late post-vaccination effect of the vaccine, and would be a result of the reaction of the inflammatory process associated with the appearance of the virus, thus causing the late increase in the number of apoptotic cells (Khatri & Sharma, Citation2007a, Citationb, Kong et al., Citation2004).
Microscopic examination revealed more damage compatible with the inflammatory processes with time post inoculation. These findings are in agreement with those studies that state that part of the affected cells, mainly the macrophages, are able to release to the environment a set of chemical mediators of the cytokine types with remarkable apoptotic effects (Qureshi, Citation1998; Khatri & Sharma, Citation2007a, Citationb).
In contrast to observations of other authors (Thangavelu et al., Citation1998; Castro et al., Citation2005), we have not found a remarkable atrophy due to the vaccination strains inside the bursa and in the period under study. On the contrary, the indicators that relate the weight of the bursa to the body weight of the chicken reveal signs of bursa enlargement coincidental with the inflammatory phenomena seen at the macroscopic level.
Marks for mediators of the apoptotic process such as proteins Bax, Bcl-2 and active caspase-3 had kinetics coincidental with the observed phenomenon, whereas anti-apoptotic proteins such as Bcl-2 did not show expression by IHC. This increase in positive Bax cells coincided with the results obtained by the TUNEL assay; pro-apoptotic proteins such as Bax and effector proteins such as active caspase 3 did show this with higher marks not only in the vaccinated groups but also in much later stages post vaccination.
In conclusion vaccination with a partially attenuated virus corresponding to the intermediate or mild intermediate strains of infectious bursitis or Gumboro disease produces an increase in the number of apoptotic cells in the medullar areas of the bursa of Fabricius together with its enlargement and development of an inflammatory phenomenon. As there are a large number of lymphoid cells lost after vaccination, we have still to clarify questions such as whether this phenomenon might affect the normal functioning of the immunological system of the chicken.
Finding that injury at the cellular level of the bursa is significant, warning must be given about the use of these strains and new strategies of vaccination treatment probably suggested, such as recombinant vaccines, in ovo vaccination or other ways in which the risk of harm and/or reversion is diminished (Rautenschlein et al., Citation2003; Perozo et al., Citation2008; Taghavian et al., Citation2013).
Considering that our country registers an extensive use of intermediate vaccine strains in poultry production, it is essential to control the effects of the vaccines used and check if these effects are a cause of immunosuppression that could make way for other diseases and consequently affect production at an economic level.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Castro, M., Saume, E., Díaz, C. & García, J. (2005). Producción de anticuerpos y cambios morfológicos de la bolsa de fabricio en pollos vacunados con cepas intermedia e intermedia plus de la enfermedad de gumboro. Veterinaria Tropical, 29–30, 83–98.
- Henry, C.W., Brewer, R.N., Edgar, S.A. & Gray, B.W. (1980). Studies on infectious bursal disease in chickens: 2. Scoring microscopic lesions in the bursa of Fabricius, thymus, spleen, and kidney in gnotobiotic and battery reared White Leghorns experimentally infected with infectious bursal disease virus. Poultry Science, 59, 1006–1017. doi: 10.3382/ps.0591006
- Jungmann, A., Nieper, H. & Muller, H. (2001). Apoptosis is induced by infectious bursal disease virus replication in productively infected cells as well as in antigen-negative cells in their vicinity. Journal of General Virology, 82, 1107–1115. doi: 10.1099/0022-1317-82-5-1107
- Khatri, M. & Sharma, J.M. (2007a). Modulation of macrophages by infectious bursal disease virus. Cytogenetic and Genome Research, 117, 388–393. doi: 10.1159/000103202
- Khatri, M. & Sharma, J.M. (2007b). Replication of infectious bursal disease virus in macrophages and altered tropism of progeny virus. Veterinary Immunology and Immunopathology, 117, 106–115. doi: 10.1016/j.vetimm.2007.02.002
- Kong, L.L., Omar, A.R., Hair-Bejo, M., Aini, I. & Seow, H.F. (2004). Comparative analysis of viral RNA and apoptotic cells in bursae following infection with infectious bursal disease virus. Comparative Immunology, Microbiology & Infectious Diseases, 27, 433–443. doi: 10.1016/j.cimid.2004.01.006
- Lam, K. M. (1997). Morphological evidence of apoptosis in chickens infected with infectious bursal disease virus. Journal of Comparative Pathology, 116, 367–377. doi: 10.1016/S0021-9975(97)80053-9
- Lucio, B. & Hitchner, S.B. (1979). Infectious bursal disease emulsified vaccine: effect upon neutralizing-antibody levels in the dam and subsequent protection on the progeny. Avian Diseases, 23, 466–478. doi: 10.2307/1589577
- Mahgoub, H. A. (2012). An overview of infectious bursal disease. Archives of Virology, 157, 2047–2057. doi: 10.1007/s00705-012-1377-9
- Müller, H., Mundt, E., Eterradossi, N. & Islam, M.R. (2012). Current status of vaccines against infectious bursal disease. Avian Pathology, 41, 133–139. doi: 10.1080/03079457.2012.661403
- Nieper, H., Teifke, J.P., Jungmann, A., Löhr, C.V. & Müller, H. (1999). Infected and apoptotic cells in the IBDV-infected bursa of Fabricius, studied by double-labelling techniques. Avian Pathology, 28, 279–285. doi: 10.1080/03079459994777
- Perozo, F., Villegas, P., Estevez, C., Alvarado, I.R., Purvis, L.B. & Williams, S. (2008). Protection against infectious bursal disease virulent challenge conferred by a recombinant avian adeno-associated virus vaccine. Avian Diseases, 52, 315–319. doi: 10.1637/8122-100207-ResNote.1
- Qureshi, M. A. (1998). Role of macrophages in avian health and disease. Poultry Science, 77, 978–982. doi: 10.1093/ps/77.7.978
- Rautenschlein, S., von Samson-Himmelstjerna, G. & Haase, C. (2007). A comparison of immune responses to infection with virulent infectious bursal disease virus (IBDV) between specific-pathogen-free chickens infected at 12 and 28 days of age. Veterinary Immunology and Immunopathology, 115, 251–260. doi: 10.1016/j.vetimm.2006.11.002
- Rautenschlein, S., Yeh, H.Y. & Sharma, J.M. (2003). Comparative immunopathogenesis of mild, intermediate, and virulent strains of classic infectious bursal disease virus. Avian Diseases, 47, 66–78. doi: 10.1637/0005-2086(2003)047[0066:CIOMIA]2.0.CO;2
- Rodríguez-Lecompte, J.C., Niño-Fong, R., Lopez, A., Frederick Markham, R.J. & Kibenge, F.S.B. (2005). Infectious bursal disease virus (IBDV) induces apoptosis in chicken B cells. Comparative Immunology, Microbiology & Infectious Diseases, 28, 321–337. doi: 10.1016/j.cimid.2005.08.004
- Sayegh, C.E. & Ratcliffe, M.J.H. (2000). Perinatal deletion of B cells expressing surface Ig molecules that lack V(D)J-encoded determinants in the bursa of Fabricius is not due to intrafollicular competition. The Journal of Immunology, 164, 5041–5048. doi: 10.4049/jimmunol.164.10.5041
- Schat, K. & Skinner, M. 2008. Avian immunosuppressive diseases and immune evasion. In F. Davison, B. Kasper, & K. Schat (Eds.). Avian Immunology 1st edn (pp. 299–322). London: Academic Press.
- Taghavian, O., Spiegel, H., Hauck, R., Hafez, H.M., Fischer, R. & Schillberg, S. 2013. Protective oral vaccination against infectious bursal disease virus using the major viral antigenic protein VP2 produced in Pichia pastoris. PLoS ONE 8, e83210. doi: 10.1371/journal.pone.0083210
- Thangavelu, A., Raj, G.D., Elankumaran, S., Manohar, B.M., Koteeswaran, A. & Venugopalan, A.T. (1998). Pathogenicity and immunosuppressive properties of infectious bursal disease virus field isolates and commercial vaccines in India. Tropical Animal Health and Production, 30, 167–176. doi: 10.1023/A:1005059619825
- Van den Berg, T.P. (2000). Acute infectious bursal disease in poultry: a review. Avian Pathology, 29, 175–194. doi: 10.1080/03079450050045431
- Wei, L., Zhu, S., Ruan, G., Hou, L., Wang, J., Wang, B. & Liu, J. (2011). Infectious bursal disease virus-induced activation of JNK signaling pathway is required for virus replication and correlates with virus-induced apoptosis. Virology, 420, 156–163. doi: 10.1016/j.virol.2011.08.027