ABSTRACT
Enterococcus faecalis is the major pathogen found in field cases of amyloid arthropathy in chickens. Given the need for a better understanding of the virulence mechanisms of the causative strains, the embryo lethality assay (ELA) is proposed in the present study as a model to evaluate the virulence of E. faecalis strains, specifically the pathogenic avian strain K923/96, which was previously related with amyloid arthropathy. Hence, 0.2 ml of five doses of the cited strain (from 2.5 to 2500 colony-forming units (CFU) per ml) were inoculated into the allantoic cavity of 10-day-old embryos. The embryo mortality rate (EMR) was determined by daily candling of the eggs over a period of seven days and based on this information the median lethal dose (LD50) was calculated. The ELA was repeated four times on a sample of 3443 eggs. The infectious dose showed a significant effect on the EMR. The EMR with the doses of 2.5, 5, 25, 250 and 2500 CFU/ml was 43%, 45%, 63%, 90% and 93%, respectively. The estimated dose at LD50 was 6.6 CFU/ml. As expected, the higher the infectious dose, the greater the EMR and the lower the embryo survival time. The highest EMR was recorded after three and four days post-inoculation in all doses. In conclusion, these results can be used as a basis for further researches on the E. faecalis virulence. In order to corroborate its model capacity to predict the virulence of this bacterium, more ELAs with different E. faecalis strains are required.
Introduction
Systemic amyloid-A amyloidosis is known in chickens as amyloid arthropathy (AA). This disease is characterized by AA amyloid depositions in the knee and hock joint. Chickens show reduced weight gain and lameness, which compromises the welfare of chickens. AA affects about 20–30% of all European chicken flocks, resulting in substantial economic losses (Landman et al., Citation1998a).
AA is associated with chronic infections, which appear to be induced by arthropathic and amyloidogenic Enterococcus faecalis (E. faecalis) strains (Landman et al., Citation1997). This bacterium is considered as an opportunistic pathogen because it constitutes a part of the endogenous intestinal microflora of humans and birds, but can nonetheless become pathogenic and infect hosts with a deficient immune system (Sava et al., Citation2010). As recently reviewed by Blanco et al. (Citation2016), E. faecalis is gaining more attention, not only because it has been associated with several poultry diseases (Gregersen et al., Citation2010), but also because of its ability to acquire antimicrobial resistance and its capacity to transfer pathogenicity islands, which encode virulence genes (Coburn et al., Citation2007). Consequently, E. faecalis could acquire the ability to survive in an environment in which antimicrobial agents are heavily used and, therefore, the enterococcal infection treatments are increasingly limited (Hayes et al., Citation2003). Hence, a better understanding of the virulence mechanisms of the E. faecalis strains is needed to develop preventive measures.
In order to study the role of E. faecalis on the pathogenesis of AA, chicken lethality assay is the most used animal model. AA has been induced experimentally with E. faecalis in one-day-old chickens (Landman, Citation1999) and in six-week-old brown layer chickens (Landman et al., Citation1997; Landman et al., Citation1998b; Landman et al., Citation1999b; Landman et al., Citation1999a). However, relatively high costs, complexity and ethical considerations limit the application of such challenge tests. Therefore, it seems appropriate to use the chicken embryo lethality assay (ELA), which can provide an alternative tool to study the virulence of different strains in a faster, more sensitive, less expensive, more specific and relatively simple form (Wooley et al., Citation2000; Gibbs et al., Citation2003; Gibbs & Wooley, Citation2003; Oh et al., Citation2012; Seo et al., Citation2013).
Chicken ELA has been proposed to determine the degree of virulence of isolated fungi (Jacobsen et al., Citation2010), and bacteria such as Escherichia coli (Wooley et al., Citation2000; Gibbs et al., Citation2003; Gibbs & Wooley, Citation2003; Montgomery et al., Citation2005; Oh et al., Citation2012), Francisella spp. (Nix et al., Citation2006), Yersinia enterocolítica (Townsend et al., Citation2008), Campylobacter jejuni (Stewart-Tull et al., Citation2009), Staphylococcus aureus (Polakowska et al., Citation2012), Riemerella anatipestifer (Seo et al., Citation2013) and Listeria monocytogenes (Andersson et al., Citation2015). In the case of Enterococcus spp., Borst et al. (Citation2014) reported that the ELA is able to differentiate between virulent and avirulent strains, whose genotype has been closely related to each other through pulsed-field gel electrophoresis. Rudolph (Citation2004) reported that different E. faecalis strains produced different degrees of mortality when the embryos were infected in the allantoic cavity (AC) with a single infectious dose (2500 colony-forming units (CFU)/ml). Furthermore, they were able to compare and confirm the results obtained by the ELA with the results obtained by the chicken lethality assay in a following experiment, where six-week-old chicks were infected with an infectious dose of 1010 CFU of two E. faecalis strains tested previously in embryos.
According to the revised literature cited above, it seems necessary to develop a methodology in accordance with the isolate types and the purpose of the inoculation, taking into account that the embryo mortality rate (EMR) produced during an ELA is influenced by several factors such as:
The source of fertilized eggs and good embryo quality, which are necessary to ensure that the inoculation is the only cause of mortality (Wooley et al., Citation2000; Gibbs et al., Citation2003; Gibbs & Wooley, Citation2003).
The age of the embryos at inoculation, since strains’ virulence appears to differ based on embryonic development (Seo et al., Citation2013).
The route of infection, which depends largely on the isolate type and the purpose of the inoculation (Wooley et al., Citation2000).
The infectious dose, which appears to be related to the embryo survival time and rate (Nix et al., Citation2006).
Therefore, the main goal of this study was the evaluation of the ELA as a diagnostic model that could be used to establish the pathogenicity of E. faecalis strains cultured from field samples of AA. Of particular interest was to assess the median lethality dose (LD50), which is defined as the dose required to kill half of the embryos at a defined time for a tested population, and the virulence of a well-known pathogenic avian E. faecalis strain (K923/96), which has been previously related with avian amyloidosis (Rudolph, Citation2004; Petersen et al., Citation2009) and which will be used as the reference strain in further analyses.
Materials and methods
Ethical statement
All experiments were performed in compliance with the German animal protection law (TierSchG). The trials with fertile commercial chicken eggs were finished four days prior to hatching, on developmental day 17 at the latest.
Sample
A total of 3443 eggs of White Layers at an age of 47–51 weeks were used for the different trials (see for further details). The tested flocks were free from infections such as Salmonella and Mycoplasma. Only first quality hatching eggs were used, and soiled eggs as well as eggs with hairline cracks were discarded. Eggs were stored at 15°C for a maximum of four days prior to incubation and incubated in an auto-rotating egg incubator at 37.8°C and 52–56% relative humidity.
Table 1. Characteristics of the trials I–IV carried out in order to evaluate the ELA with the E. faecalis strain K923/96.
Experimental design
The overall scope of this study included four trials (I–IV) that are illustrated in . White fertilized eggs of a commercial layer programme of Lohmann Tierzucht GmbH were inoculated via the AC after 10 days of incubation. A single known and well-characterized pathogenic avian E. faecalis strain K923/96 (Rudolph, Citation2004; Petersen et al., Citation2009) was used in all these trials. Different doses of the E. faecalis strain mentioned above were used in the present study in order to determine the LD50. As shown in , the doses used in trial I were reduced in the following trials due to the high mortality recorded with the higher doses. In addition to the infected embryos, there was always one control group in each trial, which was inoculated in the AC with 0.2 ml of sterile phosphate-buffered saline (PBS).
Inoculum
The E. faecalis strain K923/96 isolated from the amyloidogenic joint of a brown layer, which was already used by Rudolph (Citation2004) and Petersen et al. (Citation2009), was used in all trials. The preparation of the inoculum was applied in accordance with the following protocol (Rudolph, Citation2004).
The bacteria, which were preserved in a CRYOBANK™ tube, were recovered by removing the cryobank tube from the freezer. Sterile forceps were used to roll one bead on a sheep blood agar plate. The agar plate was incubated aerobically for 24 h at 37°C. After incubation, colonies were scraped off and a suspension of 10 ml was prepared in PBS. This suspension was placed in a photometer, set at a wavelength of 650 nm. To obtain a suspension around 5 × 107 CFU/ml (culture I), it was adjusted to an absorbance of approximately 0.164 ± 0.01. To adjust the suspension, 100 µl of the culture I were added to 10 ml of PBS (culture II). For the culture III another 50 µl of the culture II were added to 10 ml of PBS (dilution 1:1 with an infectious dose of 2500 CFU/ml). From this dose, different dilutions were obtained: 1/10, 1/100, 1/500 and 1/1000 with infectious doses of 250, 25, 5 and 2.5 CFU/ml, respectively (). Then, by using an inoculation volume of 0.2 ml/embryo, 500, 50, 5, 1 and 0.5 CFU/embryo were inoculated, respectively. However, Rudolph (Citation2004) reported that the number of colonies counted in their experiments could vary between 500 and 5000 CFU/ml. Therefore, in order to confirm that the infectious dose was correctly prepared, the number of CFU contained in each dose was verified by viable count on a sheep blood agar plate. For this purpose, 0.1 ml of inoculum per dose was incubated aerobically. Viable counts were determined two times per dose. After 24 h at 37°C the exact number of bacteria grown was counted with a traditional click-counter. The real number of CFU contained in each infectious dose is shown in .
Embryo lethality assay
According to the previously described methods for AC inoculation of chicken embryos, the ELA was assayed as follows.
Prior to inoculation, the unfertilized eggs and eggs with dead embryos were removed by candling. Hatching eggs, which had been incubated for 10 days and were in good condition, were inoculated after disinfection of the egg surface with Bacillol AF (Bode Chemie GmbH, Hamburg, Germany). Each egg was opened carefully at the blunt end with a punch and inoculated with a disposable sterile syringe into the AC either with 0.2 ml of bacterial suspension or with 0.2 ml of PBS in the case of the control group. All holes were sealed with wood glue and the embryos returned to incubation at 37.8°C for another seven days. The incubated eggs were candled every 24 h for seven days and embryonic mortalities were daily recorded per group. The number of dead embryos was used to classify the pathogenicity of E. faecalis according to the infectious dose.
At the end of each trial, at 17 days of incubation, all remaining embryos were stored for 48 h at 4°C.
Macroscopic lesions and re-isolation
Random selections of dead, infected embryos, as well as some surviving embryos at the end of the trials, were used for further examinations. After disinfection of the egg surface with Bacillol AF (Bode Chemie GmbH), the eggshell was opened with sterile material. The embryo was carefully separated from its surrounding membranes in order to evaluate macroscopic tissue changes in embryos caused by E. faecalis. In addition, smear samples from the allantoic fluid of some dead embryos were cultivated on a blood plate for 24 h at 37°C in order to re-isolate the E. faecalis strain.
Statistical analysis
Statistical analyses of mortality data were carried out by applying a linear logistic model for repeated measurements with a binary response variable, which was modelled as a binomial random variable (yi). The dependent variable (yi) can take the value 1 with a probability for embryonic mortality πi or the value 0 with a probability to survive of 1 – πi. The logistic model uses a link function g (μi), linking the expected value to the linear predictors ηi. The logit link function is defined by log [πi/(1 – πi)] = ηi, where πi is the probability of mortality until the end of the experiment at 17 days of incubation. The data were then analysed with the GLIMMIX procedure (SAS Institute Inc., Citation2011) using the following generalized linear model (Littell et al., Citation1999):where ηij denotes the linear predictor, πij is a binary outcome (i.e. probability of embryonic mortality), μ is the overall mean effect, αi is the fixed effect of the infectious dose (from 2.5 to 2500 CFU/ml) and ßj is the random effect of repeated measurement of trial. Least squares means (LSMEANS) were estimated on the logit scale and then back-transformed using the inverse link function π = exp(xß)/[1 + exp(xß)] to the original scale (probability) applying the LSMEANS statement. Significant differences between LSMEANS were tested using a t-test procedure by inclusion of the DIFF option in the LSMEANS statement. Differences were considered significant when P < 0.05.
Different statistical models using different probability distributions, normal, logistic and extreme value distribution, were tested in order to compare its fit statistics for the infectious dose. Underlying normal distribution using the coefficient of determination was found to be the best fit (results are not shown) and therefore was applied to estimate the LD50.
The LD50 was estimated by the SAS PROBIT procedure (SAS Institute Inc., Citation2011) using the maximum likelihood method of regression parameters. Since the response Y is binary, the probit equation is:where p is the probability of a response, C is the natural response rate, F is the cumulative distribution function, x′ is the vector of explanatory variables and ß is the vector of parameter estimates. The response rate was calculated using the INVERSECL statement option by applying the following formula:
The course of embryonic survivability over the seven post-inoculation (p.i.) days within each treatment group was illustrated according to the Kaplan–Meier method (survival analysis) by applying the LIFETEST procedure of SAS System 9.3 (SAS Institute Inc., Citation2011) and using the following model:where Ŝ(t) is the survivor function and t is the lifetime of a randomly selected experimental unit. For each j: tj ≥ t, let
represent the different event times. nj is the number of individuals at risk just prior to ti, and dj is the number of individuals that die at time tj.
Results
Embryo lethality assay
The ELA showed a different EMR according to the infectious dose. The infectious dose showed a significant effect (P < 0.0001) on the EMR. As shown in , the infectious doses of 250 and 2500 CFU/ml used in the trial I resulted in a high embryonic mortality of more than 90% lethality after seven days p.i. and did not differ significantly from each other. Therefore, neither of these doses were used in the following trials in order to avoid a massive embryonic mortality that would not allow discrimination between the E. faecalis strains. The dose of 25 CFU/ml produced an average EMR of 63%, which was significantly different than all other used doses. The infectious dose of 5 CFU/ml was added into trials II, III and IV ( and ) in order to estimate the lethal effect of the low infectious dose and calculate the proper LD50. The average EMR estimated for the doses of 5 and 2.5 CFU/ml was 45% and 43%, respectively, and did not differ significantly from each other. Moreover, in trial III a higher embryonic mortality was reported with the infectious dose of 2.5 CFU/ml (64%) as compared to the infectious dose of 5 CFU/ml (51%).
Table 2. Percentage of chicken embryo mortality with increasing infectious dose of the E. faecalis strain K923/96 following inoculation of 0.2 ml into the AC.
The average EMR of the control group was 2.5%, with variation from 0% to 7%. Thus, these low mortalities in the control group confirm the appropriate environment in the incubator during the trial, as well as the low negative impact of the injection. Therefore, the mortalities obtained in the different groups can be unequivocally associated to the effect of the infectious doses. The control group differed significantly from all other infectious doses.
Median lethality dose (LD50)
shows probit analysis on infectious dose of E. faecalis strain K923/96. The results of the dose level effect on the embryonic mortality (, left) indicate a mean tolerance for the embryos inoculated with the E. faecalis strain K923/96 of 6.6 CFU/ml, i.e. the dose corresponding to an embryonic mortality probability of 0.5 (LD50) was 6.6 CFU/ml, with a 95% confidence interval of 3.5 and 11.4 CFU/ml. It can be observed that infectious doses of this strain above 100 CFU/ml (with a 95% confidence interval of 45 and 500 CFU/ml) can produce 75% or greater embryonic mortality.
Figure 1. Probit analysis on infectious dose of E. faecalis strain K923/96. On the left, the dose level effect on the embryonic mortality and, on the right, the distribution dependent on the dose.
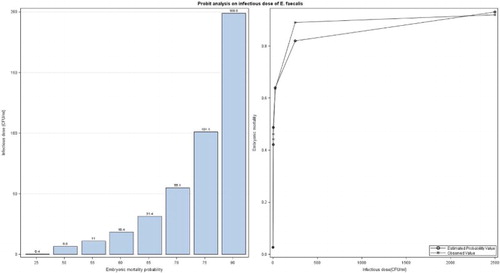
on the right shows the relationship between infectious doses, observed embryonic mortality values, and estimated probability values of the E. faecalis strain K923/96. Embryonic mortality produced with the infectious doses of 2.5 and 250 CFU/ml was higher than the estimated probability. However, the embryonic mortality observed with the infectious doses of 5 and 2500 CFU/ml was lower than the estimated probability. The observed and estimated embryonic mortality with the infectious dose of 25 CFU/ml was the same.
Embryo survival
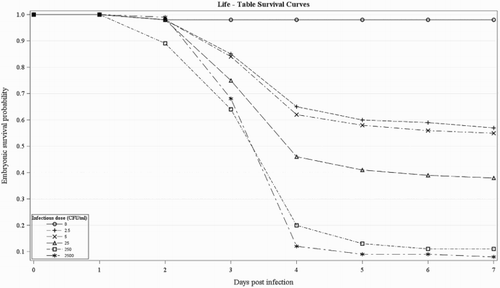
The survival curve for embryos infected with the E. faecalis strain K923/96 is presented in . Higher infectious doses of this strain were significantly more virulent than the lower doses (P < 0.0001). The highest embryonic mortality occurred three and four days p.i. for all doses. As expected, the higher the infectious dose, the lower the embryonic survival time, i.e. the embryos infected with the infectious doses of 250 and 2500 CFU/ml had less survival probability at day 3 p.i. than the embryos infected with doses of 25, 5 and 2.5 CFU/ml. As shown in , after four day p.i. the embryonic mortality declined substantially.
Figure 2. Survival curves of 10-day-old chicken embryos inoculated into the AC with different infectious doses of the E. faecalis strain K923/96.
Around 98% of the embryos in the control group survived until the end of the experiment duration.
Macroscopic lesions
No lesions were observed in the embryos of the control group. However, the embryos infected with E. faecalis succumbed to bacterial infection. Malformed and underdeveloped embryos were observed in all treated groups. Bodies of the analysed infected embryos showed profound subcutaneous oedema, cranial and skin haemorrhages and loss of the plumage regardless of the infectious dose. Therefore, a clear relationship between the embryo lesions and the respective infectious dose was not observed. It should be noted that some dead and surviving infected embryos at the end of the trials did not show any visible signs.
Re-isolation
Smear samples from the allantoic fluid of random analysed dead embryos as well as from surviving embryos were taken in order to re-isolate the E. faecalis strain. The bacterial counts of the infected embryos were always positive and their allantoic fluid contained more CFU/ml compared to the infectious doses administered to the embryos, although some of them did not show any signs or abnormalities (data not shown). In the case of the control group, the bacterial count was negative for a analyses.
Discussion
Based on the revised literature (Landman et al., Citation1999b; Wooley et al., Citation2000; Gibbs et al., Citation2003; Gibbs & Wooley, Citation2003; Rudolph, Citation2004; Montgomery et al., Citation2005; Townsend et al., Citation2008; Stewart-Tull et al., Citation2009; Jacobsen et al., Citation2010; Oh et al., Citation2012; Polakowska et al., Citation2012; Seo et al., Citation2013; Borst et al., Citation2014) an ELA was carried out as a diagnostic model to establish the pathogenicity of a single E. faecalis strain.
The results on the use of SPF embryos in the ELA obtained by Borst et al. (Citation2014) with Enterococcus cecorum showed that the SPF embryos are as susceptible as normal embryos, suggesting that SPF embryos have no innate resistance to bacterial infection. Therefore, in the present study no SPF embryos were used. Additionally, only embryos of the best quality were used in order to avoid biased results, i.e. to ensure that the inoculation is the only cause of mortality (Wooley et al., Citation2000).
According to Jacobsen et al. (Citation2010) and Seo et al. (Citation2013), the embryo age at inoculation has an influence on the embryonic lethality rate. The younger the embryos (seven days old), the more susceptible they were to the virulent strains compared to embryos at later stage of embryonic development (13 and 15 days old) when they were inoculated with R. anatipestifer (Seo et al., Citation2013) and Aspergillus fumigatus (Jacobsen et al., Citation2010). However, the greatest differences in EMR between the virulent and avirulent R. anatipestifer strains were most evident in 10-day-old chicken embryos (Seo et al., Citation2013). Therefore, for the present study 10-day-old embryos were used.
The AC route was chosen as the method of inoculation because other administration routes like yolk sac route and on the egg albumen with E. faecalis (Landman et al., Citation1999b), as well as the infection on the chorioallantoic membrane with C. jejuni (Stewart-Tull et al., Citation2009) produced massive embryonic mortality, which would not allow a clear differentiation between the E. faecalis strains or the estimate of the LD50. Landman et al. (Citation1999b) studied the induction of AA and its possible routes of infection through chicken embryo infections with E. faecalis. These authors observed that the dipped fertilized eggs and the inoculation of the air chamber with E. faecalis were ineffective, the inoculation of egg albumen hardly produced any embryonic deaths; on the other hand, yolk sac inoculation resulted in massive mortality within two days. Additionally, according to the results of Wooley et al. (Citation2000) and Seo et al. (Citation2013), the differentiation among avirulent and virulent strains of avian E. coli and R. anatipestifer, respectively, is possible and more effective through the AC route than into the yolk sac or on the chorioallantoic membrane, respectively.
According to Landman (Citation1999) and Ciftci and Diker (Citation2009), regardless of the route of administration, the infectious dose of E. faecalis in a chicken lethality assay also plays an important role in the development of avian amyloidosis. Landman (Citation1999) reported that the pathological and clinical lesions of AA can be reproduced through inoculation with high doses of E. faecalis. Ciftci and Diker (Citation2009) observed that arthritis formation in chickens inoculated with E. faecalis depends on the infectious dose independent of the inoculation route. Besides, Nix et al. (Citation2006) reported that embryos inoculated with higher infectious doses of Francisella tularensis strains (around 2.3 × 104 CFU) showed lower survival time and rate than those inoculated with lower infectious doses (around 1.3 × 101 CFU). Hence, different infectious doses of the E. faecalis strain K923/96 were used in the present study in order to estimate the LD50 of this strain in chicken embryos.
As shown in , our results showed a clear relationship between infectious dose and EMR in accordance with the results obtained by Seo et al. (Citation2013) and Borst et al. (Citation2014), who observed higher EMR with higher infectious doses than lower infectious doses. The EMR produced with the infectious dose of 2500 CFU/ml used in the present study (93%) is consistent with the values reported by Rudolph (Citation2004), who observed an EMR between 90% and 100% with the same strain and infectious dose. This indicates that the ELA results of this study can be considered reliable.
The estimated doses at LD50 for the inoculation of chicken embryos with the E. faecalis strain K923/96 was 6.6 CFU/ml (). This result is the first to report the appropriate infectious dose of an E. faecalis strain to inoculate these chicken embryos lines. Borst et al. (Citation2014) reported that the lowest dose of E. cecorum strains able to produce around 50% embryonic mortality was 103 CFU/ml, and Nix et al. (Citation2006) observed that infectious doses of F. tularensis from 30 to 200 CFU produced 100% embryonic mortality. This observation suggests the importance to determine an appropriate infectious dose according to the type of isolate and probably the strain.
In addition, Nix et al. (Citation2006) reported that the embryo survival time is usually correlated with the infectious dose; the lower the infectious dose, the greater the survival time of the embryos, which was confirmed in this study ().
In accordance with the results obtained by Wooley et al. (Citation2000) and Gibbs et al. (Citation2003), our results showed a fast decrease in survival rate from two to four days p.i., whereas the survival rate decreased more slowly afterwards, as shown in . The lowest embryonic survival probability was observed at three and four days p.i. in all doses, which confirms the results of Wooley et al. (Citation2000), Gibbs et al. (Citation2003), Gibbs and Wooley (Citation2003), Stewart-Tull et al. (Citation2009), Seo et al. (Citation2013) and Borst et al. (Citation2014) with other bacterial strains.
Rudolph (Citation2004) confirmed the ELA results with the chicken infection challenge model. Both models have the ability to distinguish among the virulence of different strains, although there are more deaths in the ELA model compared to the chicken challenge study. This author reported that 2500 CFU/ml of the E. faecalis strain K923/96 (the same strain used in the present study) produced between 90% and 100% embryonic mortality in the ELA while no deaths in the chicken model (1010 CFU/chicken) were reported, although 66% of the chickens showed a growth decline and swelling of the joints. These mortality differences may be due to the fact that the embryos succumb to bacterial infection and in part do not withstand the infection while the chickens are able to survive (Gibbs et al., Citation2003; Gibbs & Wooley, Citation2003; Borst et al., Citation2014).
The embryos in this study also succumbed to E. faecalis infection. They suffered sepsis, cutaneous haemorrhages and subcutaneous oedema like the infected embryos in the study of the authors mentioned above. Besides, Wooley et al. (Citation2000) observed that avirulent E. coli strains produced fewer lesions than virulent strains, which could not be confirmed in this study due to the use of only one single strain. All dead infected embryos in our study showed the same signs regardless of the infectious dose and, therefore, no relationship between the embryo lesions and the infectious doses was observed.
No lesions and no re-isolates were observed in the embryos of the control group, thus indicating that the experiment was reliable and the embryonic mortality was due to the infection and not due to external effects. On the contrary, the re-isolation of E. faecalis in all analysed infected embryos was always positive, although some of these embryos did not show any visible signs. Besides, an increase in the viable count of the allantoic fluid of dead embryos was observed compared to the initial number of CFU/ml contained in each infectious dose, confirming the results obtained by Wooley et al. (Citation2000), Rudolph (Citation2004), Townsend et al. (Citation2008) and Stewart-Tull et al. (Citation2009). Even an increase in the viable count was observed in surviving embryos, which is in accordance with the results obtained by Rudolph (Citation2004) and Townsend et al. (Citation2008). Therefore, the differences in bacterial growth after the inoculation cannot explain the differences in EMR because the number of CFU can fluctuate and not produce clear differences in the lethality, which is consistent with the results of Wooley et al. (Citation2000) with E. coli.
It should be taken into account that the EMR produced in the ELA depends on the dose. It is therefore important to determine an appropriate infectious dose which should not be too high to avoid a massive embryonic mortality. In conclusion, the methodology used in the present study, as well as the obtained LD50 of the E. faecalis strain K923/96, can be used as reference in further ELAs on E. faecalis virulence.
In order to corroborate the ELA capacity for discriminating E. faecalis strains, more studies with different strains are required.
Acknowledgements
We gratefully acknowledge the technical assistance of the laboratory staff of Lohmann Tierzuch GmbH (Cuxhaven, Germany). We also wish to thank the editor and the anonymous reviewers for positive inputs and suggestions.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Ana E. Blanco http://orcid.org/0000-0001-8949-5035
References
- Andersson, C., Gripenland, J. & Johansson, J. (2015). Using the chicken embryo to assess virulence of Listeria monocytogenes and to model other microbial infections. Nature Protocols, 10, 1155–1164. doi: 10.1038/nprot.2015.073
- Blanco, A.E., Barz, M., Icken, W., Cavero, D., Mazaheri, A., Voss, M., Schmutz, M. & Preisinger, R. (2016). Twenty years of amyloid arthropathy research in chickens. World’s Poultry Science Journal, 72, 495–508. doi: 10.1017/S0043933916000453
- Borst, L.B., Suyemoto, M.M., Keelara, S., Dunningan, S.E., Guy, J.S. & Barnes, H.J. (2014). A chicken embryo lethality assay for pathogenic Enterococcus cecorum. Avian Diseases, 58, 244–248. doi: 10.1637/10687-101113-Reg.1
- Ciftci, A. & Diker, K.S. (2009). The role of enterococcal virulence factors on experimental amyloid arthropathy in chickens. Kafkas Üniversitesi Veteriner Fakültesi Dergisi, 15, 903–908.
- Coburn, P.S., Baghdayan, A.S., Dolan, G.T. & Shankar, N. (2007). Horizontal transfer of virulence genes encoded on the Enterococcus faecalis pathogenicity island. Molecular Microbiology, 63, 530–544. doi: 10.1111/j.1365-2958.2006.05520.x
- Gibbs, P.S., Maurer, J.J., Nolan, L.K. & Wooley, R.E. (2003). Prediction of chicken embryo lethality with the avian Escherichia coli traits complement resistance, colicin V production, and presence of the increased serum survival gene cluster (iss). Avian Diseases, 47, 370–379. doi: 10.1637/0005-2086(2003)047[0370:POCELW]2.0.CO;2
- Gibbs, P.S. & Wooley, R.E. (2003). Comparison of the intravenous chicken challenge method with the embryo lethality assay for studies in avian colibacillosis. Avian Diseases, 47, 672–680. doi: 10.1637/7011
- Gregersen, R.H., Petersen, A., Christensen, H. & Bisgaard, M. (2010). Multilocus sequence typing of Enterococcus faecalis isolates demonstrating different lesion types in broiler breeders. Avian Pathology, 39, 435–440. doi: 10.1080/03079457.2010.517250
- Hayes, J.R., English, L.L., Carter, P.J., Proescholdt, T., Lee, K.Y., Wagner, D.D. & White, D.G. (2003). Prevalence and antimicrobial resistance of Enterococcus species isolated from retail meats. Applied and Environmental Microbiology, 69, 7153–7160. doi: 10.1128/AEM.69.12.7153-7160.2003
- Jacobsen, I.D., Große, K., Slesiona, S., Hube, B., Berndt, A. & Brock, M. (2010). Embryonated eggs as an alternative infection model to investigate Aspergillus fumigatus virulence. Infection and Immunity, 78, 2995–3006. doi: 10.1128/IAI.00268-10
- Landman, W.J.M. (1999). Amyloid arthropathy in chickens: (summary of thesis, Utrecht University, Faculty of Veterinary Medicine, 1998). Veterinary Quarterly, 21, 78–82. doi: 10.1080/01652176.1999.9694998
- Landman, W.J.M., Feberwee, A., Mekkes, D.R., Veldman, K.T. & Mevius, D.J. (1999a). A study on the vertical transmission of arthropathic and amyloidogenic Enterococcus faecalis. Avian Pathology, 28, 559–566. doi: 10.1080/03079459994344
- Landman, W.J.M., Gruys, E. & Gielkens, A.L.J. (1998a). Avian amyloidosis. Avian Pathology, 27, 437–449. doi: 10.1080/03079459808419367
- Landman, W.J.M., Mekkes, D.R., Chamanza, R., Doornenbal, P. & Gruys, E. (1999b). Arthropathic and amyloidogenic Enterococcus faecalis infections in brown layers: a study on infection routes. Avian Pathology, 28, 545–557. doi: 10.1080/03079459994335
- Landman, W.J.M., Peperkamp, N.H.M.T., Koch, C.A.M., Tooten, P.C.J., Crauwels, P.A.P. & Grays, E. (1997). Induction of amyloid arthropathy in chickens. Amyloid: The International Journal of Experimental and Clinical Investigation, 4, 87–97. doi: 10.3109/13506129708995276
- Landman, W.J.M., Van Den Bogaard, A.E.J.M., Doonenbal, P., Tooten, P.C.J., Elbers, A.R.W. & Gruys, E. (1998b). The role of various agents in chicken amyloid arthropathy. Amyloid: The International Journal of Experimental and Clinical Investigation, 5, 266–278. doi: 10.3109/13506129809007300
- Littell, R., Milliken, G., Stroup, W. & Wolfinger, R. (1999). SAS system for mixed models. Raleigh, NC: SAS Institute.
- Montgomery, R.D., Jones, L.S., Boyle, C.R., Luo, Y. & Boyle, J.A. (2005). The embryo lethality of Escherichia coli isolates and its relationship to various in vitro attributes. Avian Diseases, 49, 63–69. doi: 10.1637/7211-052004R
- Nix, E.B., Cheung, K.K., Wang, D., Zhang, N., Burke, R.D. & Nano, F.E. (2006). Virulence of Francisella spp. in chicken embryos. Infection and Immunity, 74, 4809–4816. doi: 10.1128/IAI.00034-06
- Oh, J.Y., Kang, M.S., Yoon, H., Choi, H.W., An, B.K., Shin, E.G., Kim, Y.J., Kim, M.J., Kwon, J.H. & Kwon, Y.K. (2012). The embryo lethality of Escherichia coli isolates and its relationship to the presence of virulence-associated genes. Poultry Science, 91, 370–375. doi: 10.3382/ps.2011-01807
- Petersen, A., Christensen, H., Philipp, H.C. & Bisgaard, M. (2009). Clonality of Enterococcus faecalis associated with amyloid arthropathy in chickens evaluated by multilocus sequence typing (MLST). Veterinary Microbiology, 134, 392–395. doi: 10.1016/j.vetmic.2008.08.014
- Polakowska, K., Lis, M.W., Helbin, W.M., Dubin, G., Dubin, A., Niedziolka, J.W., Miedzobrodzki, J. & Wladyka, B. (2012). The virulence of Staphylococcus aureus correlates with strain genotype in a chicken embryo model but not a nematode model. Microbes and Infection, 14, 1352–1362. doi: 10.1016/j.micinf.2012.09.006
- Rudolph, B. (2004). Variations investigations to Enterococcus faecalis as possible factor for etiology of amyloid arthropathy of brown layers (PhD thesis). Journal-Nr. 2846, Berlin: Freie Universität Berlin.
- SAS Institute Inc. (2011). SAS/STAT 9.3 user’s guide. Cary, NC: Author.
- Sava, I.G., Heikens, E. & Huebner, J. (2010). Pathogenesis and immunity in enterococcal infections. Clinical Microbiology and Infection, 16, 533–540. doi: 10.1111/j.1469-0691.2010.03213.x
- Seo, H.S., Cha, S.Y., Kang, M. & Jang, H.K. (2013). Chicken embryo lethality assay for determining the virulence of Riemerella anatipestifer isolates. Avian Pathology, 42, 387–392. doi: 10.1080/03079457.2013.816654
- Stewart-Tull, D.E.S., Coote, J.G., Thompson, D.H., Candlish, D., Wardlaw, A.C. & Candlish, A. (2009). Virulence spectra of typed strains of Campylobacter jejuni from different sources: a blinded in vivo study. Journal of Medical Microbiology, 58, 546–553. doi: 10.1099/jmm.0.005611-0
- Townsend, M.K., Carr, N.J., Iyer, J.G., Horne, S.M., Gibbs, P.S. & Prüß, B.M. (2008). Pleiotropic phenotypes of a Yersinia enterocolitica flhD mutant include reduced lethality in a chicken embryo model. BMC Microbiology, 8, 1–12. doi: 10.1186/1471-2180-8-1
- Wooley, R., Gibbs, P., Brown, T. & Maurer, J. (2000). Chicken embryo lethality assay for determining the virulence status of avian Escherichia coli isolates. Avian Diseases, 44, 318–324. doi: 10.2307/1592546
