ABSTRACT
Bornaviruses are considered to be the causative agent of proventricular dilatation disease (PDD) in psittacine birds. In order to detect haematological and blood chemistry changes during the development of PDD and a possible correlation with clinical signs and the virological status, six African grey parrots (Psittacus erithacus) were experimentally infected with parrot bornavirus 4 (PaBV-4) by subcutaneous route. All six parrots developed clinical signs of varying extent and successful infection was confirmed in all the birds by seroconversion or detection of RNA of the PaBV-4 infection strain. Based on population-based and intra-individual reference ranges established during 12 months prior to experimental infection, only minor haematological changes were detected in individual birds after infection. Changes in blood chemistry were restricted to aspartate aminotransferase, creatine kinase, total protein, glucose and uric acid. Plasma protein electrophoresis revealed marked changes starting 10 weeks post infection characterized by an increase in the γ-globulin fraction and a gradual decrease to normal values during weeks 22–34. Indications of an acute-phase reaction at the initial stages of infection were not detected. While three birds suffered from clinical signs of PDD, which included weight loss and neurological disorders and died before development of haematological and plasma protein changes, recovery of clinical disease was paralleled in the remaining birds by an increase in γ-globulins and bornavirus-specific antibody titres.
Introduction
In 2008 two independent research groups identified a new bornavirus (genus Bornavirus, family Bornaviridae, order Mononegavirales), in psittacine birds affected with proventricular dilatation disease (PDD) (Honkavuori et al., Citation2008; Kistler et al., Citation2008), a disease which is also known as avian ganglioneuritis (Korbel & Rinder, Citation2012), macaw wasting disease, neuropathic gastric dilatation disease or splanchnic neuropathy. Association of this so-called avian bornavirus with PDD was later confirmed in naturally infected psittacines (Lierz et al., Citation2009; Rinder et al., Citation2009; Weissenböck et al., Citation2009) and the signs of PDD had been induced by experimental infection of cockatiels, Patagonian conures and lovebirds, using tissue homogenates from diseased birds or using purified virus (Gancz et al., Citation2009; Gray et al., Citation2010; Mirhosseini et al., Citation2011; Piepenbring et al., Citation2012; Gentry et al., Citation2015; Piepenbring et al., Citation2016) fulfilling the Koch’s postulates. Bornaviruses are now generally accepted as the causal pathogens of PDD.
Based on investigations on the phylogeny and biology of the viruses, the taxonomy of the family Bornaviridae and the genus Bornavirus was recently revised. Two species occurring in Psittaciform birds were designated, Psittaciform 1 bornavirus including parrot bornavirus (PaBV-) 1, 2, 3, 4 and 7 and Psittaciform 2 bornavirus including PaBV-5 (Kuhn et al., Citation2015; Afonso et al., Citation2016).
PDD was first described in the 1970s and affects primarily psittacine birds (Clark, Citation1984). The disease was named after the enlargement of the proventriculus associated with infiltration of lymphocytes and plasma cells in ganglia of the gastrointestinal walls. The pathologically affected innervation and thus impaired peristalsis of the gastrointestinal tract are the cause of maldigestion and malabsorption leading to clinical signs of crop stasis, regurgitation, diarrhoea, shedding of undigested seeds in the faeces and finally death because of starvation. Central nervous system (CNS) signs such as tremor, ataxia, opisthotonus or seizure may occur as the only signs or in combination with gastrointestinal signs (Mannl et al., Citation1987; Bond et al., Citation1993; Berhane et al., Citation2001). Ophthalmological lesions have also been detected in affected birds (Korbel & Rinder, Citation2011). Subclinical forms have been observed, in which birds infected with bornavirus showed none or only slight signs of disease (De Kloet & Dorrestein, Citation2009; Lierz et al., Citation2009; Ouyang et al., Citation2009; Herzog et al., Citation2010; Hoppes et al., Citation2010; Villanueva et al., Citation2010).
Diagnosis of bornavirus infection in the living bird is mainly based on detection of anti-bornavirus antibodies using enzyme-linked immunosorbent assays (ELISAs) (De Kloet & Dorrestein, Citation2009; Rinder et al., Citation2010), indirect immunofluorescent tests (Herzog et al., Citation2010) or Western blot (De Kloet & Dorrestein, Citation2009; Lierz et al., Citation2009), as well as on detection of viral RNA by reverse transcription (RT)-PCR or realtime reverse transcription (rRT) PCR (Honkavuori et al., Citation2008; Kistler et al., Citation2008). Diagnosis of the clinical disease, PDD, still depends on the detection of characteristic lymphoplasmacytic infiltrations in affected organs by histopathology (Lierz, Citation2016).
Blood tests including blood cell counts and blood chemistry serve the purpose to analyse the patient’s physical condition and may provide important information on various diseases (Polo et al., Citation1998). Protein electrophoresis has been successfully used to gain first insights into relative or absolute changes of blood protein fractions during diverse physiological or pathological conditions. In the past, this method has already been used for the diagnosis of infectious diseases in small mammals (Kaneko, Citation1997) and was also applied in avian medicine (Quesenberry & Moroff, Citation1991; Cray et al., Citation1995; Cray & Tatum, Citation1998). In birds, protein electrophoresis was shown to be capable not only of disease detection and assessment of courses of illness, but also of improvements of the health status during therapy. Characteristic protein concentration curves have already been established for aspergillosis, acute chlamydiosis and sarcocystosis (Cray & Tatum, Citation1998).
The pathogenesis of PDD remains unclear, and so far, changes in blood biochemistry or blood cells specific for bornavirus infection or elucidating development of disease have not yet been found (Gancz et al., Citation2010). However, conclusions were generally based on results obtained from naturally infected birds in various phases of disease. Additionally, in most cases, further infectious or non-infectious diseases were present or could not be excluded. Investigations on bornavirus-induced changes in blood values under standardized conditions, with specified pathogen-free birds over a long period and during defined stages of infection are completely lacking so far.
In order to gain first information on the occurrence of possible blood protein changes in psittacine birds during development of disease, plasma protein electrophoresis was performed in addition to blood cell counts and blood chemistry in regular intervals in African grey parrots during the course of an experimental bornavirus infection. Changes were then analysed with regard to a temporal context or coincidence with clinical signs, formation of bornavirus-specific antibodies and shedding of viral RNA. Since it is known that reference values in birds of the same species can vary individually and between different population depending on the living conditions (Scope et al., Citation2002), intra-individual and population-based reference ranges were established for all the laboratory parameters. They were based on investigations of the parrots during a period of one year before the experimental infection was performed and were used in order to detect minor effects of bornavirus infection.
Materials and methods
The experiments were conducted in accordance with the German animal welfare regulations and under permission by the German authorities (reference number 55.2-1-54-2531.3-60-10).
Experimental birds
Six Congo African grey parrots (Psittacus erithacus), two years old (at the beginning of the investigation period) and numbered P7–P12, were used. They originated from bornavirus-free parents and were hand-raised for the study purpose as a group, separated from other psittaciform birds. In order to avoid natural infection with bornavirus they were housed in an isolated indoor aviary with high biosecurity standards. All birds were free of any clinical signs. Infections with primary pathogenic bacteria or fungi were excluded using standard culturing tests. Viral infections with avian bornavirus, psittacine beak and feather disease virus, avian polyomavirus and psittacine herpesvirus were excluded using PCR assays and serological tests as described before (VanDevanter et al., Citation1996; Tomaszewski et al., Citation2001; Ogawa et al., Citation2005; Halami et al., Citation2008; Honkavuori et al., Citation2008; Kistler et al., Citation2008; Rinder et al., Citation2010). The parrots were maintained on a pellet-based diet (Harrison’s High Potency Coarse; Avifood, Gräfelfing, Germany) enriched with fresh fruits and vegetables, fresh drinking water was provided ad libitum. During the whole experiment the parrots were kept together in their group and all housing conditions, like food or light, remained unchanged.
Experimental infection
The infection was performed by subcutaneous inoculation of PaBV-4 (isolate #6758) using an infection dose of 105 fluorescence forming units per bird. Virus stocks have been produced in CEC-32 quail fibroblast cells and were purified as described before (Rinder et al., Citation2009; Rubbenstroth et al., Citation2012, Citation2014).
Clinical investigation
The birds underwent surveillance over a period of one year before and one year after infection. Data obtained in the first year were used to determine intra-individual reference ranges and served as controls for each individual bird. General clinical conditions of each individual were controlled daily by inspection and clinical scores ranging from 5 to 0 were determined for each bird by the week. An unaffected general condition without any signs of disease was scored as 5. Unspecific signs such as slight trembling and a fluffed up plumage were scored as 4, more severe trembling, closed eyes and mild ataxia as 3. When a bird showed moderate ataxia and other signs indicating distinct CNS disorders as well as a severely reduced general condition, it was categorized at a score of 2. A score of 1 was given to birds with high-grade apathy and ataxia. The score of 0 was defined as the humane endpoint of the experiment and was given when massive neurological deficits occurred like failure of the positional reflexes or incapability of sitting on a perch, or when a loss of 30% of the original body mass was detected. Body weights were recorded once a week.
Haematology
Every four weeks, blood samples were obtained from the birds before feeding. They were taken from the jugular vein with heparinized 22-gauge needles using standard techniques (König & Korbel, Citation2016) and were collected in lithium-heparin tubes. All haematological examinations were conducted directly after collection. For the determination of the packed cell volume (PCV) the blood was centrifuged in a microhematocrit centrifuge using standard capillary tubes. Thin blood smears dyed with Haema Quick-Stain (Diff-Quick, LT-SYS®, Eberhard Lehmann, Berlin, Germany) were used for analysing the morphology and number of red blood cells and thrombocytes, the white blood cell count (WBC) and the differentiation between granulocytes and mononuclear cells. The WBC was determined by evaluating 20 fields of vision in a 1000-fold magnification. The leukocytes were counted and the cell counts were deduced from the mean values per one field of vision. Corrections were made for very low or very high PCVs. In case of a PCV lower than 30% or higher than 55%, the number of leukocytes was multiplied by this percentage and divided by 45 to obtain a corrected value. Based on the mean leukocyte counts per field of vision, the WBC was estimated as follows: a mean leukocyte number lower than 0.3 corresponded to a WBC of 0–5 × 10³ cells/µl, a mean value of 0.3–<1.0 to a WBC of 5–15 × 10³ cells/µl, and a mean value of 1.0–<2.5 to a WBC of 15–35 × 10³ cells/µl, while a mean value of 2.5–<4.0 corresponded to a WBC of 35–50 × 10³ cells/µl and, finally, a mean value of 4.0 or higher corresponded to a WBC of >50 × 10³ cells/µl.
Blood chemistry
Blood chemistry was investigated in parallel to the haematological tests and accomplished with a VetScan VS 2 (ABAXIS, Scil, Griesheim, Germany), according to the manufacturer’s instructions. The following parameters were measured: aspartate aminotransferase (AST), creatine kinase (CK), glucose, uric acid (UA), bile acid (BA), sodium, potassium, calcium, phosphorus and total proteins (TPs).
Calculation of reference ranges
Intra-individual and population-based reference ranges were calculated for each parrot and for the group based on measurements of blood samples taken at intervals of four weeks during the year before infection. The 0.025 and the 0.975 fractiles and thus the ranges containing 95% of the reference distribution were defined (Solberg, Citation1987). Additionally, mean, median and standard deviation (SD) were determined. For each parrot and parameter the index of individuality (IoI = CV(individual)/CV(population)) was calculated using the coefficient of variation (CV = SD × 100/mean) of all individuals and the group (population). If the IoI <0.6, which indicates marked individuality, the intra-individual reference ranges were used for evaluating the measured values, otherwise those of the population (Harris, Citation1974; Fraser & Harris, Citation1989; Scope et al., Citation2006). Eventually, the critical difference (CD = 1.96 × 2SD) was calculated to analyse a post infection (p.i.) change in values with regard to biological significance (Harris, Citation1974; Scope et al., Citation2006). Thus, a value deviation larger than the CD was assessed as significant. If a value lay outside the intra-individual or population-based references ranges, but within the CD, this change was regarded as non-significant.
ELISA
ELISA for the detection of P40 (N protein)-specific antibodies was carried out in intervals of four weeks, except during the first 46 weeks after infection when it was done at intervals of two weeks. The ELISA was performed with plasma obtained from lithium-heparin blood after centrifugation for 5 min at 3500 × g as described before using a recombinant N protein as antigen, in addition to anti-grey parrot-immunoglobulin G (IgG) and peroxidase-linked goat anti-rabbit-IgG (Jackson ImmunoResearch, Newmarket, UK) (Reuter et al., Citation2010; Rinder et al., Citation2010). Absorption higher than the mean value of three negative controls plus threefold SD was considered positive. For semi-quantification of the ELISA results the absorption values were scaled so that an absorption at the cut-off corresponded to a relative value of 0 and the value obtained from a standardized positive control sample corresponded to a relative value of 1.
rRT-PCR
Swab samples from choana and cloaca as well as blood samples with enriched contents of white blood cells were tested for the presence of PaBV-4 RNA. The swabs were obtained from every bird monthly for one year before infection and biweekly over one year p.i. Buffy coat material (blood cells with enriched content of white blood cells) originated from the blood samples used for ELISA and was harvested from heparinized blood samples as the upper cell layers after centrifugation as described above. In three birds that died during the investigation period, brain samples taken during necropsy were also included.
Total RNA purification from choanal and cloacal swabs was performed using QIAamp Viral RNA Mini Kit® (Qiagen, Hilden, Germany) and from buffy coat material as well as from brain samples using the RNeasy Mini Kit® (Qiagen). Extraction was carried out according to the manufacturer’s protocol. RNA samples were stored at –80°C until further investigation. Random hexamer primers were used to reverse transcribe total RNA into first strand complementary DNA. For the real-time PCR, a primer set (forward 5′-CAGACAGCACGTCGAGTGAGA-3′ and reverse 5′-AGTTAGGGCCTCCCTGGGTAT-3′) and a probe (6FAM-AGGTCCCCGCGAAGGAAGCGA-TMR) according to Honkavuori et al. (Citation2008) were used targeting the amino-terminal region of phosphoprotein (P) gene of PaBV-4. Cycling conditions were as follows: initial denaturation at 95°C for 15 min, followed by 50 cycles, each consisting of denaturation at 95°C for 30 s, then annealing at 60°C for 30 s and finally extension at 72°C for 30 s. Positive and negative controls were included in each run. Threshold cycle (Ct) values smaller than 32.0 and from 32.0 to 35.9 were considered strongly positive (++) and positive (+), respectively, while Ct values from 36.0 to 39.9 and greater 40.0 were assessed as borderline (o) and negative (−), respectively.
Using RNA obtained from brain samples of parrots P7, P8 and P9, a conventional PCR protocol targeting a fragment of the N gene (Kistler et al., Citation2008) was applied followed by agarose gel electrophoresis according to standard procedures. Amplified DNA was extracted using the QIAquick gel extraction kit (Qiagen). Direct sequencing was performed by GATC Biotech (Cologne, Germany) and using the PCR primers. Sequence identity was determined by BLAST analyses (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Plasma protein electrophoresis
Plasma samples of all six parrots (P7–P12) were taken 18, 10 and 2 weeks before infection and 2, 4 and 6 weeks after infection. In week 8 p.i. additional protein electrophoresis of plasma from P7 and P9 was performed. In week 10, 14, 18, 22, 26, 34 and 42 p.i. plasma of parrots P7, P8 and P9 was not available anymore because of death of these birds, and thus only plasma of P10, P11 and P12 was included.
Care was taken that the plasma samples were of high quality and did not show signs of lipaemia or haemolysis. Protein electrophoresis was undertaken at IDEXX laboratories (Ludwigsburg, Germany), and for transportation the frozen samples were packed on dry ice. The analyses were conducted in two sample batches using the same technology and consistent fraction delimits. TP was measured by the Biuret method (Beckman Coulter AU 5800). Then, plasma samples were analysed with the Sebia Hydrasys 2, an automatic, multiparametric agarose gel electrophoresis system and according to the instructions provided by the manufacturer. Gels were run for 20 min at 240/270 V. The relative concentrations for each protein fraction (prealbumin, albumin, α-, β- and γ-globulins) were acquired from densiometric scans. Absolute values (g/dl) for each fraction were then calculated by multiplying the percentages by the TP concentration. The albumin to globulin (A/G)-ratio was calculated by dividing the sum of prealbumin and albumin by the sum of α-, β- and γ-globulins.
Results
Clinical observations
Experimental infection resulted in clinical signs in all six birds. The clinical course of the disease varied among the birds. The general condition before and shortly after infection was very good and was scored as 5 in all birds. Parrots P7 and P8 were taken ill as early as 3 weeks p.i. and showed constantly aggravating signs like fluffing, trembling, closed eyes, incoordination, orientation problems and finally emaciation despite good appetite. According to animal welfare regulations, parrot P8 was euthanized 6 weeks p.i. and parrot P7 was euthanized 9 weeks p.i. because of severely reduced general condition, which was scored as 1 during the week before euthanasia in both birds. Parrot P9 developed similar signs, but not until 5 weeks p.i., descended to a lowest score of 2 and died 9 weeks p.i. In the postmortem examination a dilated proventriculus with a thin wall was found in all three birds.
In parrot P10 severe disease signs were detected from week 4 until week 14 p.i. and the general condition worsened constantly and reached a minimum score of 1 in week 9 p.i. During this period, automutilation on both wings also occurred. Afterwards the bird recovered and its health score varied between 3 and 5 until the end of the investigation period. Parrot P11 showed only few and short periods of slight and unspecific signs including reduced activity and fluffing during the whole investigation period. Its clinical score never decreased below a value of 4. In parrot P12, clinical signs were observed from 10 weeks p.i. onwards and its general condition worsened to a score of 2. Signs of disease were detected until week 18 p.i., including depression and ataxia. In addition, feather plucking of the thoracic and abdominal region occurred in this bird. During the further course of the experiment the clinical score of parrot P12 was assessed as a 4, interrupted by a short phase of a clinical score of 3 between 32 and 33 weeks p.i. After 48 weeks p.i. the bird showed a completely undisturbed appearance rated with a clinical score of 5 until the end of the examination period.
Blood cell and blood chemistry analysis
The population-based reference ranges for the African grey parrots which were calculated based on measurements during a 12 months period before infection are listed in . The individual values and reference ranges of all parrots before infection are listed in supplementary Tables S1–S11 and the individual values after infection can be found in supplementary Tables S12–S22.
Table 1. Population-based reference ranges for parrots P7–P12, mean value, median, standard deviation (SD), 2.5% and 97.5% quantile, critical difference (CD) and coefficient of variation (CV) (PCV, packed cell volume, TP, total protein, AST, aspartate aminotransferase, BA, bile acid, CK, creatine kinase, UA, uric acid, Glu: glucose, Ca: calcium, Phos: phosphorus, Pot: potassium, Sod: sodium).
After experimental infection with PaBV-4, PCV stayed within the intra-individual ranges except for parrots P10 and P11 where mild anaemia was detected between weeks 2 and 14 p.i. indicated by a reduction of PCV to 31% in P10 and to 34% (not significantly reduced) in P11.
Abnormalities in the morphology of erythrocytes or in the appearance and number of thrombocytes were not found during the investigation period. Likewise, the number of the thrombocytes remained stable. Slight to moderate increased numbers of leukocytes were occasionally detected in all parrots, but without differences between time points before and after experimental infection with PaBV.
Blood chemistry analyses revealed no significant changes in BA, calcium, phosphorus, sodium and potassium after experimental infection. Similar to the leukocyte numbers, a high variation of the AST and CK blood values and sometimes very high values were detected in the period before infection already resulting in relatively high reference ranges and large SDs. Nevertheless, the highest values measured p.i. exceeded the population-based reference ranges significantly. Parrot P8 exhibited an AST value of 475U/l and a CK value of 6430U/l 6 weeks p.i and parrot P12 had an AST value up to 385U/l (50 weeks p.i.) and a CK value of 7116U/l (18 weeks p.i.).
A slight elevation of TP up to 4.9 g/dl occurred in parrot P11 in week 18 p.i. Besides, single deviations from the reference ranges were found in parrot P10 for UA with an elevated value of 10.7 mg/dl 14 weeks p.i. and in parrot P11 for glucose with hyperglycaemic values of 505 mg/dl 22 weeks p.i. and 310 mg/dl 42 weeks p.i.
Detection of anti-bornavirus antibodies and PaBV-4 RNA
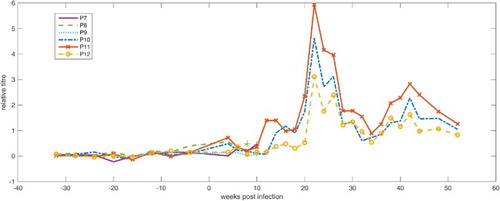
In , the course of the relative antibody titres of parrots P7–P12 starting from 34 weeks before infection is shown. Parrots P7 and P8 exhibited small but constant titre increases until their deaths 9 and 6 weeks after infection, respectively. The relative titre amounted to 0.46 in P7 and 0.48 in P8 in the last measurements. Significant increase in the antibody titre was not detected in parrot P9 until its death 9 weeks after infection; all values after infection were close to the calculated cut-off. The antibody titres of parrots P10 and P12 ascended slightly after 12 weeks p.i., then rose sharply after 18 weeks p.i. and remained at high levels until 26 weeks after infection. Afterwards, the relative titre began to decrease but still stayed on a high level. In parrot P11 an already marked increase in antibody titre was recorded 12 weeks after infection. Thereafter, the antibody titre showed a temporal pattern similar to those of parrots P10 and P12, but always at higher levels throughout the investigation period.
Figure 1. Relative antibody titres for parrots P7–P12 from 36 weeks a.i. to 52 weeks p.i. (week 0 = time of experimental infection).
As shown in , viral RNA was not detected in cloacal and pharyngeal swabs and in the buffy coat material of parrots P7, P8 and P9 until their death and in parrot P10 until the end of the investigation period. In parrots P7, P8 and P9, RNA of the bornavirus infection strain was, however, detected in brain samples taken at necropsy, as confirmed by sequencing of the PCR products. In parrot P11 and P12, PaBV-4 RNA was first detected in swabs 12 and 20 weeks p.i., respectively. Afterwards, pharyngeal and cloacal swabs from parrots P11 and P12 remained strongly positive, positive or at least borderline with regard to bornavirus RNA until the end of the investigation period. Analyses of the buffy coat showed only once, 16 weeks p.i., a positive result in parrot P11. Values considered as borderline were measured seven times in buffy coats of parrot P11 and once in parrot P12.
Table 2. Semi-quantitative results of the rRT PCR for detection of bornavirus RNA in parrots P7–P12 from pharyngeal swabs (P), cloacal swabs (C) and buffy coat (BC) (−: negative, o: borderline, +: positive, ++: strongly positive). For parrots P7–P9 results have been obtained until their death after 6 or 9 weeks p.i., respectively.
Plasma protein electrophoresis
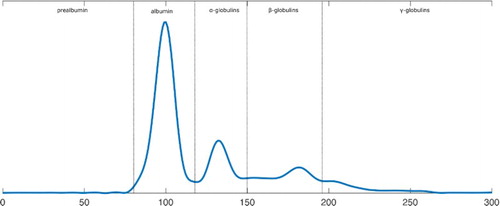
The results of the protein electrophoresis (as shown in supplementary Tables S23–S28) were given as the absolute and relative amounts of the different protein fractions and in addition, as a graphical representation of their quantitative distribution. Plasma protein patterns before experimental infection of parrots, as shown in for parrot P10, were used as individual reference curves for the analyses of changes during infection. Before infection, there was no detectable prealbumin fraction, while albumin constituted the predominating fraction followed by α-, β- and γ-globulins. The distribution of the different protein fractions obtained before infection varied only slightly between the six parrots.
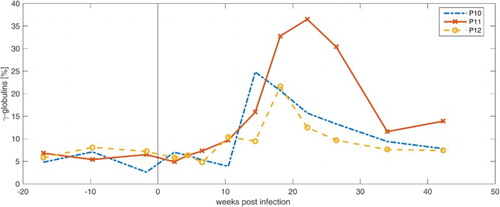
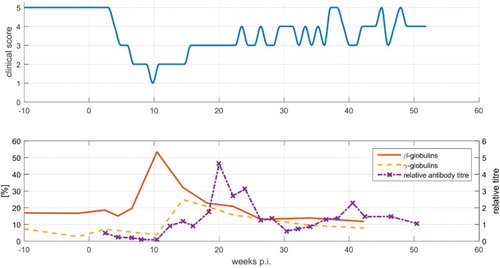
For parrots P7, P8 and P9, which deceased 6 and 9 weeks, respectively, p.i., the results of the plasma protein electrophoresis showed no considerable changes during the time after the experimental infection. For parrots P10, P11 and P12, and from 10 weeks p.i. onwards, absolute and relative values of plasma protein fractions revealed major changes, as shown in using parrot P10 as an example. Noteworthy is the massive boost of β-globulins 10 weeks p.i, followed by a likewise marked increase in γ-globulins from 14 to 18 weeks p.i. Afterwards, the values decreased and were close to the values a.i. at week 34 p.i. At the same time (10–18 weeks p.i.) a hypoalbuminaemia occurred in this bird. Accordingly, the A/G ratio was decreased in the period between 10 and 18 weeks p.i., with a minimum at week 14 p.i. However, there was no significant increase in TP during the examination period.
Figure 3. Development of plasma protein values of parrot P10 after infection for (a) 4 weeks p.i, (b) 6 weeks p.i, (c) 10 weeks p.i., (d) 14 weeks p.i., (e) 18 weeks p.i. and (f) 34 weeks p.i.
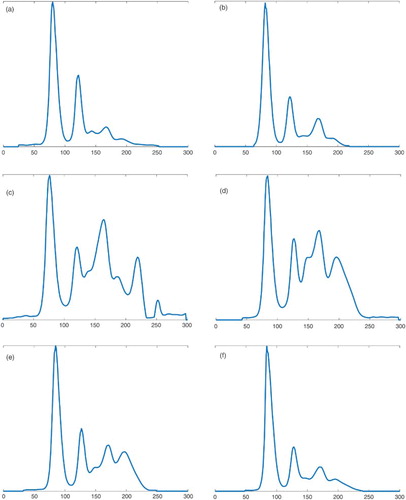
Parrot P11 showed, like parrot P10, an increase in γ-globulins starting 14 weeks p.i. The A/G-ratio of parrot P11 was decreased in the period between 18 and 26 weeks p.i., simultaneously with lower albumin values. The TP was increased in the sample taken 18 weeks after infection. An increase in β-globulins, however, was not recorded for this bird.
Parrot P12 showed the least alterations of the protein fractions in comparison with parrots P10 and P11. An increase in γ-globulins was detected between 10 and 26 weeks p.i., which turned out small compared to parrots P10 and P11. Neither a significant increase in β-globulins, as for P10, nor an increase in TP, as for P11, could be recorded. The A/G ratio stayed in a constant range over the examination period. In , the percentage γ-globulin concentrations of parrots P10, P11 and P12 are shown.
Temporal coincidence
When the temporal changes of general condition, antibody titres and plasma protein parameters were compared and displayed in a single chart (shown for parrot P10 in ), it became evident that P7, P8 and P9 died before a significant change of anti-bornavirus antibody titres and of protein electrophoresis pattern could be detected. In P10, the peak of the β-globulin concentration coincided with the lowest general condition score. After week 14 p.i, there was an improvement of its general condition which co-occurred with a marked increase in γ-globulin concentration and anti-bornavirus antibody titres. Similar results were obtained for parrot P12. Nearly simultaneously with a marked increase in antibody titres and a maximum γ-globulin amount detected after week 18 p.i., a progressive improvement of the clinical appearance of this bird occurred. The clinical course was slightly different for parrot P11, compared to the five other parrots. P11 did not develop serious disease signs but, in contrast, reached a high antibody titre already 12 weeks after infection. Viral RNA was not detected in swabs of parrot P7–P10. In parrots P11 and P12, the amount of RNA, evaluated in a semi-quantitative way, did not correlate with the clinical, serological or protein parameters mentioned before.
Discussion
In the study presented here, all six African grey parrots were successfully infected with PaBV-4 by subcutaneous route as indicated by the detection of anti-bornavirus antibodies in all six birds and of viral RNA identical to the infection strain in five of six birds. The clinical signs of the disease varied considerably between the six infected birds. Various incubation periods, illness severities and progresses of disease have also been documented for naturally infected psittacines ranging from a subclinical carrier status and nearly asymptomatic courses to a sudden onset of clinical signs followed by death (Phalen, Citation1986; Rich, Citation1992; Gregory et al., Citation1994; De Kloet & Dorrestein, Citation2009; Gancz et al., Citation2010; Kistler et al., Citation2010; Heffels-Redmann et al., Citation2011), and thus, the clinical course of the experimentally induced disease corresponded rather well to natural infections.
The results consistently obtained from this experiment using laboratory diagnostic tests are therefore regarded as valuable and well transferable to natural conditions. Until now haematological changes associated with experimental bornavirus infections in birds have not been published. Former reports on blood parameters are based on single cases and observations of naturally infected birds and documented changes differ between cases and might be attributed to other associated diseases. Specific reference ranges of the blood values for each parrot and for the group (Scope et al., Citation2002, Citation2005, Citation2006) allowed a very precise and individualized assessment even of minor changes. A reduced PCV as detected in P10 and P11 has also been described in several cases of natural bornavirus infection (Ridgway & Gallerstein, Citation1983; Joyner et al., Citation1989; Degernes et al., Citation1991; Keller et al., Citation2010). Gastrointestinal malabsorption was quoted as an explanation for the non-regenerative anaemia in birds suffering from PDD, as it is generally noted in starving birds (Suedmeyer, Citation1992; Gancz et al., Citation2010).
With regard to WBC, it has to be noted that the birds in this study showed reappearing elevated cell counts not only after infection, but also during the year before infection already. Thus, it seems unlikely that it was caused by the bornavirus infection. Elevated WBCs, often in combination with heterophilia, have occasionally been reported in birds with PDD (Rich, Citation1992; Suedmeyer, Citation1992; Bond et al., Citation1993; Gancz et al., Citation2010; Keller et al., Citation2010). Gancz et al. (Citation2010) regarded leucocytosis and heterophilia as inconsistent changes, related to stress or the existence of secondary infections. As most of those cases were naturally infected birds not kept under special hygienic conditions, additional infections with other pathogens cannot be excluded and may have led to altering leucocyte counts. Mycotic or bacterial opportunistic infections were even regarded as common in natural bornavirus infections (Phalen, Citation1986; Degernes et al., Citation1991; Lutz & Wilson, Citation1991; Rich, Citation1992) and may complicate the interpretation of laboratory findings (Gregory et al., Citation1994).
Blood chemistry analyses revealed deviations in AST, CK, TP, UA and glucose values. These changes are not necessarily induced by bornavirus infection or PDD. The increase in the enzymes AST and CK observed especially in parrots P8 and P12, partly coincided with the clinical disease period. Mild to moderate plasma elevations of enzymes of muscle origin, like AST, CK and lactate dehydrogenase were mentioned before in reports on bornavirus infections in Psittaciformes (Gancz et al., Citation2010; Keller et al., Citation2010). It was expected that elevated plasma CK values could serve as an unspecific diagnostic indicator of splanchnic neuropathy and could therefore indicate an upcoming disease (Jenkins, Citation1991). However, CK values could also rise because of patient excitement and handling, muscle damage or trauma (Fudge, Citation2000). The elevation of the TPs in parrot P11 might be due to the increase in immunoglobulins. Hyperglycaemia and an increased UA level as documented singularly for parrots P11 and P10, respectively, have not yet been described in literature about PDD or bornaviruses. Glucose values up to 600 mg/dl are most likely related to stress (Fudge, Citation1997). Slight increases in UA concentration, as documented for P10, have often been observed to occur because of dehydration (Fudge, Citation1997).
Other changes, which were occasionally and inconsistently seen in other studies on PDD in Psittaciformes and which were interpreted as indicators of a negative metabolic status, for example hypoglycaemia (Ridgway & Gallerstein, Citation1983) and increased lipase levels (Boutette & Taylor, Citation2004), were not detected in this investigation.
Protein electrophoresis revealed important changes in protein parameters. Similar to many other investigations about protein electrophoresis in birds, plasma was used instead of serum (Cray et al., Citation1995, Citation2007, Citation2009a; Cray & Tatum, Citation1998; Ivey, Citation2000; Tatum et al., Citation2000; García-Montijano et al., Citation2002; Rosenthal et al., Citation2005; Briscoe et al., Citation2010) allowing comparison and a larger usable volume. It has to be noted that plasma, unlike serum, contains the coagulation enzyme fibrinogen, which usually migrates into the β-fraction and can therefore complicate interpretation. In the present study, three electrophoreses for each parrot at different dates before infection were performed and used as a base for individual changes occurring after infection. It is also important to consider that results of electrophoresis can also differ significantly depending on the type of instrument used for the examination, the composition of the gel, the running time and the buffer (Cray et al., Citation2011b). In a comparative study with three different electrophoresis systems, the use of Sebia Hydrasys, a preceding model to the one employed in the study presented here, resulted in higher values of the α-globulin fraction compared to other systems (Cray et al., Citation2011b). We did consistently ascertain a relatively high amount of proteins of the α-globulin fraction in all performed electrophoreses as well (20–30% of TP of measurements before infection). These peculiarities are surely attributed to technical reasons and have to be taken into account during analyses and comparisons of the results.
We expected to see changes in protein patterns due to innate or acquired immune reactions very soon after the experimental infection of the African grey parrots. However, changes appeared only in parrots P10, P11 and P12 and not before 10 weeks p.i., thus at a time when parrots P7, P8 and P9 had already died. Descriptions of the main plasma protein changes are thus based on three African grey parrots, which is a consequence of a limited number of experimental birds because of animal welfare reasons. Nevertheless the high consistency of changes among the birds supports the relevance of the results, and similar plasma protein changes were also observed in naturally infected parrots by one of the authors (R.R., unpublished results).
Consistent detection of elevations of the γ-globulin fraction whose main components are the immunoglobulins did not take place earlier than week 10 or 14 p.i. An early increase in the β-globulin fraction or the α-globulin fraction reflecting an increase in acute-phase proteins and thus indicating an acute phase of the disease had not been observed. Since the first protein electrophoresis p.i. was performed with blood taken 2 weeks p.i., possible acute changes before might be missed. However, because there was not the slightest abnormality in the electrophoresis patterns of any of the six parrots during the time of an eventual acute-phase reaction, compared to the curves before infection, this possibility is regarded as unlikely. In parrot P10, an increased level of β-globulins was detected, but not before 10 weeks p.i. and it is remarkable that the peak of the β-globulins coincided with the worst clinical condition in this bird. It is unknown whether this increase in β-globulins was due to an unusually late increase in acute-phase proteins, or whether immunoglobulins were already generated and immigrated in this fraction. In avian species, serum amyloid A, transferrin, haptoglobin, fibrinogen, ceruloplasmin and alpha 1 acid glycoprotein were identified as putative positive acute-phase proteins (Cray et al. Citation2011a), which have multiple functions in modulating the immune system, protein transport and tissue protection (Cray et al., Citation2009b). Albumin, which was also described as a negative acute-phase protein (Kaneko, Citation1997; Cray et al., Citation2011b), did not, in this investigation, reveal any variation during the first weeks after infection, but was reduced after 10 weeks p.i. in parrot P10. Hypoproteinaemia, respectively, hypoalbuminaemia (Ridgway & Gallerstein, Citation1983; Degernes et al., Citation1991; Suedmeyer, Citation1992; Keller et al., Citation2010), as observed in parrots P10 and P11, have already been described for birds suspected of suffering from PDD before. Hypoalbuminaemia was a consistent finding in birds with advanced and severe disease (Boutette & Taylor, Citation2004). Thus, the results of protein electrophoresis did not support the existence of an early immune reaction in the experimentally infected parrots. High-resolution-electrophoresis or immuno-electrophoresis as performed by Cray (Citation1997) might provide further insights into proteins involved in the reactions during bornavirus infection.
When comparing the results of ELISA and protein electrophoresis with actual clinical condition, it was apparent that parrots P10 and P11, like P7 and P8, developed disease signs soon after infection, and also had similar antibody titres during this first phase of disease. But in contrast to P7 and P8 which succumbed to the disease, they showed a recovery which coincided with a marked increase in the γ -globulins and of bornavirus-specific antibodies. Parrot P11 exhibited high antibody titres and a marked increase in the γ-globulin (immunoglobulin) fraction sooner than the other parrots and showed only slight clinical signs during the whole investigation period.
Our observation of the time course of anti-bornavirus antibody development contradicts the theories that antibodies do not protect from disease (Heffels-Redmann et al., Citation2012) or might even parallel the development of clinical PDD (Villanueva et al., Citation2010). Many reports on the detection of anti-bornavirus antibodies in clinically healthy birds (De Kloet & Dorrestein, Citation2009; Lierz et al., Citation2009; Hoppes et al., Citation2010; Villanueva et al., Citation2010; Heffels-Redmann et al., Citation2012; Piepenbring et al., Citation2012) also argue against this theory that they take part in inducing the development of clinical signs.
In experimentally infected cockatiels, the outcome of clinical disease was not influenced by the height of antibody titres determined by immunofluorescence tests. It was therefore concluded that the existence of antibodies does not indicate antiviral immunity (Piepenbring et al., Citation2012). However, cockatiels, which were carriers of PaBV-4 showed a prolonged survival time after experimental infection with a different strain of PaBV-4 (Payne et al., Citation2011) compared with cockatiels of the same flock inoculated with PaBV-2 (Mirhosseini et al., Citation2011). This was interpreted as a reflection of a lack of complete immunity to superinfection but still as some resistance (Payne et al., Citation2011). Differences between the strains or failure, in naturally infected birds, to develop a strong protective immune response were discussed as possible reasons for the lack of complete protection (Payne et al., Citation2011). Information on antibody concentrations in the cockatiels before and after experimental bornavirus inoculation however is not available.
A possible involvement of anti-bornavirus antibodies in the pathogenesis of the disease was also discussed in the Mammalian 1 Bornavirus, Borna disease virus (BoDV). In a study conducted on rats, animals inoculated with low doses (102–104TCID50) of BoDV showed typical clinical disease and severe encephalitis. In contrast, infection with a high dose (105–106 TCID50) only resulted in a mild encephalitic response, the development of high titres of anti-BoDV antibodies and a concurrent protection against virulent challenge (Oldach et al., Citation1995). It was concluded that a high-dose BoDV inoculation triggered an early virus-specific reaction of the immune system, as demonstrated by strong cellular and humoral responses. There was no evidence that antibodies might contribute to neuropathology, although neutralizing antibodies apparently controlled virus tropism and could prevent the spread of virus from peripheral infection sites to the CNS (Hatalski et al., Citation1998; Furrer et al., Citation2001a, b).
It still has to be clarified to which extent the knowledge on BoDV can be transferred to bornaviruses occurring in Psittaciformes or other birds. It is obvious that there is a large amount of apparently healthy birds being seropositive. These can be infected birds about to develop disease or recovered birds immune to disease (Villanueva et al., Citation2010). Future work should be directed towards determining whether passive administration of specific antibodies can alter pathogenesis or clinical outcome of bornavirus infections.
Supplemental data
Download Zip (127.1 KB)Acknowledgements
The authors thank Peter Staeheli, University of Freiburg, Germany, for sharing the virus strain used for infection.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Charlotte Högemann http://orcid.org/0000-0002-0062-9824
Rüdiger Korbel http://orcid.org/0000-0002-1937-7305
Monika Rinder http://orcid.org/0000-0003-3269-7407
References
- Afonso, C.L., Amarasinghe, G.K., Banyai, K., Bao, Y., Basler, C.F., Bavari, S., Bejerman, N., Blasdell, K.R., Briand, F.X., Briese, T., Bukreyev, A., Calisher, C.H., Chandran, K., Cheng, J., Clawson, A.N., Collins, P.L., Dietzgen, R.G., Dolnik, O., Domier, L.L., Durrwald, R., Dye, J.M., Easton, A.J., Ebihara, H., Farkas, S.L., Freitas-Astua, J., Formenty, P., Fouchier, R.A., Fu, Y., Ghedin, E., Goodin, M.M., Hewson, R., Horie, M., Hyndman, T.H., Jiang, D., Kitajima, E.W., Kobinger, G.P., Kondo, H., Kurath, G., Lamb, R.A., Lenardon, S., Leroy, E.M., Li, C.X., Lin, X.D., Liu, L., Longdon, B., Marton, S., Maisner, A., Muhlberger, E., Netesov, S.V., Nowotny, N., Patterson, J.L., Payne, S.L., Paweska, J.T., Randall, R.E., Rima, B.K., Rota, P., Rubbenstroth, D., Schwemmle, M., Shi, M., Smither, S.J., Stenglein, M.D., Stone, D.M., Takada, A., Terregino, C., Tesh, R.B., Tian, J.H., Tomonaga, K., Tordo, N., Towner, J.S., Vasilakis, N., Verbeek, M., Volchkov, V.E., Wahl-Jensen, V., Walsh, J.A., Walker, P.J., Wang, D., Wang, L.F., Wetzel, T., Whitfield, A.E., Xie, J.T., Yuen, K.Y., Zhang, Y.Z. & Kuhn, J.H. (2016). Taxonomy of the order Mononegavirales: update 2016. Archives of Virology, 161, 2351–2360. doi: 10.1007/s00705-016-2880-1
- Berhane, Y., Smith, D.A., Newman, S., Taylor, M., Nagy, É, Binnington, B. & Hunter, B. (2001). Peripheral neuritis in psittacine birds with proventricular dilatation disease. Avian Pathology, 30, 563–570. doi: 10.1080/03079450120078770
- Bond, M.W., Downs, D. & Wolf, S. (1993). Screening for psittacine proventricular dilatation syndrome. In J. Jenkins, R.B. Altmann, & S. Orosz (Eds.), Proceedings of the Annual Conference of the Association of Avian Veterinarians (pp. 92–97). Nashville, TN.
- Boutette, J.B. & Taylor, M. (2004). Proventricular dilatation disease: a review of research, literature, species differences, diagnostics, prognosis, and treatment. In E. Bergmann (Ed.), Proceedings of the Annual Conference of the Association of Avian Veterinarians (pp. 175–181). New Orleans, LO.
- Briscoe, J.A., Rosenthal, K.L. & Shofer, F.S. (2010). Selected complete blood cell count and plasma protein electrophoresis parameters in pet psittacine birds evaluated for illness. Journal of Avian Medicine and Surgery, 24, 131–137. doi: 10.1647/2007-047.1
- Clark, F.D. (1984). Proventricular dilatation syndrome in large psittacine birds. Avian Diseases, 28, 813–815. doi: 10.2307/1590255
- Cray, C. (1997). Plasma protein electrophoresis: an update. In M. Doolen (Ed.), Proceedings of the Annual Conference of the Association of Avian Veterinarians (pp. 209–212), Reno, NV.
- Cray, C., Bossart, G.D. & Harris, D. (1995). Plasma protein electrophoresis: principles and diagnosis of infectious disease. In M.J. Kornelsen (Ed.) Proceedings of the Annual Conference of the Association of Avian Veterinarians (pp. 55–59). Philadelphia, PA.
- Cray, C., Dickey, M. & Rodriguez, M. (2011a). Quantitation of acute phase proteins in psittacine species. In S. Orosz (Ed.), Proceedings of the Annual Conference of the Association of Avian Veterinarians (pp. 31–32). Seattle, WA.
- Cray, C., King, E., Rodriguez, M., Decker, L.S. & Arheart, K.L. (2011b). Differences in protein fractions of avian plasma among three commercial electrophoresis systems. Journal of Avian Medicine and Surgery, 25, 102–110. doi: 10.1647/2010-019.1
- Cray, C., Reavill, D., Romagnano, A., Sant, F.V., Champagne, D., Stevenson, R., Rolfe, V., Griffin, C. & Clubb, S. (2009a). Galactomannan assay and plasma protein electrophoresis findings in psittacine birds with aspergillosis. Journal of Avian Medicine and Surgery, 23, 125–135. doi: 10.1647/2007-041.1
- Cray, C., Rodriguez, M. & Zaias, J. (2007). Protein electrophoresis of psittacine plasma. Veterinary Clinical Pathology, 36, 64–72. doi: 10.1111/j.1939-165X.2007.tb00184.x
- Cray, C. & Tatum, L.M. (1998). Applications of protein electrophoresis in avian diagnostics. Journal of Avian Medicine and Surgery, 12, 4–10.
- Cray, C., Zaias, J. & Altmann, N.H. (2009b). Acute phase response in animals: a review. Comparative Medicine, 59, 517–526.
- De Kloet, S.R. & Dorrestein, G.M. (2009). Presence of avian bornavirus RNA and anti-avian bornavirus antibodies in apparently healthy macaws. Avian Diseases, 53, 568–573. doi: 10.1637/8828-040209-Reg.1
- Degernes, L.A., Flammer, K. & Fisher, P. (1991). Proventricular dilatation syndrome in a Green-winged macaw. In D.J. Harris (Ed.), Proceedings of the Annual Conference of the Association of Avian Veterinarians (pp. 45–49). Chicago, IL.
- Fraser, G.G. & Harris, E.K. (1989). Generation and application of data on biological variation in clinical chemistry. Critical Reviews in Clinical Laboratory Sciences, 27, 409–437. doi: 10.3109/10408368909106595
- Fudge, A.M. (1997). Avian clinical pathology – hematology and chemistry. In R.B. Altmann (Ed.), Avian medicine and Surgery (1st ed., pp. 142–157). Philadelphia, PA: W.B. Saunders Company.
- Fudge, AM. (2000). Laboratory medicine – avian and exotic pets. Philadelphia, PA: W.B. Saunders Company.
- Furrer, E., Bilzer, T., Stitz, L. & Planz, O. (2001a). Neutralizing antibodies in persistent Borna disease virus infection: prophylactic effect of gp94-specific monoclonal antibodies in preventing encephalitis. Journal of Virology, 75, 943–951. doi: 10.1128/JVI.75.2.943-951.2001
- Furrer, E., Bilzer, T., Stitz, L. & Planz, O. (2001b). High-dose Borna disease virus infection induces a nucleoprotein-specific cytotoxic T-lymphocyte response and prevention of immunopathology. Journal of Virology, 75, 11700–11708. doi: 10.1128/JVI.75.23.11700-11708.2001
- Gancz, A.Y., Clubb, S. & Shivaprasad, H.L. (2010). Advanced diagnostic approaches and current management of proventricular dilatation disease. Veterinary Clinics of North America: Exotic Animal Practice, 13, 471–494.
- Gancz, A.Y., Kistler, A.L., Greninger, A.L., Farnoushi, Y., Mechani, S., Perl, S., Berkowitz, A., Perez, N., Clubb, S., DeRisi, J.L., Ganem, D. & Lublin, A. (2009). Experimental induction of proventricular dilatation disease in cockatiels (Nymphicus hollandicus) inoculated with brain homogenates containing avian bornavirus 4. Virology Journal, 6, 100. doi: 10.1186/1743-422X-6-100
- García-Montijano, M., García, A., Lemus, J.A., Montesinos, A., Canales, R., Luaces, I. & Pereira, P. (2002). Blood chemistry, protein electrophoresis, and hematologic values of captive Spanish Imperial eagles (Aquila adalberti). Journal of Zoo and Wildlife Medicine, 33, 112–117. doi: 10.1638/1042-7260(2002)033[0112:BCPEAH]2.0.CO;2
- Gentry, J., Heatley, J.J. & Tizard, I. (2015). Experimental infection of Peach-faced lovebirds (Agapornis roseicollis) with avian bornavirus genotypes II und IV. In C. Kirk Baer (Ed.), Proceedings of the ExoticsCon (p. 91). San Antonio, TX.
- Gray, P., Hoppes, S., Suchodolski, P., Mirhosseini, N., Payne, S., Villanueva, I., Shivaprasad, H.L., Honkavuori, K.S., Lipkin, W.I., Briese, T., Reddy, S.M. & Tizard, I. (2010). Use of avian bornavirus isolates to induce proventricular dilatation disease in conures. Emerging Infectious Diseases, 16, 473–479. doi: 10.3201/eid1603.091257
- Gregory, C.R., Latimer, K.S., Niagro, F.D., Branson, W.R., Campagnoil, R.P., Norton, T.M., McManamon, R. & Greenacre, C.B. (1994). A review of proventricular dilatation syndrome. Journal of the Association of Avian Veterinarians, 8, 69–75. doi: 10.2307/27671120
- Halami, M.Y., Nieper, H., Müller, H. & Johne, R. (2008). Detection of a novel circovirus in Mute swans (Cygnus olor) by using nested broad-spectrum PCR. Virus Research, 132, 208–212. doi: 10.1016/j.virusres.2007.11.001
- Harris, E.K. (1974). Effects of intra- and interindividual variation on the appropriate use of normal ranges. Clinical Chemistry, 20, 1535–1542.
- Hatalski, C.G., Hickey, W.F. & Lipkin, W.I. (1998). Humoral immunity in the central nervous system of Lewis rats infected with Borna disease virus. Journal of Neuroimmunology, 90, 128–136. doi: 10.1016/S0165-5728(98)00066-6
- Heffels-Redmann, U., Enderlein, D., Herzog, S., Herden, C., Piepenbring, A., Neumann, D., Muller, H., Capelli, S., Muller, H., Oberhauser, K., Gerlach, H., Kaleta, E.F. & Lierz, M. (2011). Occurrence of avian bornavirus infection in captive psittacines in various European countries and its association with proventricular dilatation disease. Avian Pathology, 40, 419–426. doi: 10.1080/03079457.2011.589825
- Heffels-Redmann, U., Enderlein, D., Herzog, S., Piepenbring, A., Bürkle, M., Neumann, D., Herden, C. & Lierz, M. (2012). Follow-up investigations on different courses of natural avian bornavirus infections in psittacines. Avian Diseases, 56, 153–159. doi: 10.1637/9844-062811-Reg.1
- Herzog, S., Enderlein, D., Heffels-Redmann, U., Piepenbring, A., Neumann, D., Kaleta, E.F., Müller, H., Lierz, M. & Herden, C. (2010). Indirect immunofluorescence assay for intra vitam diagnosis of avian bornavirus infection in psittacine birds. Journal of Clinical Microbiology, 48, 2282–2284. doi: 10.1128/JCM.00145-10
- Honkavuori, K.S., Shivaprasad, H.L., Williams, B.L., Quan, P.L., Hornig, M., Street, C., Palacios, G., Hutchison, S.K., Franca, M., Egholm, M., Briese, T. & Lipkin, W.I. (2008). Novel Borna virus in psittacine birds with proventricular dilatation disease. Emerging Infectious Diseases, 14, 1883–1886. doi: 10.3201/eid1412.080984
- Hoppes, S., Gray, P.L., Payne, S., Shivaprasad, H.L. & Tizard, I. (2010). The isolation, pathogenesis, diagnosis, transmission, and control of avian bornavirus and proventricular dilatation disease. Veterinary Clinics of North America: Exotic Animal Practice, 13, 495–508.
- Ivey, E.S. (2000). Serologic and plasma protein electrophoretic findings in 7 psittacine birds with aspergillosis. Journal of Avian Medicine and Surgery, 14, 103–106. doi: 10.1647/1082-6742(2000)014[0103:SAPPEF]2.0.CO;2
- Jenkins, T. (1991). Creatinine kinase as a diagnostic indicator of splanchnic neuropathy. Journal of the Association of Avian Veterinarians, 5, 49. doi: 10.2307/27671011
- Joyner, K.L., Kock, N. & Styles, D. (1989). Encephalitis, proventricular and ventricular myositis, and myenteric ganglioneuritis in an Umbrella cockatoo. Avian Diseases, 33, 379–381. doi: 10.2307/1590862
- Kaneko, J.J. (1997). Serum proteins and dysproteinemias. In J. J. Kaneko (Ed.), Clinical Biochemistry of Domestic Animals (5th ed., pp. 117–138). San Diego, CA: Academic Press.
- Keller, D.L., Honkavuori, K.S., Briese, T., Lipkin, W.I., Muthuswamy, A., Steinberg, H. & Sladky, K.K. (2010). Proventricular dilatation disease associated with avian bornavirus in a Scarlet macaw (Ara macao). Journal of Veterinary Diagnostic Investigation, 22, 961–965. doi: 10.1177/104063871002200619
- Kistler, A., Gancz, A., Clubb, S., Skewes-Cox, P., Fischer, K., Sorber, K., Chiu, C., Lublin, A., Mechani, S., Farnoushi, Y., Greninger, A., Wen, C., Karlene, S., Ganem, D. & DeRisi, J. (2008). Recovery of divergent avian bornaviruses from cases of proventricular dilatation disease: identification of a candidate etiologic agent. Virology Journal, 5, 88. doi: 10.1186/1743-422X-5-88
- Kistler, A.L., Smith, J.M., Greninger, A.L., DeRisi, J.L. & Ganem, D. (2010). Analysis of naturally occurring avian bornavirus infection and transmission during an outbreak of proventricular dilatation disease among captive psittacine birds. Journal of Virology, 84, 2176–2179. doi: 10.1128/JVI.02191-09
- König, H.E. & Korbel, R. (2016). Medication and blood collection techniques. In H.E. König, R. Korbel & H.G. Liebich (Eds.), Avian Anatomy – Textbook and Colour Atlas (2nd ed., pp. 289–303). Sheffield: 5m Publishing.
- Korbel, R. & Rinder, M. (2011). Ocular findings in psittacine birds infected with avian bornavirus (ABV). In S. Orosz (Ed.), Proceedings of the Annual Conference of the Association of Avian Veterinarians (p. 21). Seattle, WA.
- Korbel, R. & Rinder, M. (2012). Update on psittacine proventricular disease (PDD) and avian Bornavirus infection. In E. Cross & R. Johnson (Eds.), Proceedings of the Annual Conference of the Australasian Committee Association of Avian Veterinarians and Unusual Exotic Pet Veterinarians (pp. 103–105). Melbourne, Australia.
- Kuhn, J.H., Durrwald, R., Bao, Y., Briese, T., Carbone, K., Clawson, A.N., deRisi, J.L., Garten, W., Jahrling, P.B., Kolodziejek, J., Rubbenstroth, D., Schwemmle, M., Stenglein, M., Tomonaga, K., Weissenbock, H. & Nowotny, N. (2015). Taxonomic reorganization of the family Bornaviridae. Archives of Virology, 160, 621–632. doi: 10.1007/s00705-014-2276-z
- Lierz, M. (2016). Avian bornavirus and proventricular dilatation disease. In B. Speer (Ed.), Current therapy in avian medicine and surgery (pp. 28–46). St. Louis, MI: Elsevier.
- Lierz, M., Hafez, H.M., Honkavuori, K.S., Gruber, A.D., Olias, P., Abdelwhab, E.M., Kohls, A., Lipkin, W.I., Briese, T. & Hauck, R. (2009). Anatomical distribution of avian bornavirus in parrots, its occurrence in clinically healthy birds and ABV-antibody detection. Avian Pathology, 38, 491–496. doi: 10.1080/03079450903349238
- Lutz, M. & Wilson, R. (1991). Psittacine proventricular dilatation syndrome in an Umbrella cockatoo. Journal of American Veterinary Medical Association, 198, 1962–1964.
- Mannl, A., Gerlach, H. & Leipold, R. (1987). Neuropathic gastric dilatation in psittaciformes. Avian Diseases, 31, 214–221. doi: 10.2307/1590799
- Mirhosseini, N., Gray, P.L., Hoppes, S., Tizard, I., Shivaprasad, H. & Payne, S. (2011). Proventricular dilatation disease in cockatiels (Nymphicus hollandicus) after infection with a genotype 2 avian bornavirus. Journal of Avian Medicine and Surgery, 25, 199–204. doi: 10.1647/2010-030.1
- Ogawa, H., Yamaguchi, T. & Fukushi, H. (2005). Duplex shuttle PCR for differential diagnosis of budgerigar fledgling disease and psittacine beak and feather disease. Microbiology and Immunology, 49, 227–237. doi: 10.1111/j.1348-0421.2005.tb03724.x
- Oldach, D., Zink, M.C., Pyper, J.M., Herzog, S., Rott, R., Narayan, O. & Clements, J.E. (1995). Induction of protection against Borna disease by inoculation with high-dose-attenuated Borna disease virus. Virology, 206, 426–434. doi: 10.1016/S0042-6822(95)80058-1
- Ouyang, N., Storts, R., Tian, Y., Wigle, W., Villanueva, I., Mirhosseini, N., Payne, S., Gray, P. & Tizard, I. (2009). Histopathology and the detection of avian bornavirus in the nervous system of birds diagnosed with proventricular dilatation disease. Avian Pathology, 38, 393–401. doi: 10.1080/03079450903191036
- Payne, S., Shivaprasad, H.L., Mirhosseini, N., Gray, P., Hoppes, S., Weissenböck, H. & Tizard, I. (2011). Unusual and severe lesions of proventricular dilatation disease in cockatiels (Nymphicus hollandicus) acting as healthy carriers of avian bornavirus (ABV) and subsequently infected with a virulent strain of ABV. Avian Pathology, 40, 15–22. doi: 10.1080/03079457.2010.536978
- Phalen, D.N. (1986). An outbreak of psittacine proventricular dilatation syndrome (PPDS) in a private collection of birds and an atypical form of PPDS in a Nanday conure. In R.D. Axelson (Ed.), Proceedings of the Annual Conference of the Association of Avian Veterinarians (pp. 27–34). Miami, FL.
- Piepenbring, A.K., Enderlein, D., Herzog, S., Al-Ibadi, B., Heffels-Redmann, U., Heckmann, J., Lange-Herbst, H., Herden, C. & Lierz, M. (2016). Parrot Bornavirus (PaBV)-2 isolate causes different disease patterns in cockatiels than PaBV-4. Avian Pathology, 45, 156–168. doi: 10.1080/03079457.2015.1137867
- Piepenbring, A.K., Enderlein, D., Herzog, S., Kaleta, E.F., Heffels-Redmann, U., Ressmeyer, S., Herden, C. & Lierz, M. (2012). Pathogenesis of avian bornavirus in experimentally infected cockatiels. Emerging Infectious Diseases, 18, 234–241. doi: 10.3201/eid1802.111525
- Polo, F.J., Peinado, V.I., Viscor, G. & Palomeque, J. (1998). Hematologic and plasma chemistry values in captive psittacine birds. Avian Diseases, 42, 523–535. doi: 10.2307/1592679
- Quesenberry, K. & Moroff, S. (1991). Plasma electrophoresis in psittacine birds. In D.J. Harris (Ed.), Proceedings of the Annual Conference of the Association of Avian Veterinarians (pp. 112–117). Chicago, IL.
- Reuter, A., Ackermann, A., Kothlow, S., Rinder, M., Kaspers, B. & Staeheli, P. (2010). Avian bornaviruses escape recognition by the innate immune system. Viruses, 2, 927–938. doi: 10.3390/v2040927
- Rich, G.A. (1992). Classic and atypical cases of proventricular dilatation disease. In J.R. Jenkins (Ed.), Proceedings of the Annual Conference of the Association of Avian Veterianrians (pp. 119–125). New Orleans, LA.
- Ridgway, R.A. & Gallerstein, G.A. (1983). Proventricular dilatation in psittacines. In S.L. Clubb (Ed.), Proceedings of the Annual Conference of the Association of Avian Veterinarians (pp. 228–233). San Diego, CA.
- Rinder, M., Ackermann, A., Kempf, H., Kaspers, B., Korbel, R. & Staeheli, P. (2009). Broad tissue and cell tropism of avian bornavirus in parrots with proventricular dilatation disease. Journal of Virology, 83, 5401–5407. doi: 10.1128/JVI.00133-09
- Rinder, M., Adrian, K., Staeheli, P., Kaspers, B. & Korbel, R. (2010). Bornavirus infections: development of serological tests for psittacine birds. In E. Bergmann (Ed.), Proceedings of the Annual Conference of the Association of Avian Veterinarians (p. 17). San Diego, CA.
- Rosenthal, K.L., Johnston, M.S. & Shofer, F.S. (2005). Assessment of the reliability of plasma electrophoresis in birds. American Journal of Veterinary Research, 66, 375–378. doi: 10.2460/ajvr.2005.66.375
- Rubbenstroth, D., Rinder, M., Kaspers, B. & Staeheli, P. (2012). Efficient isolation of avian bornaviruses (ABV) from naturally infected psittacine birds and identification of a new ABV genotype from a Salmon-crested cockatoo (Cacatua moluccensis). Veterinary Microbiology, 161, 36–42. doi: 10.1016/j.vetmic.2012.07.004
- Rubbenstroth, D., Schmidt, V., Rinder, M., Legler, M., Corman, V.M. & Staeheli, P. (2014). Discovery of a new avian bornavirus genotype in Estrildid finches (Estrildidae) in Germany. Veterinary Microbiology, 168, 318–323. doi: 10.1016/j.vetmic.2013.11.032
- Scope, A., Schwendenwein, I. & Frommlet, F. (2005). Influence of outlying values and variations between sampling days on reference ranges for clinical chemistry in budgerigars (Melopsittacus undulatus). Veterinary Record, 156, 310–314. doi: 10.1136/vr.156.10.310
- Scope, A., Schwendenwein, I. & Frommlet, F. (2006). Biological variation, individuality and critical differences of eight biochemical blood constituents in budgerigars (Melopsittacus undulatus). Veterinary Record, 159, 839–843.
- Scope, A., Schwendenwein, I. & Gabler, C. (2002). Short-term variations of biochemical parameters in racing pigeons (Columba livia). Journal of Avian Medicine and Surgery, 16, 10–15. doi: 10.1647/1082-6742(2002)016[0010:STVOBP]2.0.CO;2
- Solberg, H.E. (1987). Approved recommendation (1987) on the theory of reference values. Part 5. Statistical treatment of collected reference values. Determination of reference limits. Clinica Chimica Acta, 170, S13–S32. doi: 10.1016/0009-8981(87)90151-3
- Suedmeyer, W.K. (1992). Diagnosis and clinical progression of three cases of proventricular dilatation syndrome. Journal of the Association of Avian Veterinarians, 6, 159–163. doi: 10.2307/30136722
- Tatum, L.M., Zaias, J., Mealey, B.K., Cray, C. & Bossart, G.D. (2000). Protein electrophoresis as a diagnostic and prognostic tool in raptor medicine. Journal of Zoo and Wildlife Medicine, 31, 497–502. doi: 10.1638/1042-7260(2000)031[0497:PEAADA]2.0.CO;2
- Tomaszewski, E., Wilson, V.G., Wigle, W.L. & Phalen, D.N. (2001). Detection and heterogeneity of herpesviruses causing Pacheco’s disease in parrots. Journal of Clinical Microbiology, 39, 533–538. doi: 10.1128/JCM.39.2.533-538.2001
- VanDevanter, D.R., Warrener, P., Bennett, L., Schultz, E.R., Coulter, S., Garber, R.L. & Rose, T.M. (1996). Detection and analysis of diverse herpesviral species by consensus primer PCR. Journal of Clinical Microbiology, 34, 1666–1671.
- Villanueva, I., Gray, P., Mirhosseini, N., Payne, S., Hoppes, S., Honkavuori, K.S., Briese, T., Turner, D. & Tizard, I. (2010). The diagnosis of proventricular dilatation disease: use of a Western blot assay to detect antibodies against avian Borna virus. Veterinary Microbiology, 143, 196–201. doi: 10.1016/j.vetmic.2009.11.041
- Weissenböck, H., Bakonyi, T., Sekulin, K., Ehrensperger, F., Doneley, R.J., Dürrwald, R., Hoop, R., Erdélyi, K., Gál, J. & Kolodziejek, J. (2009). Avian bornaviruses in psittacine birds from Europe and Australia with proventricular dilatation disease. Emerging Infectious Diseases, 15, 1453–1459. doi: 10.3201/eid1509.090353