ABSTRACT
Aspergillosis is a common and life-threatening respiratory disease in raptors with acute and chronic courses. Among raptors, gyrfalcons (Falco rusticolus) and their hybrids are often declared to be highly susceptible with juvenile individuals being the most susceptible. However, species- and age-specific experimental studies are lacking and minimal infective doses (IDs) for Aspergillus spp. conidia are unknown.Therefore, 8-week-old, healthy gyr-hybrid falcons (F. rusticolus X F. cherrug) (N = 18) were experimentally infected with Aspergillus fumigatus using a single intratracheal inoculation with varying dosages of conidia (102 to 107 conidia). Over 28 days, clinical signs were monitored as well as haematological and serological parameters. Following euthanasia, necropsy, histopathology, bacteriology, and mycology were performed. Re-isolated fungi were compared to the inoculum using microsatellite length polymorphisms. During the trial, clinical signs and dyspnoea correlated significantly with the ID. Necropsy revealed fungal lesions in the upper and lower airways of 10/18 inoculated falcons, but not in the control birds. In 9/18 inoculated falcons, fungal granulomas were confirmed in histopathology and A. fumigatus was re-isolated from these granulomas. Except one nasal isolate all re-isolated fungal strains were identical to the inoculum strain. Based on mycology and histopathology a minimal ID of 50% was calculated to be MID50% (±S.E.) = 104.52±0.67 for a single tracheal inoculation of A. fumigatus conidia. This study demonstrates for the first time that a single exposure is able to cause acute aspergillosis in juvenile falcons.
Introduction
Aspergillosis is a life-threatening and common disease in raptors (Forbes, Citation1991; Joseph, Citation2000; Deem, Citation2003; Beernaert et al., Citation2010) affecting individuals in captivity (Van Cutsem & Fransen, Citation1987; Redig, Citation2007; Rahim et al., Citation2013) and free-ranging birds of prey worldwide (Redig et al., Citation1980; Redig, Citation1993; Caliendo & McKinney, Citation2013). The intra-vitam diagnosis is difficult as pathognomonic signs are lacking and a combination of various diagnostics is required to detect avian aspergillosis (Jones & Orosz, Citation2000; Fischer & Lierz, Citation2015). The disease is caused by fungal agents mainly belonging to the genus Apergillus sp. with Aspergillus fumigatus being the most prevalent and ubiquitous species (Latgé, Citation1999). The agent is spread by fungal conidia, subsequently aerosolized and inhaled by the avian host (Nardoni et al., Citation2006).
Acute, subacute, and chronic courses of avian aspergillosis are described, as well as localized and systemic forms (Redig, Citation1993). Chronic courses are regularly reported in veterinary practice, developing over a prolonged time (more than four weeks) and being caused by a combination of the fungal agent and various co-factors (Beernaert et al., Citation2010). To this end, parasitic, bacterial or viral co-infections, vitamin A deficiency, immunosuppression, long-term antibiosis or corticosteroid therapy, genetic factors, and insufficient management or husbandry conditions are discussed as potential co-factors for chronic aspergillosis (Van Cutsem & Fransen, Citation1987; McMillan & Petrak, Citation1989; Rautenschlein & Legler, Citation2006; Redig, Citation2007; Caliendo & McKinney, Citation2013).
In contrast, acute and subacute courses of aspergillosis are thought to be caused by a high amount of fungal conidia which overwhelm the host’s defence mechanisms and induce the disease within a few days to maximum 2–4 weeks, even in previously healthy individuals (Kunkle & Rimler, Citation1996; Redig, Citation2007). Such high loads of fungal conidia may be emitted from waste or harvested grains and hay, litter and moldy foodstuff (Dyar et al., Citation1984; Glare et al., Citation2014), and especially when a period of high humidity is followed by a drying period (Dykstra et al., Citation1997). However, the number of spores necessary to induce the acute or subacute course of aspergillosis is presently unknown in most raptor species. In general, these courses of avian aspergillosis including the associated clinical, haematological, and pathologic findings are still not well understood in birds. There are only few case reports available from parrots (Van der Heyden, Citation1993), ducks (Adrian et al., Citation1978), ostriches (Perelman & Kuttin, Citation1992), gulls (Friend & Trainer, Citation1969), penguins (Carrasco et al., Citation1993), and storks (Olias et al., Citation2010).
Detailed experimental studies on aspergillosis have been performed mostly in livestock poultry (O’Meara & Chute, Citation1959; Austwick, Citation1969; Friend & Trainer, Citation1969; Ghori & Edgar, Citation1973; Redig, Citation1980; Richard et al., Citation1981; Carrasco et al., Citation1993; Kunkle & Rimler, Citation1996; Olias et al., Citation2010), and only in a few exotic or companion bird species such as starlings (Atasever & Gümüşsoy, Citation2004), quail (Ghori & Edgar, Citation1973; Chaudhary & Sadana, Citation1988; Gümüşsoy et al., Citation2004), pigeons (Beernaert et al., Citation2008; Van Waeyenberghe et al., Citation2012), hawks (Redig, Citation1980), and falcons (Van Waeyenberghe et al., Citation2012). Different application schemes have been used altering comparability of these experimental studies. Aspergillus spp. spores have been injected in the air sacs or the lungs of turkeys and pigeons (Peden & Rhoades, Citation1992; Kunkle & Rimler, Citation1996; Femenia et al., Citation2007; Beernaert et al., Citation2008), injected intravenously in pigeons (Elmubarak & Fadlelmula, Citation1991), and injected intracoelomically in chickens (Okoye & Okeke, Citation1986). As a less invasive alternative, simulating the natural infection route, an inoculation via aerosol has been performed in chickens and turkeys (O’Meara & Chute, Citation1959; Taylor & Burroughs, Citation1973; Richard et al., Citation1981; Richard & Thurston, Citation1983; Julian & Goryo, Citation1990), and an intratracheal application has been used in ducks, quail, starlings, pigeons, turkeys, red-tailed hawks, and falcons (Chaudhary & Sadana, Citation1988; Graczyk et al., Citation1998; Atasever & Gümüşsoy, Citation2004; Gümüşsoy et al., Citation2004; Beernaert et al., Citation2008; Tell et al., Citation2010; Van Waeyenberghe et al., Citation2012; Wlaz et al., Citation2015). Only the tracheal inoculation allows for detailed determination of the inoculated conidia via the natural infection route, as the conidia concentration inside the inhalation chambers is not constant and the respiratory volume of the individuals differs (Femenia et al., Citation2007). However, a detailed determination of the amount of fungal conidia is important for the determination of husbandry guidelines including limit values for Aspergillus spp. conidia in the air.
Besides the amount of the infectious agent, a variable susceptibility of bird species to Aspergillus spp. has been discussed. In particular, mainly based on the number of clinical presentations, certain raptor species such as gyrfalcons (Falco rusticolus), merlins (Falco columbarius), rough-legged hawks (Buteo lagopus), red-tailed hawks (Buteo jamaicensis), golden eagles (Aquila chrysaetos), sea eagles (Haliaeethus spp.), ospreys (Pandion haliaetus), and goshawks (Accipiter gentilis) and their hybrids are thought to be highly susceptible to aspergillosis (Forbes, Citation1991; Redig, Citation1993, Citation2007; Heidenreich, Citation1997; Joseph, Citation2000; Naldo & Samour, Citation2004). An experimental study confirmed differences in susceptibility between chickens, ducks and Cortunix quail (Ghori & Edgar, Citation1973). The first experiment, using 3258 fungal conidia per cubic foot of air, resulted in clinical effects in 20%, 60% and 67% of these three avian species respectively. In a second experiment using 9840 conidia, disease was produced in 60%, 80% and 93% of the chickens, ducks and Cortunix quail respectively. Interestingly, the pathologic lesions such as congested lungs or fungal granulomas in lungs and air sacs were more severe and have been more frequently found in turkeys compaired to quails and chickens. In raptors such experimental trials are very limited. Only one study in hybrid gyrfalcons and pigeons is available, where no differences in susceptibility have been demonstrated between the two species, although pigeons are generally considered to be less susceptible than raptors (Van Waeyenberghe et al., Citation2012). This demonstrates that the transfer of susceptibility as well as clinical and pathologic results from one avian species to another species is limited and that species-specific research is required.
Independently from the species, numerous reports indicate that young birds are more susceptible than adults (O’Meara & Chute, Citation1959; Taylor & Burroughs, Citation1973; Adrian et al., Citation1978; Morris & Fletcher, Citation1988; Redig, Citation1993, Citation2007; Sidor et al., Citation2003; Femenia et al., Citation2007). Therefore, young individuals should be examined in order to define limit values for fungal conidia concentrations in the ambient air of falcon enclosures. In large falcons, only immature/adult individuals have been investigated yet (Van Waeyenberghe et al., Citation2012). Therefore, the potentially higher susceptible group of young individuals has not been investigated, representing the age of first trade, transport, and training.
The aim of this study was to evaluate the susceptibility of juvenile gyr-hybrid falcons to A. fumigatus conidia and to evaluate clinical, haematological, pathologic, and histopathologic findings under controlled, experimental conditions. Furthermore, the minimal ID to induce aspergillosis in these birds and the influence of the ID on the clinical and pathologic course of the disease were investigated.
We hypothesized that aspergillosis can be induced in healthy juvenile falcons by a single, experimental inoculation of A. fumigatus conidia. Moreover, we hypothesized that juvenile falcons are more susceptible to A. fumigatus conidia, measurable by more severe clinical signs, higher mortality, and more severe pathologic lesions, compared to immature/adult individuals, which have been investigated in a previous trial (Van Waeyenberghe et al., Citation2012).
Materials and methods
Birds, husbandry, and health check
Twenty-one captive-bred, 2-month-old, male gyr-saker hybrid falcons (F. rusticolus × F. cherrug) were obtained from a commercial falcon breeding centre. Upon arrival, general examination, faecal parasitology (direct smear and flotation), haematology, radiologic examination, and endoscopic laparoscopy (including thoracic and abdominal air sacs) were performed in all birds to ensure absence from diseases. All falcons were found healthy before the trial, especially free of respiratory signs typical for aspergillosis (Fischer & Lierz, Citation2015).
Birds were housed on screen perches according to standard methods in falconry () (Heidenreich, Citation1997) and the diet consisted of one-day-old chicks ad libitum once daily. All perches were placed in one air-filtered room, which had been thoroughly cleaned and disinfected (F10 SC Veterinary DisinfectantTM, Health & Hygiene, Florida Hills, South Africa; Mold Bomb Fogger, Biocide Laboratories, Cumming, GA, USA) previously. The inside air was continuously monitored for environmental mould contamination using an air-sampler twice a week (MAS-100 Eco impaction air-sampler®, Merck, Whitehouse Station, NJ, USA) and subsequent aerobic culture at 37°C on Sabouraud dextrose agar (SAB) (CM0041; Oxoid Ltd, Basingstoke, UK) agar plates as described earlier (Van Waeyenberghe et al., Citation2012). Eight measurements ranged between 50 and 140 CFU (colony forming units)/m³ and an average concentration of 91.43 ± 32.70 A. fumigatus CFU/m³ air was detected during the trial.
Fungal strain and inoculum preparation
The A. fumigatus strain K125 (accession no. of rRNA and ITS genes HE864321), isolated from a granuloma of a gyr-saker hybrid falcon which died from aspergillosis, was prepared for inoculation according to previous studies (Van Waeyenberghe et al., Citation2012). Briefly, the isolate was cultured for five days at 37°C on SAB. To harvest fungal conidia the culture was washed with 5 ml 0.01% Tween 20 in Hank’s balanced salt solution (HBSS). Conidia were washed three times in 0.01% Tween 20 HBSS (centrifugation: 3200 × g for 10 min at 4°C) before the suspension was adjusted to 107, 106, 105, 104, 103, and 102 conidia/0.5 ml HBSS by haemocytometer count (Van Waeyenberghe et al., Citation2012). To determine numbers of viable conidia 10-fold serial dilutions were cultured on SAB in 0.01% Tween 20 in HBSS at 37°C and the number of CFU per ½ millilitre was calculated after 24 h of incubation. The final conidia suspension had viable counts of 1.20 × 107, 0.78 × 106, 0.88 × 105, 0.66 × 104, 0.80 × 103, and 1.50 × 102 CFU/0.5 ml.
Challenge studies
The trial was performed with the permission of the ethical committee of the competent regional authority (GI 18/9 Nr. 69/2011 – Regierungspraesidium Giessen, Germany) and in accordance with the European animal welfare regulations.
Six groups of three falcons (B1–B6) were each inoculated intratracheally with 107 (B1), 106 (B2), 105 (B3), 104 (B4), 103 (B5), and 102 (B6) A. fumigatus conidia, respectively, in 0.5 ml HBSS, and one group of three falcons was sham-inoculated intratracheally with 0.5 ml HBSS (B7). The inoculation was performed under general anaesthesia with isoflurane (Isoflo CP®, CP-Pharma, Burgdorf 31303, Germany) using a paediatric endotracheal tube (inner diameter 2.5 mm, outer diameter 4.1 mm, length 165 mm) (Vygon, Ecouen 95440, France). Blood samples were collected in 1.3 ml lithium-heparinized tubes (Sarstedt AG & Co., Nümbrecht 51582, Germany) from the wing vein (Vena basilica) before the onset of inoculation, smeared for haematology, and centrifuged (2400 × g for 10 min at 21°C) immediately after collection. Haematology smears were stained with Wright-Giemsa stain and evaluated according to standard procedures (Pendl, Citation2008a, Citation2008b). Plasma samples were stored at −20°C and forwarded for further analysis of A. fumigatus antibodies, A. fumigatus antigens (Galactomannan and beta-(1,3)-D-glucan), A. fumigatus toxin (Fumigaclavin A), acute phase proteins (Haptoglobin and serum amyloid A), and plasma protein electrophoresis which have been published elsewhere (Fischer et al., Citation2014).
All falcons were examined daily for posture, plumage (ruffled feathers), behaviour, quality of excrements, respiration, and food uptake (Beernaert et al., Citation2008). The investigator did not know which bird belonged to which experimental group (blinded evaluation). Respiration was monitored more closely for the occurrence of abdominal respiration, tail bobbing, increased wing movement, open-beak breathing and stridors, and documented using a dyspnoea score ranging from 0 to 1 (average of abdominal respiration and tail bobbing): a dyspnoea score 0 ≥ dyspnoea score < 0.2 referred to a normal respiration, 0.2 ≥ dyspnoea score < 0.4 to a slightly increased, but not affected (unsuspicious) respiration, dyspnoea score 0.4 ≥ dyspnoea score < 0.6 to a low-grade affected respiration, 0.6 ≥ dyspnoea score < 0.8 to a middle-grade affected respiration, 0.8 ≥ dyspnoea score < 1 to a high-grade affected respiration, and dyspnoea score = 1 to the highest grade of respiratory depression. Respiration, posture, feeding behaviour, and the presence of ruffled feathers, vomitus, stridors, and open-beak breathing were scored and combined to a clinical score ranging from 0 to 4 according to . Clinical score = 0 referred to a healthy/inconspicuous status, clinical score = 1 to a mildly/low-grade affected status, clinical score = 2 to a moderately/middle-grade affected status, and clinical score = 3 to a severely/high-grade affected status. Deceased birds were given a clinical score = 4. Birds were euthanized on the 28th day post inoculation (dpi) or earlier, when falcons showed severe dyspnoea (dyspnoea score = 1), extreme weight loss (>20%), seizures, torticollis, or somnolence.
Table 1. Definition of the clinical scores (CS) applied to 21 juvenile falcons during the 28 days of the infection trial.
Pathological, histopathological, and immunohistochemical examination
Immediately after euthanasia a complete gross pathologic examination was performed and macroscopic aspergillosis lesions were noted using a pathology score (PS). PS = 0 referred to the absence of any lesions and PS = 1 to mild lesions, such as a slightly swollen spleen or reddened lung tissue without specific hints at aspergillosis. PS = 2 referred to mild to medium lesions, such as air sac opacity or thickness with neovascularization possibly associated with aspergillosis. PS = 3 referred to granulomatous lesion or other severe lesions, most likely associated with aspergillosis, e.g. fungal mycelium. Samples from the nasal concha, trachea, lung, heart, liver, spleen, proventriculus, ventriculus, intestines, kidney, cloacal bursa, pectoral muscle, brain, and granulomas were fixed in phosphate-buffered formaldehyde solution, sectioned and stained with haematoxylin–eosin (H&E), periodic acid Schiff reagent, and concanavalin A (ConA), according to previous studies (Greenfield et al., Citation1988; Van Waeyenberghe et al., Citation2012).
Microbiological examination and microsatellite length polymorphism
Samples from the trachea, syrinx, lung, cranial and caudal thoracic air sacs, abdominal air sacs, heart blood, liver, spleen, kidney, cloacal bursa, nose, brain, and potential fungal lesions were cultured on SAB (Oxoid) at 37°C for 72 h. Samples from the heart blood, liver, lungs, and granulomatous lesions were additionally cultured on Columbia sheep-blood agar (CSB) (Oxoid) under the same conditions.
Each culture from re-isolated fungi (re-isolated from birds and from ambient air) as well as the experimentally inoculated conidia was analysed and compared using microsatellite length polymorphism according to previous studies (Van Waeyenberghe et al., Citation2011). This was to confirm that the fungal re-isolates from the birds originated from the inoculated strain.
Statistical analysis
The statistical analyses were done by means of the software packages BMDP (BioMeDical Package, BMDP Statistical Solutions, Stonehill Corporate Center, Saugus, MA, USA) and StatXact (CYTEL Inc., Cambridge, MA, USA). To evaluate potential correlations of the ID with the clinical score and the dyspnoea score, respectively, the area under the curve of both scores over the complete observation time of the trial was plotted against the ID using bivariate scatter plots in the programme BMDP6D. As the scores are ordinal scaled variables, appropriate Spearman’s rank correlation coefficients (rs) of both scores (clinical score and dyspnoea score) and ID were calculated including levels of significance (P) of both scores using the programme BMD3D. P-levels < 0.05 were regarded as statistically significant. Based on the results of the histopathological confirmation of aspergillosis, specificity and sensitivity of haematological results and pathology scores including a 95% confidence interval were calculated using the programme BiAS for Windows Version 9.14 (Dr Hanns Ackermann, Goethe-University Frankfurt, Germany).
Two-dimensional frequency tables of ID against severe pathologic lesions were calculated using the programme BMDP4F. Additionally, the corresponding Spearman’s rank correlation coefficients for the relation between ID and pathologic lesions, histologic findings and mycological isolation, respectively, were calculated using the exact method by means of the programme StatXact9.
The minimal infective lg-dose (MID) 10% (MID10), 50% (MID50), and 90% (MID90) with their logarithmic asymptotic standard errors (SE) were estimated using the logistic regression model by maximum likelihood estimation with the programme BMDPLE.
Results
Clinical signs demonstrated in dyspnoea scores and clinical scores
Mild clinical signs which may have been associated with aspergillosis, such as a dyspnoea score = 0.5 and a clinical score of 2, were seen in all experimental groups and the control group at least on single days during the trial. Thus, the sensitivity of dyspnoea score ≥ 0.5 and clinical score ≥ 2 was low, being less helpful for the diagnosis of aspergillosis.
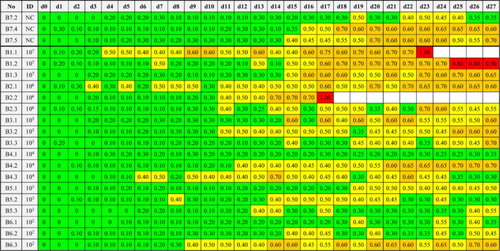
A medium/middle-grade affected respiration, such as a dyspnoea score = 0.6 was seen in 14/18 and a dyspnoea score = 0.7 in 11/18 inoculated falcons, respectively. However, in 2/3 control birds (B7.4, B7.5) respiration was also medium/middle-grade affected (0.6 ≥ dyspnoea score < 0.8) during the trial at 6 or 10 days, respectively. In three birds from the high-dosage groups (B1.1, B1.2, B2.2) dyspnoea score worsened to a severely/high-grade affected respiration with dyspnoea score ≥ 0.8. The area under the curve of dyspnoea score over the complete time of the trial correlated significantly with the ID rs = 0.52 (P = 0.017). The dyspnoea score of all falcons are displayed in .
Figure 2. Dyspnoea scores (average of abdominal respiration and tail bobbing) of falcons during the trial.
During the trial, the clinical signs in the infected groups included ruffled feathers, dyspnoea, mint-green coloured urates, and diarrhoea (). Inappetence and weight loss were observed in the three high-dosage groups (groups B1–B3) and vomiting and somnolence in group B1 only. Clinical score = 3 was seen in falcons from high-dosage groups (B1.1, B1.2, B1.3, B2.1) and one bird from the middle dosage group (B3.1).
Figure 3. Clinical signs of falcons following experimental inocculation of Aspergillus fumigatus intratracheally. (A) Sleeping falcon. (B) Falcon with drooping wings. (C) Falcons with open-beak breathing. (D) Falcon with sticky and dirty feathers after vomiting. (E) Mint-green urates of a falcon. (F) Falcon with ruffled feathers.

Figure 4. Clinical scores (CS) of falcons during the trial calculated by scores for respiration, posture, feeding behaviour, and plumage.
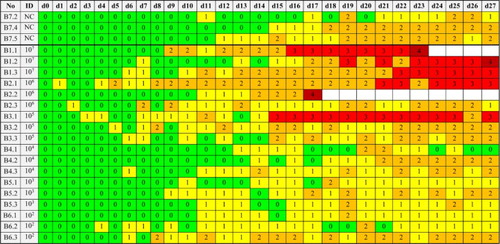
Three birds from the high-dosage groups (B1.1, B1.2, B2.2) had to be euthanized before the end of the trial, including one bird (B2.2) that did not reach clinical score = 3 previously.
The area under the curve of clinical score over the complete time of the trial correlated significantly with the ID rs = 0.67 (P < 0.001). The clinical score of all falcons are displayed in .
Haematology
At the beginning of the trial, 6/21 falcons (B1.3, B2.3, B4.1, B5.1, B5.2, B6.1) exceeded slightly the maximum leukocyte count (leukocytosis) compared to the haematologic reference range for gyr-hybrid falcons, which has been based on 990 clinically healthy individuals (Wernery et al., Citation2004). None of the falcons remained under the minimum reference value. The percentage distribution of the leukocyte fractions differed in 8/21 individuals (B1.3, B2.2, B3.1, B3.3, B4.3, B5.2, B5.3, B6.3) from the reference values (Wernery et al., Citation2004) previous to inoculation.
At the end of the trial, 11/21 falcons demonstrated a leukocytosis (B1.3, B1.1, B2.1, B2.2, B3.1, B3.2, B3.3, B5.2, B6.3, B7.4, B7.5), including two control birds and eight falcons which demonstrated granulomatous lesions in subsequent necropsy.
In five birds leukocytosis was associated with an increased number of heterophil granulocytes (heterophilia) and in four birds with an increased lymphocyte number (lymphocytosis). Between 2% and 17% of these lymphocytes revealed toxic activation, which was demonstrated by a darkish colouration of the cytoplasm and increased size as described previously (Pendl, Citation2008a, Citation2008b). The two control birds (B7.4, B7.5) demonstrated also a leukocytosis with heterophilia and lymphocytosis, respectively, in the absence of any pathologic lesions. Monocyte count did not exceed the reference values in any of the birds. Statistically, haematology findings (EWBC, percentage heterophil granulocytes, or lymphocytes) did not significantly correlate with the ID. Sensitivity of leukocytosis regarding the diagnosis of aspergillosis was 88.9% (51.8–99.7), of heterophilia 44.4% (13.7–78.8), of lymphopenia 55.6% (21.2–86.3), and of lymphocytosis 0% (0–28.3). Specificity of leukocytosis was 75% (42.8–94.5), of heterophilia and lymphopenia 91.7% (61.5–99.8), and of lymphocytosis 66.7% (34.9–90.1). The haematology values of the gyr-saker hybrid falcons are depicted in .
Table 2. Haematological examination of 18 falcons before and 28 days after inoculation with A. fumigatus conidia and three non-inoculated control falcons.
Pathological and histopathological findings
In 3/3 falcons from the high-dosage group B1 (107 A. fumigatus conidia) PS = 3 lesions were detected in the air sacs, in two of these also in the lungs and in one bird (B1.1) even in the whole respiratory tract, in the spleen, and the kidneys. In 2/3 falcons of group B2 (106 A. fumigatus conidia) PS = 2 and PS = 3 lesions were detected in the respiratory tract, including granulomatous lesions in the trachea and syrinx associated with a severe splenitis in one bird (B2.1). However, B2.3 demonstrated an opaque pericardium and no other lesions in any organ which were categorized PS = 2. All 3/3 falcons in middle-dose group B3 (105 A. fumigatus conidia) showed PS = 2 lesions in the respiratory tract and falcon B3.1 a PS = 3-lesion in the air sacs additionally. Likewise, PS = 2 lesions were detected in air sacs and lungs of one bird in each of the groups B4 (104 A. fumigatus conidia) and B6 (102 A. fumigatus conidia), but no specific lesions were detected in the other inoculated falcons or in the three control falcons. In general, the number of PS = 2 and PS = 3 lesions in organs varied between 6 and 11 in group B1 and 0 and 6 in the other infected experimental groups. The occurrence of pathologic lesions PS = 2 and PS = 3 () correlated significantly with the ID (rs = 0.684; P < 0.001). Sensitivity of PS = 1 and PS = 2 was 100% (71.7–100) and of PS = 3 66.7% (29.9–92.5). Specificity of PS = 1 was 50% (21.1–78.9), of PS = 2 91.7% (61.5–99.8), and of PS= 3 100% (77.9–100), respectively.
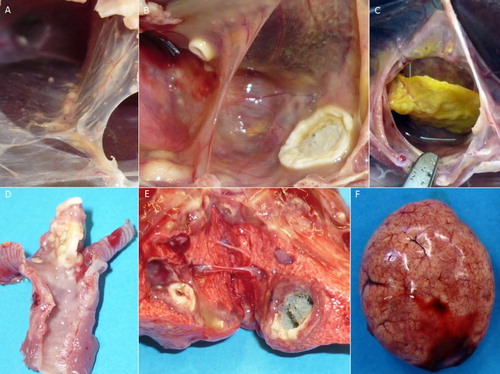
Figure 5. Pathological findings in falcons after experimental infection with Aspergillus fumigatus. (A) + (B) + (C) Fungal granuloma in the air sacs of falcons of varying sizes, from pinhead-sized nodules to complete filling of the air sac. (D) Fungal granuloma at the tracheal bifurcation. (E) Fungal granuloma with active mycelium in the lung. (F) Splenomegaly and follicular hyperplasia.
Histopathology identified purulent to granulomatous inflammatory processes in 9/18 inoculated falcons (B1.1, B1.2, B1.3, B2.1, B2.2, B3.1, B3.3, B4.2, B6.3) and none of the control birds. The purulent lesions contained heterophil granulocytes, while the granulomas demonstrated a concentric accumulation of heterophil granulocytes, epithelioid cells, polynuclear giant cells, and macrophages around a necrotic centre. 9/18 falcons demonstrated lesions inside the air sacs. Five of these nine falcons (B1.1, B1.2, B1.3, B2.1, B2.2) also showed lesions in the upper respiratory tract (trachea and syrinx) and five falcons (B1.1, B1.3, B2.2, B3.1, B6.3) also in the lungs. Moreover, in two of these nine individuals (B1.1, B1.3) additional granulomas were seen in other organs including the pericardium, liver, kidney, spleen, and Bursa cloacalis.
The control falcons and the remaining 9/18 inoculated falcons were free of histopathologic findings of aspergillosis. In general, the number of histopathologically confirmed fungal granulomas varied between 5 and 9 in group B1 and 0 and 5 in the other infected experimental groups. The occurrence of histopathologic confirmation of fungal structures inside the lesions correlated significantly with the ID (rs = 0.626; P = 0.003). The pathological findings and the histopathological confirmation of fungal hyphae in the periodic acid Schiff reagent stain are depicted in .
Table 3. Overview of pathologic lesions, histopathologic identification of fungal structures, and fungal re-isolation in 18 falcons 28 days after experimental inoculation of Aspergillus fumigatus conidia and in three non-inoculated control falcons.
Mycological results and typing of re-isolated fungal strains
In none of the falcons were bacteria cultured under aerobic conditions from the heart, liver, lung, and granulomas.
In 50% (9/18) of the inoculated falcons (B1.1, B1.2, B1.3, B2.1, B2.2, B3.1, B3.3, B4.2, B6.3) A. fumigatus was re-isolated from air sacs and in five of those (B1.1, B1.3, B2.2, B3.1, B6.3) also from the lungs, trachea, and syrinx, respectively. In two of these nine falcons (B1.3, B3.1) an additional re-isolation was possible from the nose, in two falcons from the kidneys (B1.1, B1.3), and in one falcon (B1.1) from the cloacal bursa and spleen as well. In general, the number of mycological positive re-isolation sites varied between 5 and 15 in group B1 and 0 and 5 in the other groups.
In all control birds and the remaining nine inoculated falcons (B2.3, B3.2, B4.1, B4.3, B5.1, B5.2, B5.3, B6.1, B6.2) re-isolation failed, even from PS = 2 air sac lesions. The success of fungal re-isolation from lesions correlated significantly with the ID (rs = 0.626; P = 0.003). The fungal re-isolation of A. fumigatus from various lesions in the experimentally inoculated falcons is depicted in .
The microsatellite length polymorphism confirmed that all A. fumigatus re-isolates from the internal organs of all nine histopathologically positive falcons (B1.1, B1.2, B1.3, B2.1, B2.2, B3.1, B3.3, B4.2, B6.3) were identical to the inoculum. Only one additional isolate from the nose in one falcon (B3.1) was identified as a different genotype. All fungal isolates from the ambient air inside the air-filtered room which were collected using an air-sampler differed from the inoculum and from the additional isolate in falcon B3.1.
Calculation of the minimal ID
Based on the histopathologic results as definitive diagnosis of avian aspergillosis, the logarithm of the minimal ID of A. fumigatus conidia was calculated by maximum likelihood estimation in the logistic model. This way the estimated minimal ID of A. fumigatus conidia was calculated as MID10 (±S.E.) = 101.95 ± 1.26, MID50 (±S.E.) = 104.52 ± 0.67, and MID90 (±S.E.) = 107.10 ± 1.33, respectively.
Discussion
One single deep tracheal inoculation of A. fumigatus conidia was used successfully to induce aspergillosis experimentally in juvenile falcons. Thus, the present study confirms the suitability of this infection route in accordance with previous studies in ducks (Graczyk & Cockrem, Citation1995), quail (Chaudhary & Sadana, Citation1988; Gümüşsoy et al., Citation2004; Tell et al., Citation2010; Wlaz et al., Citation2015), starlings (Atasever & Gümüşsoy, Citation2004), pigeons (Beernaert et al., Citation2008), adult falcons (Van Waeyenberghe et al., Citation2012), turkeys, and broad-winged hawks (Redig, Citation1980). Tracheal inoculation enabled application of an exactly quantified number of fungal conidia via the natural route of infection and the calculation of a minimal ID in contrast to the use of inhalation chambers (O’Meara & Chute, Citation1959; Taylor & Burroughs, Citation1973; Richard et al., Citation1981; Richard & Thurston, Citation1983; Julian & Goryo, Citation1990). The latter method would mimic the natural route of infection better, but does not offer a detailed quantification of inhaled conidia per individual (Femenia et al., Citation2007). From a clinical point of view the fact that a single inoculation is suitable to induce aspergillosis underlines the importance of good sanitation to reduce fungal spore exposure as effectively as possible (Redig, Citation1980, Citation2007).
In the present study, the minimal IDs of A. fumigatus conidia in juvenile gyr-saker hybrid falcons have been calculated as MID10 (±S.E.) 101.95 ± 1.26, MID50 (±S.E.) 104.52 ± 0.67, and MID90 (±S.E.) 107.10 ± 1.33, respectively. The MID calculation was based on histopathology results, which are currently regarded as state-of-the-art confirmation of avian aspergillosis (Jones & Orosz, Citation2000; Fischer & Lierz, Citation2015). These MID in falcons are below the lethal dose 50 (LD50) of 12.03 × 106 in quail (Chaudhary & Sadana, Citation1988), which represents to our knowledge the only calculated dose in birds. Moreover, these values are below the reported tracheal inoculation doses in turkeys (106 and 107 conidia) (Redig, Citation1980) and in starlings (1.35 × 106) (Atasever & Gümüşsoy, Citation2004) which have been associated with acute and subacute courses of aspergillosis; however, they are also below the doses in pigeons (0.2 × 108 conidia), which failed to induce aspergillosis following tracheal inoculation (Beernaert et al., Citation2008). On the other hand, the doses in falcons are higher than IDs in Pekin ducks (3.5 × 104 Aspergillus spp. conidia) which resulted in 60% mortality within 20 days post infection (Graczyk et al., Citation1998). Nevertheless, one bird (B6.3) was diseased after inoculation of a very low number of conidia (102 conidia). In contrast, in another bird belonging to the second high-dosage group (B2.3) aspergillosis was suspected in necropsy, but histopathology and mycology failed to confirm the presumptive diagnosis. Clearance of the infectious agent during the trial by the bird’s immune mechanisms may be a potential explanation besides individual susceptibility differences (Taylor & Burroughs, Citation1973; Femenia et al., Citation2007). Thus, individual differences must be considered in terms of fungal infections and limit values of A. fumigatus conidia for husbandry guidelines need to be interpreted critically.
Compared to adult gyr-saker hybrid falcons in a previous study (Van Waeyenberghe et al., Citation2012), a higher percentage of birds developed disease per group using the same IDs of the same A. fumigatus isolate K125; more precisely, aspergillosis was induced in 80% (4/5) of the adult and 100% (3/3) of the juvenile falcons using 107 conidia and in 20% (1/5) of the adults and 66.7% (2/3) of the juvenile falcons using 105 conidia. Moreover, three juvenile falcons had to be euthanized, while in adult falcons no mortality occurred and no indication for euthanasia was given (Van Waeyenberghe et al., Citation2012). This corresponds to studies in other avian species which suggested age-dependent susceptibility differences and the age-dependent development of the immune system as a possible explanation (Austwick, Citation1969; Beernaert et al., Citation2008). Therefore, an age-dependent susceptibility in falcons is very likely too. The age of the young gyr-saker hybrid falcons in the present study represents the common age of trade and transport of commercially bred falcons and therefore a challenging life period (Heidenreich, Citation1997). Thus, the results of the present study may be used to formulate threshold values for A. fumigatus conidia valid for falcons of different ages, because such values should always be based on the most susceptible group in a certain collection. However, species-specific differences between different falcon species cannot be ruled out when transferring the values of gyr-saker hybrid falcons to other falcons.
To correlate the experimental inoculation dose with the conidia concentration in ambient air one needs to account for the tidal volume of falcons per time period and set this in relation to the MID. As the experiment only covers an acute fungal exposure, a 1-hour ventilation and a 24-hour ventilation should be used as the maximum reference time period. Based on case reports, this time seems to be a realistic reference period under field conditions to inhale a temporarily increased amount of fungal conidia from ambient air during composting, harvesting, or littering work (Redig, Citation1980, Citation2007; Kunkle & Rimler, Citation1996). Repeated fungal exposure or exposure over a prolonged time were not investigated in the present study and they may induce a different course of the disease as shown in turkeys (Redig, Citation1980). In the absence of data about the respiration in gyr-saker hybrid falcons, respiratory data in closely related prairie falcons (Falco mexicanus) are used for calculation. In prairie falcons the minute ventilation varies depending on ambient temperature and body mass from 108.1–677.2 ml/min in males (weight: 444–532 g) to 155.4–717.5 ml/min in females (weight: 712–800 g) at 0–42°C ambient temperature (Kaiser & Bucher, Citation1985). Compared to the body mass of male gyr-saker hybrid falcons data in female prairie falcons seem to fit the best. To cover the highest risk in terms of a risk assessment the highest respiratory rate and the lowest MID of the most susceptible age group are used for calculation. In the case of gyr-saker hybrid falcons this would be the juvenile individuals in comparison to data in immature/adult falcons (Van Waeyenberghe et al., Citation2012). Multiplication of the maximum minute ventilation in female prairie falcons (717.5 ml/min ≙ 0.72 l/min) by 60 gave a maximum hour ventilation of 42.93 l/h (≙0.04 m3/h). Multiplication of the maximum 1-hour ventilation by 24 gave a maximum 24-hour ventilation of 1030.32 l/d (≙1.03 m3/d). The MID10 then was divided by the maximum respiratory rate, to calculate the conidia concentration in ambient air that must be inhaled by a falcon over 1 h or 24 h. To inhale the MID10 = 101.95 juvenile falcons need to inhale a concentration of 2076.06 CFU/m³ ambient air for 1 h or 86.50 CFU/m³/h for 24 h, respectively. At this point it should be noted that these respiratory data are only valid for resting birds while birds during flight may increase the respiratory volume by 2.5–20 times by increasing the respiratory frequency and the tidal volume (Hart & Roy, Citation1966; Torre-Bueno, Citation1978). Moreover, larger and heavier falcons may breathe more compared to the prairie falcons. Therefore, this highest risk scenario may not completely cover all sizes of falcons and increased respiratory rate during flight. However, respiratory rates decrease within the thermal neutral zone of birds, which is within 19–35°C for female prairie falcons, but increases outside this range (e.g. at hot temperatures). Therefore, the tolerable conidia dose may be higher within the thermal neutral zone. Nevertheless, in a highest risk scenario it is reasonable to define recommendations valid for different age groups and various temperatures during the whole year. In general, this conidia concentration seems to be below the range of normal conidia concentrations in outside air of 145–204 CFU/m³ (Ault & Schott, Citation1994), but recent studies detected much lower median values between 10 and 55 CFU/m³ for normal A. fumigatus concentration in ambient air (Trautmann et al., Citation2005). Moreover, these estimated values correspond to reports in falcons and gulls, which associated 400 CFU/m³ air (Schulz et al., Citation2007) and 450–525 CFU/m³ air (Nardoni et al., Citation2006), respectively, with an increased incidence of aspergillosis.
As a limitation of the study, it must be considered that the results were established using the A. fumigatus strain K125. The use of other strains may have led to different results, as virulence differences between fungal isolates have been stated previously (Peden & Rhoades, Citation1992; Mondon et al., Citation1996; Aufauvre-Brown et al., Citation1998). However, in this study a clinical isolate from a gyrfalcon that died due to aspergillosis was used, to mimic the most realistic epidemic conditions. Moreover, some authors stated a minor relevance of virulence differences between fungal isolates under field conditions (Olias et al., Citation2011). Therefore, the MID established in this study can be used for orientation.
The intra-vitam diagnosis of avian aspergillosis is difficult and requires in most cases invasive techniques such as endoscopy and biopsy of tissue for culture and/or histology (Jones & Orosz, Citation2000; Deem, Citation2003; Beernaert et al., Citation2010; Rahim et al., Citation2013; Fischer & Lierz, Citation2015). As mild clinical signs and mild dyspnoea occurred in all infected experimental groups and in the control birds, the results of the present study support that a clinical examination alone is regarded as not sufficient to detect avian aspergillosis. However, moderate to severe clinical signs and dyspnoea scores, such as dyspnoea score ≥0.8 and clinical score ≥3, were highly sensitive for the detection of aspergillosis. In this regard dyspnoea score and clinical score both correlated significantly with the ID. Therefore, clinicians should consider clinical signs (ruffled feathers, mint-green coloured urates, diarrhoea, inappetence, weight loss, vomiting, and somnolence) and signs for dyspnoea (tail bobbing, abdominal respiration, increased wing movement, open-beak breathing, and stridors) as indicators to initiate further diagnostic procedures (Fischer & Lierz, Citation2015). However, behavioural differences and individual differences (e.g. variation in breathing patterns) need to be taken into account in the interpretation of clinical signs.
The diagnostic procedures to detect aspergillosis commonly include serological and haematological examinations. A leukocytosis with heterophilia, lymphopenia, and monocytosis has been described as a sign of avian aspergillosis (Jones & Orosz, Citation2000; Pendl, Citation2008a). These data are mostly derived from clinical case reports with heterogeneous conditions and cannot be easily compared to experimental conditions of the present study. Experimental studies about haematologic changes associated with aspergillosis are available from quail (Coturnix coturnix japonica) only, describing a heterophilic leukocytosis with lymphopenia as a remarkable change (Pandita et al., Citation1991). This corresponds to the results of the present study, as leukocytosis occurred in 11/18 inoculated falcons, including the nine falcons with histopathologic and mycological confirmed aspergillosis. However, this leukocytosis was only in five birds associated with heterophilia and lymphopenia, but in four individuals with lymphocytosis and heteropenia. Moreover, two control birds demonstrated also a leukocytosis in the absence of any pathologic lesions at the end of the trial. Likewise, previous to inoculation, 6/21 falcons demonstrated a mild leukocytosis compared to the species-specific haematologic reference ranges (Wernery et al., Citation2004). Consequently, haematology parameters did not correlate significantly with the ID. Leukocytosis was the only parameter with a high sensitivity (88.9%) and specificity (75%) regarding the diagnosis of aspergillosis, while sensitivities of heterophilia, lymphocytosis, and lymphopenia were poor (each below 60%) Therefore, haematologic results should always be interpreted carefully and in combination with other diagnostic tools, as many factors are known to influence haematology values (Jones & Orosz, Citation2000; Fischer & Lierz, Citation2015).
In the present study, pathologic findings in 10/18 falcons included whitish-creamy, cheese-like to gelatinous coatings, nodular and confluent-diphtheroid lesions and fluffy, greenish pigmented fungal mycelium. The pathological lesions were confirmed in nine of these 10 birds in histopathology and/or mycology. The lesions corresponded to the typical aspergillosis lesions as described in previous studies (Beernaert et al., Citation2008; Cacciuttolo et al., Citation2009). In contrast to former statements, lesions of acute and subacute aspergillosis were not limited to the lungs or to the respiratory tract (Redig, Citation1993, Citation2007), but involved the kidneys, spleen, liver, pericardium, and cloacal bursa. These lesions had been mainly associated with chronic courses of avian aspergillosis (Redig, Citation1993, Citation2007), but should be considered in acute and subacute courses as well. The involvement of these organs may hint at an invasive form of aspergillosis with spreading per continuitatem, per contiguitatem, and haematogenous spreading as reported in other bird species (Richard & Thurston, Citation1983; Cacciuttolo et al., Citation2009; Barathidasan et al., Citation2013). In general, the occurrence of pathological lesions and positive results in histology and mycology correlated significantly with the ID.
In conclusion, this study proves for the first time that juvenile gyr-saker hybrid falcons develop acute to subacute aspergillosis after a single exposure of A. fumigatus conidia and it evaluates a minimal ID in these birds. That a single exposure to fungal spores may be sufficient to induce the disease needs to be taken into account when assessing aspergillosis cases in avian practice. When assessing the environment, a potential high environmental exposure should be considered as well.
These data may be used as a base for the formulation of husbandry guidelines and threshold values of fungal conidia in ambient air in zoological collections, breeding centres, and in falconry. Clinical signs, haematological values, and post-mortem findings were investigated under controlled conditions, which contributes to a better understanding of the disease in juvenile falcons, offering advantages for the diagnosis of avian aspergillosis.
Acknowledgements
The authors thank Andreas Schaubmar and Marion Sparenberg from the Unit for Biomathematics and Data Processing for assistance in statistical analysis. They also thank the staff of the Department of Pathology, Bacteriology and Avian Diseases at Ghent University (especially Prof Dr Pasmans and Dr Tom Hellebuyck) and of the Clinic for Birds, Reptiles, Amphibians, and Fish of Justus Liebig University Giessen (especially Luisa Ziegler, Helena Schneider, and Antoinette Huhn) for help and assistance during the trials and the laboratory examinations.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Dominik Fischer http://orcid.org/0000-0001-7334-6705
An Martel http://orcid.org/0000-0001-7609-5649
Additional information
Funding
References
- Adrian, W.J., Spraker, T.R. & Davies, R.B. (1978). Epornitics of aspergillosis in mallards (Anas platyrhynchos) in North Central Colorado. Journal of Wildlife Diseases, 14, 212–217. doi: 10.7589/0090-3558-14.2.212
- Atasever, A. & Gümüşsoy, K.S. (2004). Pathological, clinical and mycological findings in experimental aspergillosis infections of starlings. Journal of Veterinary Medicine Series A, 51, 19–22. doi: 10.1111/j.1439-0442.2004.00598.x
- Aufauvre-Brown, A., Brown, J.S. & Holden, D.W. (1998). Comparison of virulence between clinical and environmental isolates of Aspergillus fumigatus. European Journal of Clinical Microbiology & Infectious Diseases, 17, 778–780. doi: 10.1007/s100960050184
- Ault, S.K. & Schott, M. (1994). Aspergillus, aspergillosis, and composting operations in California. Sacramento, CA: Research Services Section, Grants and Research Branch, Markets, Research and Technology Division, California Integrated Waste Management Board, California Environmental Protection Agency.
- Austwick, P.C.K. (1969). Mycotic infections. In A. McDiarmid (Ed.), Diseases in Free-living Wild Animals - Symposia of the Zoological Society of London (pp. 249–271). London: Academic Press.
- Barathidasan, R., Singh, S.D., Saini, M., Sharma, A.K. & Dhama, K. (2013). The first case of angioinvasive pulmonary aspergillosis in a Himalayan griffon vulture (Gyps himalayensis). Avian Biology Research, 6, 302–306. doi: 10.3184/175815513X13818257354160
- Beernaert, L.A., Pasmans, F., Haesebrouck, F. & Martel, A. (2008). Modelling Aspergillus fumigatus infections in racing pigeons (Columba livia domestica). Avian Pathology, 37, 545–549. doi: 10.1080/03079450802382280
- Beernaert, L.A., Pasmans, F., Van Waeyenberghe, L., Haesebrouck, F. & Martel, A. (2010). Aspergillus infections in birds: a review. Avian Pathology, 39, 325–331. doi: 10.1080/03079457.2010.506210
- Cacciuttolo, E., Rossi, G., Nardoni, S., Legrottaglie, R. & Mani, P. (2009). Anatomopathological aspects of avian aspergillosis. Veterinary Research Communications, 33, 521–527. doi: 10.1007/s11259-008-9199-7
- Caliendo, V. & McKinney, P. (2013). Fungal airsacculitis associated with serratospiculiasis in captive falcons of the United Arab Emirates. Veterinary Record, 173, 143. doi: 10.1136/vr.101649
- Carrasco, L., Bautista, M.J., de las Mulas, J.M. & Jensen, H.E. (1993). Application of enzyme-immunohistochemistry for the diagnosis of aspergillosis, candidiasis, and zygomycosis in 3 lovebirds. Avian Diseases, 37, 923–927. doi: 10.2307/1592056
- Chaudhary, S.K. & Sadana, J.R. (1988). Experimental aspergillosis in Japanese quails (Coturnix coturnix japonica). Mycopathologia, 102, 179–184. doi: 10.1007/BF00437402
- Deem, S.L. (2003). Fungal diseases of birds of prey. Veterinary Clinics of North America: Exotic Animal Practice, 6, 363–376.
- Dyar, P.M., Fletcher, O.J. & Page, R.K. (1984). Aspergillosis in turkeys associated with use of contaminated litter. Avian Diseases, 28, 250–255. doi: 10.2307/1590149
- Dykstra, M.J., Loomis, M., Reininger, K., Zombeck, D. & Faucette, T. (1997). A comparison of sampling methods for airborne fungal spores during an outbreak of aspergillosis in the forest aviary of the North Carolina Zoological Park. Journal of Zoo and Wildlife Medicine, 28, 454–463.
- Elmubarak, A.K. & Fadlelmula, A. (1991). Pathogenesis of Aspergillus fumigatus infection in pigeons in the Sudan. Revue D’elevage Et De Medecine Veterinaire Des Pays Tropicaux, 44, 26–28.
- Femenia, F., Fontaine, J.-J., Lair-Fulleringer, S., Berkova, N., Huet, D., Towanou, N., Rakotovao, F., Granet, O.-I., Le Loc’h, G., Arné, P. & Guillot, J. (2007). Clinical, mycological and pathological findings in turkeys experimentally infected by Aspergillus fumigatus. Avian Pathology, 36, 213–219. doi: 10.1080/03079450701332337
- Fischer, D. & Lierz, M. (2015). Diagnostic procedures and available techniques for the diagnosis of aspergillosis in birds. Journal of Exotic Pet Medicine, 24, 283–295. doi: 10.1053/j.jepm.2015.06.016
- Fischer D., Van Waeyenberghe, L., Cray, C., Gross, M., Usleber, E., Pasmans, F., Martel, A. & Lierz, M. (2014). Comparison of diagnostic tools for the detection of aspergillosis in blood samples of experimentally infected falcons. Avian Diseases, 58, 587–598.
- Forbes, N.A. (1991). Aspergillosis in raptors. Veterinary Record, 128, 263. doi: 10.1136/vr.128.11.263-b
- Friend, M. & Trainer, D.O. (1969). Aspergillosis in captive herring gulls. Bulletin of the Wildlife Disease Association, 5, 271–275. doi: 10.7589/0090-3558-5.3.271
- Ghori, H.M. & Edgar, S.A. (1973). Comparative susceptibility of chickens, turkeys and Coturnix quail to aspergillosis. Poultry Science, 52, 2311–2315. doi: 10.3382/ps.0522311
- Glare, T.R., Gartrell, B.D., Brookes, J.J. & Perrott, J.K. (2014). Isolation and identification of Aspergillus spp. from brown kiwi (Apteryx mantelli) nocturnal houses in New Zealand. Avian Diseases, 58, 16–24. doi: 10.1637/10589-061013-Reg.1
- Graczyk, T.K. & Cockrem, J.F. (1995). Aspergillus spp. seropositivity in New Zealand penguins. Mycopathologia, 131, 179–184. doi: 10.1007/BF01102898
- Graczyk, T.K., Cranfield, M.R. & Klein, P.N. (1998). Value of antigen and antibody detection, and blood evaluation parameters in diagnosis of avian invasive aspergillosis. Mycopathologia, 140, 121–127. doi: 10.1023/A:1006805816537
- Greenfield, C.L., Sanders, F.S. & Dietert, R.R. (1988). Detection of avian macrophages with concanavalin A. Avian Pathology, 17, 803–820. doi: 10.1080/03079458808436503
- Gümüşsoy, K.S., Uyanik, F., Atasever, A. & Çam, Y. (2004). Experimental Aspergillus fumigatus infection in quails and results of treatment with itraconazole. Journal of Veterinary Medicine, Series B, 51, 34–38. doi: 10.1046/j.1439-0450.2003.00720.x
- Hart, J.S. & Roy, O.Z. (1966). Respiratory and cardiac responses to flight in pigeons. Physiological Zoology, 39, 291–306. doi: 10.1086/physzool.39.4.30152353
- Heidenreich, M. (1997). Birds of Prey – Medicine and Management. Oxford: Wiley-Blackwell Science.
- Jones, M.P. & Orosz, S.E. (2000). The diagnosis of aspergillosis in birds. Seminars in Avian and Exotic Pet Medicine, 9, 52–58. doi: 10.1053/AX.2000.4619
- Joseph, V. (2000). Aspergillosis in raptors. Seminars in Avian and Exotic Pet Medicine, 9, 66–74. doi: 10.1053/AX.2000.4617
- Julian, R.J. & Goryo, M. (1990). Pulmonary aspergillosis causing right ventricular failure and ascites in meat type chickens. Avian Pathology, 19, 643–654. doi: 10.1080/03079459008418720
- Kaiser, T.J. & Bucher, T.L. (1985). The consequences of reverse sexual size dimorphism for oxygen consumption, ventilation, and water loss in relation to ambient temperature in the prairie falcon, Falco mexicanus. Physiological Zoology, 58, 748–758. doi: 10.1086/physzool.58.6.30156078
- Kunkle, R.A. & Rimler, R.B. (1996). Pathology of acute aspergillosis in turkeys. Avian Diseases, 40, 875–886. doi: 10.2307/1592312
- Latgé, J.P. (1999). Aspergillus fumigatus and aspergillosis. Clinical Microbiology Reviews, 12, 310–350.
- McMillan, M.C. & Petrak, M.L. (1989). Retrospective study of aspergillosis in pet birds. Journal of the Association of Avian Veterinarians, 3, 211–215. doi: 10.2307/27670896
- Mondon, P., De Champs, C., Donadille, A., Ambroise-Thomas, P. & Grillot, R. (1996). Variation in virulence of Aspergillus fumigatus strains in a murine model of invasive pulmonary aspergillosis. Journal of Medical Microbiology, 45, 186–191. doi: 10.1099/00222615-45-3-186
- Morris, M.P. & Fletcher, O.J. (1988). Disease prevalence in Georgia turkey flocks in 1986. Avian Diseases, 32, 404–406. doi: 10.2307/1590903
- Naldo, J.L. & Samour, J.H. (2004). Causes of morbidity and mortality in falcons in Saudi Arabia. Journal of Avian Medicine and Surgery, 18, 229–241. doi: 10.1647/2002-013
- Nardoni, S., Ceccherelli, R., Rossi, G. & Mancianti, F. (2006). Aspergillosis in Larus cachinnans micaellis: survey of eight cases. Mycopathologia, 161, 317–321. doi: 10.1007/s11046-006-0012-2
- Okoye, J.O.A. & Okeke, C.N. (1986). Pathogenicity of an isolate of Aspergillus flavus in chickens. Avian Pathology, 15, 259–270. doi: 10.1080/03079458608436286
- Olias, P., Gruber, A.D., Hafez, H.M., Lierz, M., Slesiona, S., Brock, M. & Jacobsen, I.D. (2011). Molecular epidemiology and virulence assessment of Aspergillus fumigatus isolates from white stork chicks and their environment. Veterinary Microbiology, 148, 348–355. doi: 10.1016/j.vetmic.2010.08.029
- Olias, P., Gruber, A.D., Winfried, B., Hafez, H.M. & Lierz, M. (2010). Fungal pneumonia as a major cause of mortality in white stork (Ciconia ciconia) chicks. Avian Diseases, 54, 94–98. doi: 10.1637/9088-092509-Reg.1
- O’Meara, D.C. & Chute, H.L. (1959). Aspergillosis experimentally produced in hatching chicks. Avian Diseases, 3, 404–406. doi: 10.2307/1587580
- Pandita, A., Sadana, J.R. & Asrani, R.K. (1991). Studies on clinical signs and haematological alterations in pneumonic aspergillosis due to Aspergillus flavus in Japanese quail. Mycopathologia, 116, 119–123. doi: 10.1007/BF00436374
- Peden, W.M. & Rhoades, K.R. (1992). Pathogenicity differences of multiple isolates of Aspergillus fumigatus in turkeys. Avian Diseases, 36, 537–542. doi: 10.2307/1591746
- Pendl, H. (2008a). Für Studium und Praxis: Möglichkeiten und Grenzen einer praxisnahen Hämatologie beim Vogel - Teil 1: Methodische Einführung und Befunde beim klinisch gesunden Vogel. Tierärztliche Praxis. Ausgabe K, Kleintiere/Heimtiere, 36, 290–298.
- Pendl, H. (2008b). Für Studium und Praxis: Möglichkeiten und Grenzen einer praxisnahen Hämatologie beim Vogel - Teil 2: Ontogenetisch-physiologische und pathologische Befunde und deren Interpretation. Tierärztliche Praxis. Ausgabe K, Kleintiere/Heimtiere, 36, 368–380.
- Perelman, B. & Kuttin, E.S. (1992). Aspergillosis in ostriches. Avian Pathology, 21, 159–163. doi: 10.1080/03079459208418830
- Rahim, M., Bakhiet, A. & Hussein, M. (2013). Aspergillosis in a gyrfalcon (Falco rusticolus) in Saudi Arabia. Comparative Clinical Pathology, 22, 131–135. doi: 10.1007/s00580-012-1615-2
- Rautenschlein, S. & Legler, M. (2006). Owl herpes virus infection (Hepatosplenitis infectiosa strigum) with additionally present Trichomonadosis (Trichomonas gallinae) und Aspergillosis (Aspergillus fumigatus) in an eagle owl (Bubo bubo) from avian breeding (article in German). Der Praktische Tierarzt, 87, 688–694.
- Redig, P.T. (1980). The diagnosis and treatment of aspergillosis in birds. College of Veterinary Medicine (PhD thesis). University of Minnesota, St. Paul, MN.
- Redig, P.T. (1993). Avian aspergillosis. In M.E. Fowler (Ed.), Zoo and Wild Animal Medicine Current Therapy (pp. 178–181). Philadelphia, PA: WB Saunders.
- Redig, P.T. (2007). Fungal diseases – aspergillosis. In J.H. Samour (Ed.), Avian Medicine 2nd ed. (pp. 373–387). Amsterdam, NL: Mosby.
- Redig, P.T., Fuller, M.R. & Evans, D.L. (1980). Prevalence of Aspergillus fumigatus in free-living goshawks (Accipiter gentilis atricapillus). Journal of Wildlife Diseases, 16, 169–174. doi: 10.7589/0090-3558-16.2.169
- Richard, J.L., Cutlip, R.C., Thurston, J.R. & Songer, J. (1981). Response of turkey poults to aerosolized spores of Aspergillus fumigatus and aflatoxigenic and nonaflatoxigenic strains of Aspergillus flavus. Avian Diseases, 25, 53–67. doi: 10.2307/1589826
- Richard, J.L. & Thurston, J.R. (1983). Rapid hematogenous dissemination of Aspergillus fumigatus and A. flavus spores in turkey poults following aerosol exposure. Avian Diseases, 27, 1025–1033. doi: 10.2307/1590203
- Schulz, J., Sanchez, A., Lierz, M. & Hartung, J. (2007). Dead losses of falcons caused by aspergillosis. In A. Aland (Ed.), XIII. International Congress on Animal Hygiene ISAH (pp. 707–711). Tartu: Estonia Estonian University of Life Sciences.
- Sidor, I., Pokras, M., Major, A., Poppenga, R., Taylor, K. & Miconi, R. (2003). Mortality of common loons in New England, 1987 to 2000. Journal of Wildlife Diseases, 39, 306–315. doi: 10.7589/0090-3558-39.2.306
- Taylor, J. & Burroughs, E. (1973). Experimental avian aspergillosis. Mycopathologia et Mycologia Applicata, 51, 131–141. doi: 10.1007/BF02141104
- Tell, L.A., Clemons, K.V., Kline, Y., Woods, L., Kass, P.H., Martinez, M. & Stevens, D.A. (2010). Efficacy of voriconazole in Japanese quail (Coturnix japonica) experimentally infected with Aspergillus fumigatus. Medical Mycology, 48, 234–244. doi: 10.3109/13693780903008821
- Torre-Bueno, J.R. (1978). Respiration during flight in birds. In J. Piiper (Ed.), Respiratory function in birds, adult and embryonic (pp. 89–94). Berlin: Springer.
- Trautmann, C., Gabrio, T., Dill, I., Weidner, U. & Baudisch, C. (2005). Hintergrundkonzentrationen von Schimmelpilzen in Luft (report in German). Bundesgesundheitsblatt-Gesundheitsforschung-Gesundheitsschutz, 48, 12–20. doi: 10.1007/s00103-004-0966-5
- Van Cutsem, J. & Fransen, J. (1987). Fungal infections in birds in captivity – six case reports. Mykosen, 30, 166–171. doi: 10.1111/j.1439-0507.1987.tb03963.x
- Van der Heyden, N. (1993). Aspergillosis in psittacine chicks. In The AAV – Association of Avian Veterinarians (Ed.), Annual Conference Association Avian Veterinarians (pp. 207–212). Nashville, TN.
- Van Waeyenberghe, L., Fischer, D., Coenye, T., Ducatelle, R., Haesebrouck, F., Pasmans, F., Lierz, M. & Martel, A. (2012). Susceptibility of adult pigeons and hybrid falcons to experimental aspergillosis. Avian Pathology, 41, 563–567. doi: 10.1080/03079457.2012.733930
- Van Waeyenberghe, L., Pasmans, F., Beernaert, L.A., Haesebrouck, F., Vercammen, F., Verstappen, F., Dorrestein, G.M., Klaassen, C.H.W. & Martel, A. (2011). Microsatellite typing of avian clinical and environmental isolates of Aspergillus fumigatus. Avian Pathology, 40, 73–77. doi: 10.1080/03079457.2010.540229
- Wernery, R., Wernery, U., Kinne, J. & Samour, J.H. (2004). Haematology and blood biochemistry. In R. Wernery, U. Wernery, J. Kinne & J.H. Samour (Eds.), Colour Atlas of Falcon Medicine (pp. 12–42). Hannover: Schlütersche Verlagsgesellschaft mbH.
- Wlaz, P., Knaga, S., Kasperek, K., Wlaz, A., Poleszak, E., Jezewska-Witkowska, G., Winiarczyk, S., Wyska, E., Heinekamp, T. & Rundfeldt, C. (2015). Activity and safety of inhaled itraconazole nanosuspension in a model pulmonary Aspergillus fumigatus infection in inoculated young quails. Mycopathologia, 180, 35–42. doi: 10.1007/s11046-015-9885-2

