ABSTRACT
An adult female emu (Dromaius novaehollandiae) presented with anorexia, maldigestion, weight loss, and various subtle nervous deficits. After four months of unrewarding diagnostics, treatments, and supportive care, the emu was euthanized due to lack of clinical improvement and progressive weight loss. Gross pathology revealed a very narrow pylorus and multiple flaccid diverticula of the small intestines. Histopathological findings included severe lymphoplasmacytic encephalomyelitis and multifocal lymphocytic neuritis associated with the gastrointestinal tract. Immunohistochemistry and polymerase chain reaction on the brain were positive for an avian bornavirus (ABV), and partial sequencing of the matrix gene identified aquatic bird bornavirus-1 (ABBV-1), 100% identical to viruses circulating in wild Canada geese (Branta canadensis). As wild geese frequently grazed and defaecated in the emu’s outdoor exhibit, natural transmission of ABBV-1 from free-ranging waterfowl to the emu was presumed to have occurred.
Introduction
Since the identification of the avian bornaviruses (ABVs) in psittacine birds in 2008, the number of viruses and range of affected avian species have expanded greatly. To date, there are six species of ABV encompassing 15 virus members (Kuhn et al., Citation2014; Philadelpho et al., Citation2014; Afonso et al., Citation2016; Rubbenstroth et al., Citation2016). The natural route(s) of infection have not been established for ABVs, but the main route of transmission has been suggested to be via ingestion of viral particles; virus has been isolated from the choana, oral cavity, kidneys, gastrointestinal tract, urates, faeces, and even skin and feathers of infected birds (Raghav et al., Citation2010; de Kloet et al., Citation2011; Heatley, Citation2012; Delnatte et al., Citation2014)
There appears to be moderate host/virus species fidelity, with psittacine bornaviruses predominantly found in psittacine birds, passerine bornaviruses in passerine birds, and waterfowl bornaviruses in geese, swans, ducks, and gulls (Delnatte et al., Citation2013; Guo et al., Citation2014, Citation2015; Rubbenstroth et al., Citation2016). There have been, however, several examples of infections in birds outside the usual host range (Payne et al., Citation2012; Bourque et al., Citation2015). In one case, the parrot bornavirus 4 (PaBV-4) was diagnosed in a symptomatic Himalayan monal sharing an aviary with multiple ABV-positive parrots, one of which was confirmed to have been infected with PaBV-4. Faecal oral route of transmission from parrots to the monal was postulated (Bourque et al., Citation2015). In another case, a wild bald eagle with neurologic signs was diagnosed with aquatic bird bornavirus 1 (ABBV-1); it was speculated that transmission occurred by the eagle preying on infected waterfowl (Payne et al., Citation2012).
Lesions caused by ABVs appear to be very similar across both avian and viral species. Clinical signs, when present, include central and peripheral nervous system deficits, e.g. blindness, ataxia, and motor abnormalities, as well as regurgitation, maldigestion, gastrointestinal stasis, and subsequent wasting due to autonomic nervous system dysfunction (Hoppes et al., Citation2013). Gross necropsy findings are generally non-specific and include poor body condition, dilatation of gastrointestinal segments, and food impaction in the oesophagus, proventriculus, or ventriculus. Until the identification of the ABVs, histopathology was the gold standard for identification of what was known as proventricular dilatation disease in psittacine birds. Lymphoplasmacytic ganglioneuritis, particularly of the gastrointestinal plexuses and nerves, encephalitis, and myelitis are the hallmark lesions. Infiltration of parenchymatous organs, primarily the adrenal and kidney, are also frequent histologic findings (Berhane et al., Citation2001; Gancz et al., Citation2010; Raghav et al., Citation2010; Delnatte et al., Citation2013). Antemortem diagnosis is currently based on identification of shedding of viral RNA by PCR, particularly in the urofaeces, and in some instances combined with serologic testing. Sensitivity of intra vitam detection of ABVs is low, due to intermittent urofaecal shedding of viral RNA (Hoppes et al., Citation2013; Zimmermann et al., Citation2014). Serology has been promoted by a number of authors to be the most sensitive method for detecting exposure of birds to ABVs (Herzog et al., Citation2010; de Kloet et al., Citation2011). In a large study on wild waterfowl, Delnatte et al. found that most, but not all, birds shedding viral RNA were ELISA positive (Citation2014). Zimmermann et al. concluded that sensitivity of the immunofluorescent antibody test is high when testing for a known bornavirus antigen, but cross-reactivity with genotypes of other phylogenetic groups can be significantly reduced due to the divergence of Bornaviridae (Citation2014).
ABV infection has not previously been reported in ratite species to the authors’ knowledge, although a series of papers from the 1990s describe an outbreak of a transmissible spongiform encephalopathy in ostriches that was attributed to infection with the Borna disease virus (Ashash et al., Citation2015). The pathologic lesions in those cases were not consistent with what is seen with ABV infection in birds; however, molecular methods of viral identification were not available at the time of the outbreak.
This case report describes the post-mortem identification of ABBV-1 in an emu (Dromaius novaehollandiae) at the Toronto Zoo, after a long course of anorexia, maldigestion, weight loss, and variable neurologic deficits.
Materials and methods
Case history
An adult female emu living in a large zoological park was examined after five days of partial inappetance (rejecting pellets, but still eating small amounts of lettuce and leafy greens) and the production of liquid dark green droppings. The bird had been housed with a male emu that was euthanized three months earlier due to severe joint disease. The emus shared an outdoor exhibit with wallabies and kangaroos. In spite of efforts to prevent this, wild raccoons as well as wild birds including high numbers of Canada geese were frequently observed foraging in this enclosure.
On initial examination, the emu was noted to be overly calm and was occasionally knuckling on the left metatarsal-phalangeal joint. Clinical work up, including physical examination, complete blood count and serum biochemistry, coelomic radiographs, and proventricular and ventricular endoscopy, did not reveal any abnormalities other than the bird being in fair to poor body condition. Faecal examination was negative for occult blood. A direct smear showed mixed Gram-positive cocci, Gram-positive cocco-bacilli, and Gram-negative bacilli. Heavy growths of Klebsiella pneumonia and Enterobacter cloacae were cultured from faeces.
Initial therapy consisting of enrofloxacin (5 mg/kg Baytril® 5% PO q12h for 7 days), meloxicam (0.25 mg/kg Metacam® 2% SC q12h for 6 days), vitamins (0.03 ml/kg Dystosel®/MD IM once, 0.03 ml/kg Vitamaster®-NF IM once and 2.5 mg/kg thiamine HCL IM once), tube feeding (Oxbow Herbivore Critical Care® 14 ml/kg once), and periodic IV fluid therapy (Plasma-Lyte® and 0.9% sodium chloride solution IV) did not result in clinical improvement. The provision of multiple feed stations and a great variety of food items did not improve appetite, and there was no response to treatment with mirtazapine (mirtazapine tablets 0.4 mg/kg PO once). As weight loss progressed, force-feeding one to three times per day was initiated. Over the following four weeks the frequency of neurologic behaviours increased, with clinical signs including intermittent knuckling over on her feet, muscle twitching, kicking before taking a step and rubbing her neck continuously against the wall for hours. Generally, however, she appeared calm and remained in sternal recumbency. With continued supportive care, her neurologic signs improved; she became brighter, more active, and demonstrated purposeful movements. Unfortunately, she continued to slowly lose weight and so, four months after the initial onset of the signs, euthanasia was elected. The bird’s final weight was 34 kg, as compared to her healthy bodyweight of between 50 and 57 kg.
Necropsy and histopathology
Necropsy was performed immediately after euthanasia. Representative tissue samples were collected and fixed in 10% neutral buffered formalin, embedded in paraffin, and sections were cut at 4–6 μm and stained with hematoxylin and eosin (H&E).
Immunohistochemistry with rabbit polyclonal antiserum for PaBV-2 nucleoprotein (N), as described by Raghav et al. (Citation2010), was performed on multiple sections of brain and on sections containing proventriculus, ventriculus, small intestine, cloaca, adrenal, kidney, pancreas, peripheral nerve, skeletal muscle, and tongue.
Polymerase chain reaction and sequencing
Total nucleic acids were extracted from the frozen brain and scrolls of formalin fixed paraffin-embedded brain, and from frozen serum, feathers, small intestine, pancreas, kidney, spleen, liver, lung, and heart, and real-time RT–PCR for ABVs was carried out as described previously (Delnatte et al., Citation2013). Real-time RT–PCR tests for avian paramyxovirus 1, eastern equine encephalitis virus, West Nile Virus, and avian influenza virus were carried out on the frozen brain at the University of Guelph Animal Health Laboratory using their standard operating procedures.
Frozen banked tissues from two emus previously housed in the same enclosure (liver, lung, kidney, spleen, brain, testis; and liver, lung, kidney, heart, joint fluid, respectively), one of which had cohabited with the emu in this case report, were also tested for ABVs by RT–PCR as described above.
Results
Necropsy and histopathology
As noted clinically, the emu was in poor body condition with minimal fat stores and reduced muscle mass. Gross abnormalities in the gastrointestinal tract included a very narrow pylorus and a very firm pancreas. Throughout the ileum and jejunum, there were many diverticula where the intestinal wall appeared thin and flaccid. Green content (consistent with lettuce at different stages of digestion) was present throughout the gastrointestinal tract. One small (2–3 mm) white scar was present on the caudo-ventral edge of the hepatic capsule.
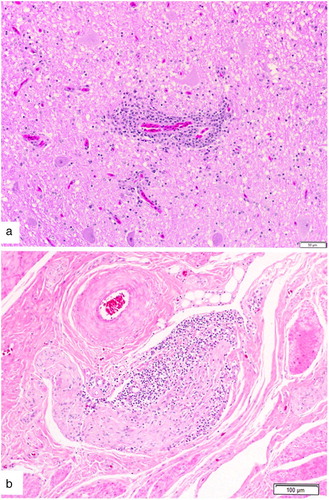
Histologic examination revealed widespread prominent lymphoplasmacytic perivascular cuffing with associated endothelial hyperplasia within multiple sections of the brain and the cervical spinal cord ((a)). Lesions were most severe at the level of the cerebellar peduncle at the junction of the grey and white matter, and were associated with numerous reactive astrocytes. In sections of the small intestine grossly identified with a diverticulum, all layers of the intestinal wall were thin and epithelial cells lining the crypts contained large intracytoplasmic eosinophilic droplets. Large numbers of lymphocytes were surrounding and within some of the serosal nerves ((b)). Marked pancreatic fibrosis was present with large numbers of heterophils in the peripancreatic connective tissue. Pancreatic exocrine cells contained little zymogen. Adrenal glands had an increased proportion of cortical cells with abundant granulated cytoplasm.
Figure 1. Histologic changes within the intestinal wall and brain of an emu (D. novaehollandiae) diagnosed with aquatic bird bornavirus 1. (a) Lymphoplasmacytic perivascular cuffing within the brain at the level of the cerebellar peduncle. H&E stain and (b) Lymphocytic infiltration of a nerve within the wall of the small intestine.
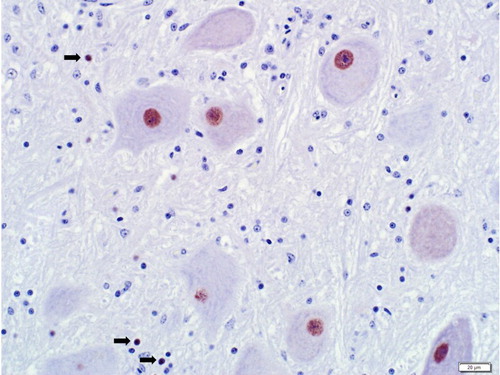
Immunohistochemistry for the PaBV-2 N protein was performed on sections of the brainstem and associated cerebellar peduncle and cerebellum, and on the cerebral cortex. Positive intranuclear and rarely intracytoplasmic staining was present in neurons and, to a much lesser extent, glial cells (). Staining was present in moderate intensity in the cerebellar peduncle and brainstem, and was sporadically positive in the granular layer of the cerebellum and in the cerebral cortex. Staining was not present in any of the nerves or ganglia associated with the additional tissues listed previously, or in those tissues themselves.
Polymerase chain reaction and sequencing
Samples of frozen and paraffin-embedded brain were positive for ABV with crossing point values of 17.38 and 21.59, respectively. Sequencing identified the virus as ABBV-1, (GenBank accession KX768138).
Real-time RT–PCR was negative for avian paramyxovirus 1, eastern equine encephalitis viruses, and West Nile Virus, and matrix PCR was negative for avian influenza virus. Frozen serum, feathers, small intestine, pancreas, kidney, spleen, liver, lung, and heart were all negative for ABV on PCR. ABV RNA was not detected in any of the banked frozen tissues from the two other emus that had occupied the same pen as the affected bird.
Discussion
The clinical signs, pathologic lesions, and molecular and immunohistochemistry testing performed on this emu lead to the conclusion that infection by ABBV-1 was the most likely cause for the neurologic deficits and possibly the cause of the wasting seen in this case. Although mesenteric ganglioneuritis is a recognized cause of gastrointestinal malfunction and wasting in birds infected with ABVs, in this emu it is possible that the pancreatic fibrosis and intestinal diverticula may also have played a role in the bird’s inappetance and weight loss. We cannot state whether the emu described in this case report was shedding virus as this was not assessed prior to necropsy; however, the virus was not identified by PCR or immunohistochemistry in any tissue other than the brain.
The ABBV-1 sequence detected in the brain from this emu was 100% identical to that of ABBV-1 circulating in Canada geese (Payne et al., Citation2012). ABBV-1 has previously been reported to be present at a high prevalence in wild Canada geese in the Toronto Zoo site in both clinically affected and apparently healthy Canada geese (Delnatte et al., Citation2013, Citation2014). We concluded that the most likely method of acquisition of ABBV-1 in this emu was by exposure to urofaecal material from the many wild waterfowl (in particular Canada geese) grazing and defaecating in the emu’s exhibit. It is possible that the indiscriminate foraging habits of the emu increased the risk of transmission. ABV RNA was not detected by PCR of various banked tissues from two emus previously housed in the same enclosure. The two emus had been euthanized due to unrelated disease. It would have been interesting to know if those birds were seropositive for ABV, but unfortunately serum samples were not available. All animals in the zoo collection that die or are euthanized are necropsied and a selection of tissues evaluated by histopathology and other diagnostic methods, as appropriate. ABV infection had not been identified in any of the other avian species, even though many are housed outdoors in enclosures also contaminated by waterfowl faeces, suggesting that transmission may require particularly intense exposure to infected material.
The finding of ABBV-1 in this emu again illustrates the broad species infectivity of at least some ABVs, and serves as a reminder to include ABVs on the list of differential diagnosis for birds of any species that present with neurologic disease and or gastrointestinal dysfunction.
Acknowledgements
The authors would like to thank Josepha DeLay for assistance with immunohistochemistry, as well as the Toronto Zoo’s Wildlife Health and Wildlife Care staff.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Afonso, C.L., Amarasinghe, G.K., Bányai, K., Bà, Y., Basler, C.F., Bavari, S., Bejerman, N., Blasdell, K.R., Briand, F., Briese, T., Bukreyev, A., Calisher, C.H., Chandran, K., Chéng, J., Clawson, A.N., Collins, P.L., Dietzgen, R.G., Dolnik, O., Domier, L., Dürrwald, R., Dye, J.M., Easton, A.J., Ebihara, H., Farkas S.L., Freitas-Astúa, J., Formenty, P., Fouchier, R.A.M., Fù, Y., Ghedin, E., Goodin, M.M., Hewson, R., Horie, M., Hyndman, T.M., Jiāng, D., Kitajima, E.W., Kobinger, G.P., Kondo, H., Kurat, G., Lamb, R.A., Lenardon, S., Leroy, E.M., Li, C., Lin, X., Liú, L., Longdon, B., Marton, S., Maisner, A., Mühlberger, E., Netesov, S.V., Nowotny, N., Patterson, J.L., Payne, S.L., Paweska, J.T., Randall, R.E., Rima, B.K., Rota, P., Rubbenstroth, D., Schwemmle, M., Shi, M., Smither, S.J., Stenglein, M.D., Stone, D.M., Takada, A., Terregino, C., Tesh, R.B., Tian, J., Tomonaga, K., Tordo, N., Towner, J.S., Vasilakis, N., Verbeek, M., Volchkov, V.E., Wahl-Jensen, V., Walsh, J.A., Walker, P.J., Wang, D., Wang, L., Wetzel, T., Whitfield, A.E., Xiè, J., Yuen, K., Zhang, Y. & Kuhn, J.H. (2016) Taxonomy of the order Mononegavirales: update 2016. Archives of Virology, 161, 2351–2360. doi: 10.1007/s00705-016-2880-1
- Ashash, E., Malkinson, M., Meir, R., Perl, S. & Weisman, Y. (2015). Causes of losses including a Borna disease paralytic syndrome affecting young ostriches of one breeding organization over a five-year period (1989–1993). Avian Diseases, 40, 240–245. doi: 10.2307/1592397
- Berhane, Y., Smith, D.A., Newman, S., Taylor, M., Nagy, E., Binnington, B. & Hunter, B. (2001). Peripheral neuritis in psittacine birds with proventricular dilatation disease. Avian Pathology, 30, 563–570. doi: 10.1080/03079450120078770
- Bourque, L., Laniesse, D., Beaufrère, H., Pastor, A., Ojkic, D. & Smith, D.A. (2015). Identification of avian bornavirus in a Himalayan monal (Lophophorus impejanus) with neurological disease. Avian Pathology, 44, 1–11. doi: 10.1080/03079457.2015.1050956
- de Kloet, A.H., Kerski, A. & de Kloet, S.R. (2011). Diagnosis of avian bornavirus infection in psittaciformes by serum antibody detection and reverse transcription polymerase chain reaction assay using feather calami. Journal of Veterinary Diagnostic Investigation, 23, 421–429. doi: 10.1177/1040638711403406
- Delnatte, P., Nagy, É., Ojkic, D., Leishman, D., Crawshaw, G., Elias, K. & Smith, D.A. (2014). Avian bornavirus in free-ranging waterfowl: prevalence of antibodies and cloacal shedding of viral RNA. Journal of Wildlife Diseases, 50, 512–523. doi: 10.7589/2013-08-218
- Delnatte, P., Ojkic, D., Delay, J., Campbell, D., Crawshaw, G. & Smith, D.A. (2013). Pathology and diagnosis of avian bornavirus infection in wild Canada geese (Branta canadensis), trumpeter swans (Cygnus buccinator) and mute swans (Cygnus olor) in Canada: a retrospective study. Avian Pathology, 42, 114–128. doi: 10.1080/03079457.2013.769669
- Gancz, A.Y., Clubb, S. & Shivaprasad, H.L. (2010). Advanced diagnostic approaches and current management of proventricular dilatation disease. Veterinary Clinics of North America – Exotic Animal Practice, 13, 471–494. doi: 10.1016/j.cvex.2010.05.004
- Guo, J., Shivaprasad, H.L., Rech, R.R., Heatley, J.J., Tizard, I. & Payne, S. (2014). Characterization of a new genotype of avian bornavirus from wild ducks. Virology Journal, 11, 1–7. doi: 10.1186/1743-422X-11-1
- Guo, J., Tizard, I., Baroch, J., Shivaprasad, H.L. & Payne, S.L. (2015). Avian bornaviruses in North American gulls. Journal of Wildlife Diseases, 51, 754–758. doi: 10.7589/2015-01-001
- Heatley, J. (2012). Avian bornavirus in the urine of infected birds. Veterinary Medicine: Research and Reports, 3, 19–23.
- Herzog, S., Enderlein, D., Heffels-Redmann, U., Piepenbring, A., Neumann, D., Kaleta, E.F., Müller, H., Lierz, M. & Herden, C. (2010). Indirect immunofluorescence assay for intra vitam diagnosis of avian bornavirus infection in psittacine birds. Journal of Clinical Microbiology, 48, 2282–2284. doi: 10.1128/JCM.00145-10
- Hoppes, S.M., Tizard, I. & Shivaprasad, H.L. (2013). Avian bornavirus and proventricular dilatation disease: Diagnostics, pathology, prevalence, and control. Veterinary Clinics of North America – Exotic Animal Practice, 16, 339–355. doi: 10.1016/j.cvex.2013.01.004
- Kuhn, J.H., Dürrwald, R., Bào, Y., Briese, T., Carbone, K., Clawson, A.N., deRisi, J.L., Garten, W., Jarhling, P.B., Kolodziejek, J., Rubbenstroth, D., Schwemmle, M., Stenglein, M., Tomonaga, K., Weissenböck, H. & Nowotny, N. (2014). Taxonomic reorganization of the family Bornaviridae. Archives of Virology, 160, 621–632. doi: 10.1007/s00705-014-2276-z
- Payne, S.L., Delnatte, P., Guo, J., Heatley, J.J., Tizard, I. & Smith, D.A. (2012). Birds and bornaviruses. Animal Health Research Reviews/Conference of Research Workers in Animal Diseases, 13, 145–156. doi: 10.1017/S1466252312000205
- Philadelpho, N.A., Rubbenstroth, D., Guimarãres, M.B. & Piantino Ferreira, A.J. (2014). Survey of bornaviruses in pet psittacines in Brazil reveals a novel parrot bornavirus. Veterinary Microbiology, 174, 584–590. doi: 10.1016/j.vetmic.2014.10.020
- Raghav, R., Taylor, M., Delay, J., Ojkic, D., Pearl, D.L., Kistler, A.L., deRisi, J.L., Ganem, D. & Smith, D.A. (2010). Avian bornavirus is present in many tissues of psittacine birds with histopathologic evidence of proventricular dilatation disease. Journal of Veterinary Diagnostic Investigation, 22, 495–508. doi: 10.1177/104063871002200402
- Rubbenstroth, D., Schmidt, V., Rinder, M., Legler, M., Twietmeyer, S., Schwemmer, P. & Corman, V.M. (2016). Phylogenetic analysis supports horizontal transmission as a driving force of the spread of avian bornaviruses. PLoS ONE, 11, 1–19. doi: 10.1371/journal.pone.0160936
- Zimmermann, V., Rinder, M., Kaspers, B., Staeheli, P. & Rubbenstroth, D. (2014). Impact of antigenic diversity on laboratory diagnosis of avian bornavirus infections in birds. Journal of Veterinary Diagnostic Investigation, 26, 769–777. doi: 10.1177/1040638714547258