ABSTRACT
Conventional serological methods for detection and differentiation of antibodies against fowl aviadenoviruses (FAdVs) are laborious and time-consuming, therefore ELISAs based upon recombinant proteins were developed in the present study to overcome this limitation for clinically relevant serotypes FAdV-1 and FAdV-4. In order to develop serotype-specific ELISAs, the two distinct fibers, fiber-1 (fib-1) and fiber-2 (fib-2), characteristically present only in FAdV-1 and FAdV-4, were applied separately as coating antigens. Sera raised against each recombinant fib-1 and fib-2 of FAdV-1 and FAdV-4 did not react with any of the heterologous fiber ELISAs, as anticipated by the low degree of amino acid identity between those FAdV fibers (23.1–41.2%), indicating that heterologous fibers do not share common epitopes. Testing of 172 monospecific sera, raised against all FAdV serotypes (1–8a and 8b–11), retrieved specificities between 99.3% and 100.0% for the ELISAs, further substantiating the serotype-specificity of fibers. Investigating sera from chickens experimentally inoculated with different FAdV-1 or FAdV-4 strains revealed that ELISAs were equally or more sensitive than the virus-neutralization (VN) test. Furthermore, strong correlations were demonstrated between fiber antibody titres and neutralization activity. Particularly, sera directed against live virus showed a pronounced fiber antibody response, which might be explained by an excessive production of fibers during infection. Application of the newly developed fiber ELISAs on field sera with heterogeneous serological status demonstrated high sensitivity and serotype-specificity of this test system, providing for the first time a diagnostic tool for mass screening of chicken flocks against FAdV serotypes, namely FAdV-1 and FAdV-4.
Introduction
Fowl aviadenoviruses (FAdVs) are classified in the genus Aviadenovirus, family Adenoviridae. Current classification recognizes five species (FAdV-A to FAdV-E) with discriminate genomic composition and 12 serotypes (FAdV-1 to -8a and FAdV-8b to -11) based on cross-neutralization tests (Harrach et al., Citation2011).
Among these, only particular species harbour virulent strains with high economic significance for the commercial poultry sector (Hess, Citation2013). Certain FAdV-1 (FAdV-A) strains are responsible for adenoviral gizzard erosions (AGE), while members of FAdV-4 (FAdV-C) strains cause hepatitis-hydropericardium syndrome (HHS) (Mazaheri et al., Citation1998). Particular strains of serotypes FAdV-8a and -8b (FAdV-E) as well as FAdV-2 and -11 (FAdV-D) are responsible for inclusion body hepatitis (IBH) (Ojkic et al., Citation2008; Marek et al., Citation2010; Steer et al., Citation2011; Schachner et al., Citation2016).
FAdV serotypes can be distinguished based upon their antigenic compositions, determined by the major structural components of the virion, hexon and penton. Hexon forms the body of the capsid, while pentons, complexes of penton base and protruding fibers, are located at the 12 vertices of the icosahedral virion (Norrby, Citation1969). Characteristically, FAdVs possess two fiber proteins of species-dependent length difference per vertex (Gelderblom & Maichle-Lauppe, Citation1982), as opposed to single fibers of mammalian adenoviruses. Notably, only fibers of species FAdV-A and FAdV-C, comprising serotypes FAdV-1 and FAdV-4/FAdV-10, respectively, are transcribed by two separate genes, which are distinct in sequence and length, and are referred to as fiber-1 (fib-1) and fiber-2 (fib-2). In contrast, the remaining FAdV species possess only one single fiber gene (Hess et al., Citation1995; Marek et al., Citation2012).
The structural proteins of the capsid represent major targets of the humoral immune response against FAdVs (Li et al., Citation1984). In domestic poultry, immunization with fiber subunits has been shown to mediate clinical protection against FAdV-4 (Schachner et al., Citation2014) as well as adenoviruses from other genera, TAdV-3 (Pitcovski et al., Citation2005) and DAdV-1 (Fingerut et al., Citation2003). However, neutralizing activity does not seem necessary for the observed protection.
Although vertical transmission of FAdVs (Yates & Fry, Citation1957) and an increased susceptibility of young chickens to clinical disease (Schachner et al., Citationforthcoming) urge serological monitoring of breeding stocks, all currently available serological tools face certain limitations. Being widely outdated in use, the agar gel precipitation test and the immunofluoresence assay detect only group-specific antibodies, impeding the differentiation of FAdV species and serotypes (McFerran, Citation1981). Instead, the cross-neutralization test represents the gold standard due to its applicability for serotyping (Hess, Citation2000), with high sensitivity and specificity, but significant trade-offs in cost and time efficiency. As an alternative to first-generation FAdV ELISAs based on whole virus lysates (Dawson et al., Citation1980; Calnek et al., Citation1982; Mockett & Cook, Citation1983), which are highly sensitive, but generate considerable cross-reactions between serotypes, indirect ELISAs based on recombinant FAdV proteins have recently been developed. Depending on the type of protein, the hitherto reported coating antigens, non-structural proteins 33K and 100K can differentiate FAdV infected from vaccinated chickens (Xie et al., Citation2013), whereas recombinant hexon proteins enabled the detection of clinically relevant serotypes FAdV-2 and FAdV-4 with no data on cross-reactivity between serotypes (Junnu et al., Citation2014; Rajasekhar & Roy, Citation2014).
In the present study we utilized for the first time recombinant fiber proteins to develop indirect ELISAs based on serotypes FAdV-1 and FAdV-4, which uniquely allowed for expression of two types of fibers and their separate investigation with regard to specificity and sensitivity in FAdV detection. The potential of the newly developed ELISAs was assessed by testing monospecific sera as well as field samples, altogether supporting the benefit of the developed assays.
Materials and methods
Virus preparation
Selected FAdV strains were propagated on primary chicken embryo liver (CEL) cells as previously reported (Schat & Sellers, Citation2008) and viral titres were determined according to the method of end-point titration (Reed & Muench, Citation1938).
Preparation of inactivated virus for inoculation of birds was performed with 1% formaldehyde (incubation at 37°C for 24 h) and mixed 1:1 with adjuvant (GERBU LQ no. 3000, GERBU Biotechnik GmbH, Heidelberg, Germany) before administration.
Cloning and protein expression
Reference strains CELO (FAdV-1) and KR5 (FAdV-4) were used for cloning of fiber genes. Viral DNA was extracted from cell culture supernatant with the DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany) and the entire fiber gene regions were amplified (). Cloning and protein expression was carried out with the baculovirus Bac-to-Bac expression system (Invitrogen, Vienna, Austria) according to manufacturer's instructions. Purification and further processing of recombinant fibers, including Coomassie-stained SDS-polyacrylamide gels and Western Blot, was performed as described recently (Schachner et al., Citation2014). Bradford assay (Thermo Scientific, Vienna, Austria) was used to determine the concentration of purified proteins.
Table 1. Overview of coating antigens.
Chicken sera
Serum samples were obtained from different sources, as outlined below. All bird trials conducted in the course of the present study were discussed and approved by the institutional ethics committee and the national authority according to §26 of the Law for Animal Experiments, Tierversuchsgesetz 2012 – TVG 2012, licence numbers: GZ 68.205/0158-WF/V/3b/2014, GZ 68.205/0196-WF/V/3b/2016 and GZ 68.205/0044-WF/V/3b/2016.
Control sera
A total of 57 sera from specific-pathogen free (SPF) chickens, aged between four and 64 weeks, served as negative controls. The SPF sera were kindly supplied by Lohmann Tierzucht GmbH (Cuxhaven, Germany), or derived from non-infected chickens kept in our facilities.
Sera positive for avian encephalomyelitis virus (AEV), avian reovirus (ARV), avian influenza virus (AIV), avian metapneumovirus (aMPV), chicken anaemia virus (CAV), fowlpox virus (FPV), egg drop syndrome virus (EDSV), infectious bronchitis virus (IBV), infectious bursal disease virus (IBDV), infectious laryngotracheitis virus (ILTV) and Newcastle disease virus (NDV) were purchased from GD Animal Health (Deventer, Netherlands).
Antisera against recombinant fiber proteins
Antisera against each individual recombinant protein (FAdV-1, fib-1 or fib-2; FAdV-4, fib-1 or fib-2) were derived from SPF chickens injected intramuscularly (i.m.) with 50 µg of purified protein mixed 1:1 with adjuvant (GERBU LQ no. 3000, GERBU Biotechnik GmbH) (, group 1).
Table 2. Overview of SPF chicken serum samples used within the present studies.
Experimentally recruited serum samples
Antisera against selected FAdV reference strains were generated in SPF chickens (VALO, Lohmann Tierzucht GmbH, Cuxhaven, Germany), kept for up to 10 weeks in isolator units. In the first experiment each group of birds (n = 10) was infected at day 14 of life, either orally with 0.5 ml of 107 median tissue culture infective dose (TCID50)/ml per chicken, or i.m. with 0.5 ml of 105 TCID50/ml per chicken (, group 2). To achieve adequate antibody development, infection routes for different FAdV strains varied, based on experience from previous trials and literature (Cook, Citation1983).
In a second experiment SPF chickens (n = 2 to 9) were inoculated i.m. at day 14 of life with 0.25 ml of 105.5 to 108 TCID50/ml per bird, using different inactivated FAdV reference strains (, group 3).
During both experiments serum samples were collected weekly post inoculation (p.i.) until termination of the trials. Birds were reinoculated i.m. at the earliest after two weeks, depending on the antibody status of the group.
Additional sera against FAdV reference and field strains were derived from previous infection experiments at our laboratory (, groups 4–7).
Virus-neutralization (VN) test
The assay was carried out on CEL cells, plated in 96-well plates (Sarstedt, Wiener Neudorf, Austria). Serum samples were heat-inactivated at 56°C for 30 min, serially diluted from 1:8 to 1:16,384 and incubated with 100 TCID50 of the homologous FAdV strain. After five days at 37°C in 5% CO2, the wells were investigated for cytopathic effect (CPE). Serum samples with antibody titres below or equal to 3 log2 were considered negative.
ELISA
Based on checkerboard titrations optimal concentrations of all required ELISA components were determined. The final protocol, applicable for all four established ELISAs, was performed on microtitre plates (Nunc, Roskilde, Denmark) coated with 100 µl of recombinant protein in 0.05 M carbonate buffer (pH 9.4) with addition of 0.7 M NaCl and an inert protein (Starting Block™ T20 PBS, Pierce Biotechnology, Rockford, USA). Following incubation overnight at 4°C, the plates were washed three times with PBS, containing 0.1% Tween 20, pH 7.4, using an automated plate washer. Serum samples diluted 1:100 in blocking buffer (Starting Block™ T20 PBS, Pierce Biotechnology) were added to the wells in duplicates and incubated for 1 h at room temperature. After washing three times, 100 µl Goat-Anti-Chicken IgY-horseradish-peroxidase (Southern Biotechnology, Birmingham, USA) were added, followed by incubation for 1 h at room temperature and a final washing step. Subsequently, 100 µl TMB (tetramethylbenzidine) substrate (Calbiochem, Darmstadt, Germany) were added to each well and the plates were incubated in the dark for 15 min. The colour reaction was stopped with 100 µl 0.5 M sulphuric acid per well and the optical density (OD) was determined at 450 nm. ODs represent the mean from duplicate wells.
Cut-off values were determined on basis of the negative control sera by calculating the arithmetic mean of all OD values plus three times the standard deviation.
Specificity
Serotype-specificity of the individual fiber ELISAs was assessed with a panel of 172 monospecific antisera against reference strains from all serotypes FAdV-1 to -8a and FAdV-8b to -11 (, groups 2, 3, 4 and 5(a)) and available field strains (, groups 5(b), 6 and 7), possessing the highest available VN titres within each serotype (mean values ranging from 8.1 log2 to 13.8 log2).
Sensitivity and correlation with VN test
Based on 145 and 186 sera obtained from chickens inoculated with FAdV-1 (, groups 1–4) and FAdV-4 strains (, groups 5–8), respectively, detection rates of the newly developed ELISAs were compared with VN tests and the correlation between titres obtained by ELISAs and VN tests was determined.
Table 3. Sensitivities of (a) FAdV-1 and (b) FAdV-4 fiber-based ELISAs in comparison with VN test.
Kinetics of antibody response following FAdV-1 or FAdV-4 infection
Upon infection of birds with CELO (FAdV-1) (, group 4(a)) or KR5 (FAdV-4) (, group 2(b)) the antibody development and neutralization was monitored for each of the fiber subpopulations. Blood samples were taken weekly until the end of the experiment (8 weeks p.i.) and investigated by serotype homologous ELISAs and VN tests.
Screening of field sera
Altogether 64 serum samples from four commercial chicken flocks of unknown disease and vaccination status were examined for the presence of FAdV antibodies by the newly developed ELISAs and compared to the VN tests for FAdV-1 and FAdV-4 (Supplementary Table 1).
Additionally, these sera were screened by single serum dilution VN tests against all other FAdV serotypes, using a serum dilution of 1:50 and 100 TCID50 of FAdV reference strain representatives of each serotype (Supplementary Table 2).
Statistical analysis
Pairwise comparison between the detection rates of the serological methods was performed with Pearson chi-square analysis. The p-values were adjusted according to Bonferroni correction for multiple comparisons.
Correlation between titres of fiber ELISAs and VN tests was calculated by pairwise Spearman correlation test and correlation coefficients were classified as described by Cohen (Citation1988). ELISA ODs were converted into log2 titres by linear regression analysis. In addition, VN and ELISA log2 titres were classified into three groups (VN titres, > 3–5 log2 (low), 6–9 log2 (intermediate) and 10–> 14 log2 (high); ELISA titres, cut-off to 7.5 log2 (low), 7.5–9.5 log2 (intermediate) and 9.5–> 12 log2 (high)). The significance level in all conducted tests was defined as p < 0.05. Statistical analyses were performed with SPSS Version 24 (IBM SPSS Statistics, IBM Corporation, Somers, USA).
Results
Fiber-based ELISAs
The recombinant fiber proteins were expressed as soluble proteins in the supernatant, as demonstrated by the presence of bands with the approximate sizes of the estimated molecular masses in SDS-PAGE and Western blot (). Yields of purified protein ranged between 9.6 and 15.9 mg per litre of insect cell culture.
Figure 1. Detection of purified recombinant FAdV fiber proteins by Coomassie-stained SDS-PAGE (a) and Western blot analysis (b): Lane M, molecular weight marker; lane 1, FAdV-1 fib-1; lane 2, FAdV-1 fib-2; lane 3, FAdV-4 fib-1; lane 4, FAdV-4 fib-2.
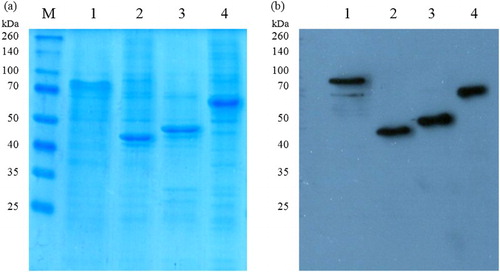
Antigen concentrations of 0.25 µg/well, serum dilutions of 1:100 and second antibody dilutions of 1:10,000 revealed optimal results with greatest ratios between positive and negative sera. To some extent, negative sera from SPF birds displayed non-specific reactions, which increased with the age of chickens. These reactions could be minimized by adding NaCl and an inert protein to the coating buffer and by increasing NaCl and Tween 20 in the washing buffer. Cut-off values between 0.45 and 0.57 were determined for the fiber-based ELISAs ().
Specificity
Sera positive for other avian pathogens remained negative in each of the four established fiber-based ELISAs. Sera raised against each of the recombinant fibers (FAdV-1 fib-1, FAdV-1 fib-2, FAdV-4 fib-1, FAdV-4 fib-2) did not react with any of the heterologous fiber ELISA systems, demonstrating that antibodies against fibers of FAdV-1 and FAdV-4 specifically recognize the correct type of fiber.
Among 172 FAdV antisera, comprising all 12 FAdV serotypes, FAdV-1 fiber ELISAs detected only serotype homologous antibodies with 100% specificity. FAdV-4 fib-1 and FAdV-4 fib-2 ELISAs showed 99.3% specificity, each detecting one antiserum against inactivated FAdV-5 as positive. Of note, both FAdV-4 fiber ELISAs additionally detected all antisera against FAdV-10.
Sensitivity and correlation with VN test
Among the sera derived from FAdV-1 inoculated chickens 71.0% (103/145) were positive by the FAdV-1 fib-1 ELISA, 62.1% (90/145) by the FAdV-1 fib-2 ELISA and 54.5% (79/145) by VN test ((a)). The FAdV-1 fib-1 ELISA proved to be significantly more sensitive compared to the VN test (χ2 = 8.498, p < 0.008).
Furthermore, 81.2% (151/186) and 79.0% (147/186) of sera derived from chickens inoculated with FAdV-4 were detected by the FAdV-4 fib-2 and fib-1 ELISAs, respectively, while 68.8% (128/186) were positive by the VN test ((b)), showing a significantly higher sensitivity of both FAdV-4 fiber ELISAs than VN test (FAdV-4 fib-1 ELISA vs. VN test, χ2 = 5.034, p < 0.049; FAdV-4 fib-2 ELISA vs. VN test, χ2 = 7.584, p < 0.012). Of note, the higher sensitivity of fiber ELISAs as compared to VN tests resulted mainly from testing of antisera against live virus (, groups 1 and 5) (group 1, FAdV-1 fib-1 ELISA vs. VN test, χ2 = 10.629, p < 0.002; group 5, FAdV-4 fib-1 ELISA vs. VN test, χ2 = 18.889, p < 0.001; FAdV-4 fib-2 ELISA vs. VN test, χ2 = 27.307, p < 0.001), whereas no significant differences between the test systems were noticed for sera against inactivated virus (, groups 2, 6 and 7).
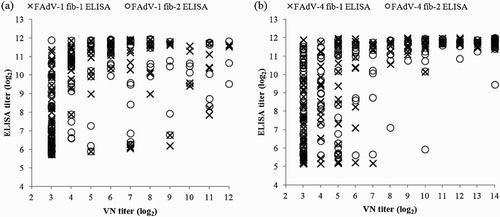
Overall, strong correlations (ranging between r = 0.641, p < 0.000 to r = 0.778, p < 0.000) were found between antibody titres of the respective ELISA and VN test ().
Figure 2. Correlation between antibody titres (log2) obtained by fiber-based ELISAs and VN tests for serum samples derived from SPF chickens experimentally inoculated with either FAdV-1 or FAdV-4 strains. (a) FAdV-1 fib-1 ELISA vs. FAdV-1 VN test, r = 0.641, p < 0.000; FAdV-1 fib-2 ELISA vs. FAdV-1 VN test, r = 0.680, p < 0.000; (b) FAdV-4 fib-1 ELISA vs. FAdV-4 VN test, r = 0.778, p < 0.000; FAdV-4 fib-2 ELISA vs. FAdV-4 VN test, r = 0.756, p < 0.000. ELISA titres > 6.76 log2 (FAdV-1 fib-1), > 6.69 log2 (FAdV-1 fib-2), > 5.96 log2 (FAdV-4 fib-1), > 6.57 log2 (FAdV-4 fib-2) and VN titres > 3 log2 are considered positive.
Kinetics of antibody response following FAdV-1 or FAdV-4 infection
In birds infected with FAdV-1 earliest detection of antibodies was noticed at 3 weeks p.i. with the greatest number of birds (75%) found positive by the FAdV-1 fib-1 ELISA, while at this time point only 50% were positive by the FAdV-1 fib-2 ELISA and only 37.5% by VN test. Throughout the experiment higher titres were recorded in the FAdV-1 fib-1 as compared to the FAdV-1 fib-2 assay ((a)). VN titres remained comparatively low.
Figure 3. Mean titres (log2) of sera collected weekly after infection evaluated by fiber-based ELISAs (continuous and dashed lines) and VN tests (dotted lines). Results are displayed for birds infected with (a) CELO (n = 8) and (b) KR5 (n = 10). ELISA titres > 6.76 log2 (FAdV-1 fib-1), > 6.69 log2 (FAdV-1 fib-2), > 5.96 log2 (FAdV-4 fib-1), > 6.57 log2 (FAdV-4 fib-2) and VN titres > 3 log2 are considered positive. Horizontal lines represent the cut-off values for the VN tests (dotted lines) and for the fiber-based ELISAs (continuous and dashed lines). Arrows indicate booster inoculation.
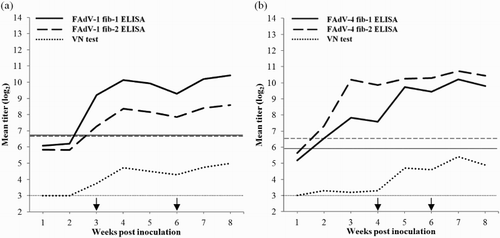
In birds infected with FAdV-4 first detection of antibodies was observed at 2 weeks p.i. with the highest number of positives obtained by the FAdV-4 fib-2 ELISA (60%), followed by the FAdV-4 fib-1 ELISA (40%) and VN test (10%). Considering the different cut-offs of ELISAs higher mean titres of FAdV-4 fib-2 antibodies compared to FAdV-4 fib-1 antibodies were observed at 3 and 4 weeks p.i. ((b)). In contrast, positive VN results were obtained for the majority of birds from 5 weeks p.i.
Screening of field sera
In field sera, the FAdV-1 fib-1 ELISA detected 39.1%, the FAdV-1 VN test 34.4% and the FAdV-1 fib-2 ELISA 21.9% positives (Supplementary Table 1). In flock 1, only one sample was positive for FAdV-1 at week 34, as detected by the fib-1 ELISA. Flock 2 was positive for FAdV-1 by all three methods, both fiber ELISAs and VN test, at week 34. While only fib-1 ELISA detected one bird of flock 3 as positive at week 23, all three methods detected seropositives in this group at week 34. Similarly, seropositive results were obtained by all three methods in flock 4.
In contrast to intermediate or high mean titres found by the FAdV-1 fiber ELISAs in flock 3 at 34 weeks, mean neutralization titres remained low in the indicated flock ((a) and (c)). In flocks 2 and 4 low mean titres were recorded by all three assays.
Figure 4. Box plot presentation of antibody titres (log2) from breeder flocks obtained by (a) FAdV-1 VN test, (b) FAdV-4 VN test, (c) FAdV-1 fib-1 and FAdV-1 fib-2 ELISA and (d) FAdV-4 fib-1 and FAdV-4 fib-2 ELISA. VN titres > 3 log2 and ELISA titres > 6.76 log2 (FAdV-1 fib-1), > 6.69 log2 (FAdV-1 fib-2), > 5.96 log2 (FAdV-4 fib-1), >6.57 log2 (FAdV-4 fib-2) are considered positive. Horizontal lines represent the cut-off values for the VN test (4(a–b), dotted lines), the FAdV-1 fib-1 ELISA (4(c), dotted line) and FAdV-1 fib-2 ELISA (4(c), continuous line) as well as for the FAdV-4 fib-1 ELISA (4(d), continuous line) and FAdV-4 fib-2 ELISA (4(d), dotted line).
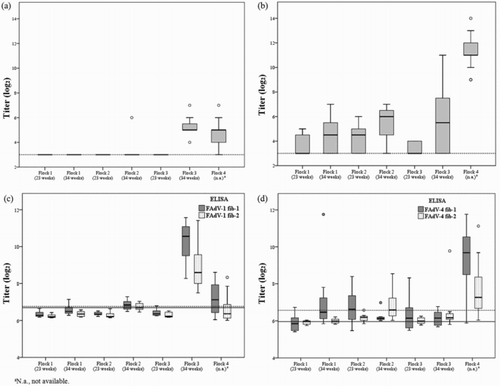
Within FAdV-4, the fib-1 ELISA detected 76.6%, the VN test 68.8% and the fib-2 ELISA 31.3% of field samples as positive (Supplementary Table 1). The FAdV-4 fiber-1 ELISA and FAdV-4 VN test provided seropositive results in all investigated flocks, with low or intermediate mean titres in flocks 1–3 and high mean titres in flock 4 ((b) and (d)). FAdV-4 fib-2 ELISA positive samples were noticed only in breeder flock 2 at 23 and 34 weeks, in flock 3 at 34 weeks, as well as in flock 4.
Furthermore, antibodies against other FAdV serotypes were found by VN test to a high extent (Supplementary Table 2).
Discussion
Methods commonly applied to detect antibodies against FAdVs are either laborious (VN test, immunofluorescence assay) or lack adequate sensitivity (agar gel precipitation test, whole virus-based ELISA). Although ELISA systems based upon whole virus as antigen prioritize a homologous versus a heterologous reaction, they possess a broad range of cross-reactivity depending on the succession of infections, which is a certain limitation considering the existence of 12 serotypes (Mockett & Cook, Citation1983; Günes et al., Citation2011). Despite the use of recombinant proteins (hexon, non-structural proteins 33K and 100K) serotype-specificity could not be demonstrated (Xie et al., Citation2013; Junnu et al., Citation2014; Rajasekhar & Roy, Citation2014). In order to overcome this deficiency the present study reports the development of indirect ELISAs based on recombinant fiber proteins of the clinically relevant serotypes FAdV-1 and FAdV-4. Furthermore, special attention was paid to the fact that these serotypes are peculiarly characterized by two distinct fiber genes, which were separately investigated for their suitability as ELISA coating antigens.
Technical impediments due to relatively high OD values in negative SPF chicken sera, mainly from older birds, correspond with previous observations (Slaght et al., Citation1979; Meulemans & Halen, Citation1982; Cunningham et al., Citation2001). They were resolved in the present study by coating of plates with an additional inert protein as well as by the increase of NaCl and Tween 20 in the washing buffer.
The final ELISA protocol revealed no cross-reactions with sera positive for other avian pathogens. Furthermore, sera raised specifically against each type of recombinant fibers recognized the analogous fiber type with no cross-reactions between heterologous fibers, as expected by their low degree of amino acid identity (23.1–41.2%). However, since it is known that fibers share a common tertiary structure (Guardado-Calvo et al., Citation2006; El Bakkouri et al., Citation2008), arguing for the presence of consistent antigenic determinants, our results corroborate that the dual fibers of each FAdV-1 and FAdV-4 do not share common epitopes.
The investigation of FAdV antisera, comprising all 12 serotypes, demonstrated specificities of 100.0% and 99.3% for the FAdV-1 and FAdV-4 fiber ELISAs, substantiating the serotype-specificity of fibers. These findings extend the results of former studies, which have already indicated an adequately large divergence of the fiber for molecular differentiation of FAdVs (Marek et al., Citation2012; Schachner et al., Citation2016).
Exceptional cross-reactions were found only in individual antisera against inactivated FAdV-5, reacting in FAdV-4 fiber ELISAs, but sera from birds infected with the equivalent live virus did not react. The process of virus inactivation by formaldehyde treatment might be an explanation for the observed cross-reactivity due to the alteration of surface proteins during the inactivation procedure (Akhtar et al., Citation2000). However, no clinical significance is attributed to FAdV-5 in contemporary reports on FAdV-induced diseases (Marek et al., Citation2010; Schachner et al., Citation2016) and inactivated viruses of this serotype are not in use. Furthermore, the finding that FAdV-4 fiber ELISAs detected antisera against inactivated FAdV-10 as positive conforms with earlier observations of a close immunological relationship between serotypes FAdV-4 and FAdV-10 (Erny et al., Citation1995; Steer et al., Citation2011), arguing for the classification within one serotype.
Results of experimentally generated FAdV-1 and FAdV-4 antisera revealed that ELISAs were more sensitive compared to VN test, with FAdV-1 fib-1 ELISA and both FAdV-4 fib ELISAs detecting significantly more samples as positive. This finding was most pronounced in sera generated against live virus, compared to birds inoculated with inactivated virus. On the one hand, this might be related to the aforementioned alteration of antigenic structures by virus inactivation which limits the detection of such induced antibodies. On the other hand, it was shown in human adenoviruses that the contact with live virus elicits a more pronounced fiber response (Cheng et al., Citation2010; Yu et al., Citation2013), a phenomenon that can possibly be related to the release of an excessive amount of fibers without capsid integrity during early stages of virus replication (Chroboczek et al., Citation1995). This also complies with our finding that the majority of FAdV-1 and FAdV-4 infected birds produced fiber antibodies already at early time points post infection, whereas neutralizing antibodies were detected by VN test with certain delay.
Overall, a strong correlation between fiber antibody titres and neutralizing activity in sera from birds infected with FAdV-1 and FAdV-4 was noticed, although previous studies have indicated that fiber antibodies alone do not confer efficient neutralization (Pitcovski et al., Citation2005; Schachner et al., Citation2014). Furthermore, a strong correlation between hexon antibodies and VN titres in FAdV-2 antisera was reported (Junnu et al., Citation2014). The required presence of both fiber and penton base antibodies for efficient neutralization of human adenovirus implies that neutralization is primarily a result of multiple antibody fractions directed against different adenovirus capsid proteins (Gahéry-Ségard et al., Citation1998). Anyhow, early induction of a vigorous fiber response favours FAdV-1 and FAdV-4 fiber-based ELISAs over VN test in the detection of FAdV antibodies.
Testing of field sera confirmed the suitability of the developed fiber ELISAs for a serotype-specific detection of antibodies in a scenario involving birds infected with a broad variety of FAdV serotypes. This finding in particular demonstrates the advantage of fiber ELISAs over whole virus-based ELISAs, as the latter ones do not enable serotype-specific detection of antibodies in chickens infected with different serotypes, due to group-specific reactions (Mockett & Cook, Citation1983). Based upon the results obtained from field sera, the FAdV-1 fib-1 and the FAdV-4 fib-1 ELISAs were found more sensitive than VN test. Particularly, detection of FAdV-1 antibodies in birds that seroconverted between the two sampling time points was most efficient by FAdV-1 fib-1 ELISA in comparison to VN test, as it reacted sooner or detected a greater number of samples as positive. This finding may be explained by a pronounced fiber response upon infection, as already mentioned above.
In conclusion, in the present study recombinant fiber proteins have been implemented in an ELISA assay and demonstrated for the first time a serotype-specific detection of certain FAdV serotypes, namely FAdV-1 and FAdV-4. The established fiber ELISAs allow testing of large sample sizes and are equal or even superior in sensitivity to VN test while being advantageous in processing and handling, hence providing a suitable basis for monitoring of antibodies against serotypes FAdV-1 and FAdV-4 in commercial chicken flocks.
Supplemental data
Download Zip (24.2 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Akhtar, M., Ahmed, R., Hayat, C.S., Hussain, I. & Ashfaque, M. (2000). Comparative immune response of formalin inactivated and binnary ethyleneimine inactivated Angara disease vaccines. Pakistan Journal of Biological Sciences, 3, 1313–1314. doi: 10.3923/pjbs.2000.1313.1314
- Calnek, B.W., Shek, W.R., Menendez, N.A. & Stiube, P. (1982). Serological cross-reactivity of avian adenovirus serotypes in an enzyme-linked immunosorbent assay. Avian Diseases, 26, 897–906. doi: 10.2307/1589878
- Cheng, C., Gall, J.G.D., Nason, M., King, C.R., Koup, R.A., Roederer, M., McElrath, M.J., Morgan, C.A., Churchyard, G., Baden, L.R., Duerr, A.C., Keefer, M.C., Graham, B.S. & Nabel, G.J. (2010). Differential specificity and immunogenicity of adenovirus type 5 neutralizing antibodies elicited by natural infection or immunization. Journal of Virology, 84, 630–638. doi: 10.1128/JVI.00866-09
- Chroboczek, J., Ruigrok, R.W. & Cusack, S. (1995). Adenovirus fiber. Current Topics in Microbiology and Immunology, 199, 163–200.
- Cohen, J. (1988). Statistical Power Analysis for the Behavioral Sciences 2nd edn. Hillsdale, NJ: Lawrence Earlbaum Associates.
- Cook, J.K.A. (1983). Fowl adenoviruses: studies on aspects of the pathogenicity of six strains for 1-day-old chicks. Avian Pathology, 12, 35–43. doi: 10.1080/03079458308436147
- Cunningham, S.C., Lew, A.M. & Tannock, G.A. (2001). The antigenicity of the chicken anaemia virus protein VP3 (Apoptin). Avian Pathology, 30, 613–619. doi: 10.1080/03079450120092107
- Dawson, G.J., Orsi, L.N., Yates, V.J., Chang, P.W. & Pronovost, A.D. (1980). An enzyme-linked immunosorbent assay for detection of antibodies to avian adenovirus and avian adenovirus-associated virus in chickens. Avian Diseases, 24, 393–402. doi: 10.2307/1589706
- El Bakkouri, M., Seiradake, E., Cusack, S., Ruigrok, R.W.H. & Schoehn, G. (2008). Structure of the C-terminal head domain of the fowl adenovirus type 1 short fiber. Virology, 378, 169–176. doi: 10.1016/j.virol.2008.05.011
- Erny, K., Pallister, J. & Sheppard, M. (1995). Immunological and molecular comparison of fowl adenovirus serotypes 4 and 10. Archives of Virology, 140, 491–501. doi: 10.1007/BF01718426
- Fingerut, E., Gutter, B., Gallili, G., Michael, A. & Pitcovski, J. (2003). A subunit vaccine against the adenovirus egg-drop syndrome using part of its fiber protein. Vaccine, 21, 2761–2766. doi: 10.1016/S0264-410X(03)00117-8
- Gahéry-Ségard, H., Farace, F., Godfrin, D., Gaston, J., Lengagne, R., Tursz, T., Boulanger, P. & Guillet, J.G. (1998). Immune response to recombinant capsid proteins of adenovirus in humans: antifiber and anti-penton base antibodies have a synergistic effect on neutralizing activity. Journal of Virology, 72, 2388–2397.
- Gelderblom, H. & Maichle-Lauppe, I. (1982). The fibers of fowl adenoviruses. Archives of Virology, 72, 289–298. doi: 10.1007/BF01315225
- Grafl, B., Prokofieva, I., Wernsdorf, P., Steinborn, R. & Hess, M. (2014). Infection with an apathogenic fowl adenovirus serotype-1 strain (CELO) prevents adenoviral gizzard erosion in broilers. Veterinary Microbiology, 172, 177–185. doi: 10.1016/j.vetmic.2014.05.020
- Guardado-Calvo, P., Llamas-Saiz, A.L., Langlois, P. & van Raaij, M.J. (2006). Crystallization of the C-terminal head domain of the avian adenovirus CELO long fiber. Acta Crystallographica Section F: Structural Biology and Crystallization Communications, 62, 449–452. doi: 10.1107/S160053680504287X
- Günes, A., Grafl, B., Berger, E., & Hess, M. (2011). Shedding of fowl adenoviruses from experimentally infected specific pathogen free layer chickens investigated by cell culture and PCR. Proceedings of the XVIIth International Congress of the World Veterinary Poultry Association (p. 182). Cancun, Mexico.
- Günes, A., Marek, A., Grafl, B., Berger, E. & Hess, M. (2012). Real-time PCR assay for universal detection and quantitation of all five species of fowl adenoviruses (FAdV-A to FAdV-E). Journal of Virological Methods, 183, 147–153. doi: 10.1016/j.jviromet.2012.04.005
- Harrach, B., Benkö, M., Both, G.W., Brown, M., Davison, A.J., Echavarria, M., Hess, M., Jones, M.S., Kajon, A., Lehmkuhl, H.D., Mautner, V., Mittal, S.K. & Wadell, G. (2011). Family Adenoviridae. In A.M.Q. King, M.J. Adams, E.B. Carstens, & E.J. Lefkowitz. Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses 9th edn (pp. 125–141). New York: Elsevier Academic Press.
- Hess, M. (2000). Detection and differentiation of avian adenoviruses: a review. Avian Pathology, 29, 195–206. doi: 10.1080/03079450050045440
- Hess, M. (2013). Aviadenovirus Infections. In D.E. Swayne, J.R. Glisson, L.R. McDougald, L.K. Nolan, D.L. Suarez, & V. Nair. Diseases of Poultry 13th edn (pp. 290–300). Ames: Wiley-Blackwell.
- Hess, M., Cuzange, A., Ruigrok, R.W.H., Chroboczek, J. & Jacrot, B. (1995). The avian adenovirus penton: two fibers and one base. Journal of Molecular Biology, 252, 379–385. doi: 10.1006/jmbi.1995.0504
- Junnu, S., Lertwatcharasarakul, P., Jala, S., Phattanakunanan, S., Moonjit, P. & Songserm, T. (2014). Developing an indirect ELISA based on recombinant hexon protein for serological detection of inclusion body hepatitis in chickens. The Journal of Veterinary Medical Science, 76, 289–293. doi: 10.1292/jvms.13-0196
- Li, P., Bellett, A.J.D. & Parish, C.R. (1984). The structural proteins of chick embryo lethal orphan virus (fowl adenovirus type 1). Journal of General Virology, 65, 1803–1815. doi: 10.1099/0022-1317-65-10-1803
- Marek, A., Günes, A., Schulz, E. & Hess, M. (2010). Classification of fowl adenoviruses by use of phylogenetic analysis and high-resolution melting-curve analysis of the hexon L1 gene region. Journal of Virological Methods, 170, 147–154. doi: 10.1016/j.jviromet.2010.09.019
- Marek, A., Nolte, V., Schachner, A., Berger, E., Schlötterer, C. & Hess, M. (2012). Two fiber genes of nearly equal lengths are a common and distinctive feature of fowl adenovirus C members. Veterinary Microbiology, 156, 411–417. doi: 10.1016/j.vetmic.2011.11.003
- Matos, M., Grafl, B., Liebhart, D., Schwendenwein, I. & Hess, M. (2016). Selected clinical chemistry analytes correlate with the pathogenesis of inclusion body hepatitis experimentally induced by fowl aviadenoviruses. Avian Pathology, 45, 520–529. doi: 10.1080/03079457.2016.1168513
- Mazaheri, A., Prusas, C., Voss, M. & Hess, M. (1998). Some strains of serotype 4 fowl adenoviruses cause inclusion body hepatitis and hydropericardium syndrome in chickens. Avian Pathology, 27, 269–276. doi: 10.1080/03079459808419335
- McFerran, J.B. (1981). Immunity to Adenoviruses. In M.E. Rose, L.N. Payne, & B.M. Freeman. Avian Immunology (pp. 187–203). Edinburgh: British Poultry Science.
- Meulemans, G. & Halen, P. (1982). Enzyme-linked immunosorbent assay (ELISA) for detecting infectious laryngotracheitis viral antibodies in chicken serum. Avian Pathology, 11, 361–368. doi: 10.1080/03079458208436111
- Mockett, A.P. & Cook, J.K. (1983). The use of an enzyme-linked immunosorbent assay to detect IgG antibodies to serotype-specific and group-specific antigens of fowl adenovirus serotypes 2, 3 and 4. Journal of Virological Methods, 7, 327–335. doi: 10.1016/0166-0934(83)90086-1
- Norrby, E. (1969). The structural and functional diversity of adenovirus capsid components. Journal of General Virology, 5, 221–236. doi: 10.1099/0022-1317-5-2-221
- Ojkic, D., Martin, E., Swinton, J., Vaillancourt, J.P., Boulianne, M. & Gomis, S. (2008). Genotyping of Canadian isolates of fowl adenoviruses. Avian Pathology, 37, 95–100. doi: 10.1080/03079450701805324
- Pitcovski, J., Fingerut, E., Gallili, G., Eliahu, D., Finger, A. & Gutter, B. (2005). A subunit vaccine against hemorrhagic enteritis adenovirus. Vaccine, 23, 4697–4702. doi: 10.1016/j.vaccine.2005.03.049
- Rajasekhar, R. & Roy, P. (2014). Recombinant hexon antigen based single serum dilution ELISA for rapid serological profiling against fowl adenovirus-4 causing hydropericardium syndrome in chickens. Journal of Virological Methods, 207, 121–127. doi: 10.1016/j.jviromet.2014.06.017
- Reed, L.J. & Muench, H. (1938). A simple method of estimating fifty percent endpoints. American Journal of Epidemiology, 27, 493–497. doi: 10.1093/oxfordjournals.aje.a118408
- Schachner, A., Marek, A., Grafl, B. & Hess, M. (2016). Detailed molecular analyses of the hexon loop-1 and fibers of fowl aviadenoviruses reveal new insights into the antigenic relationship and confirm that specific genotypes are involved in field outbreaks of inclusion body hepatitis. Veterinary Microbiology, 186, 13–20. doi: 10.1016/j.vetmic.2016.02.008
- Schachner, A., Marek, A., Jaskulska, B., Bilic, I. & Hess, M. (2014). Recombinant FAdV-4 fiber-2 protein protects chickens against hepatitis-hydropericardium syndrome (HHS). Vaccine, 32, 1086–1092. doi: 10.1016/j.vaccine.2013.12.056
- Schachner, A., Matos, M., Grafl, B. & Hess, M. (Forthcoming). Fowl aviadenovirus (FAdV) induced diseases and strategies for their control – a review on the current global situation. Avian Pathology, submitted for publication.
- Schat, K.A. & Sellers, H.S. (2008). Cell-culture Methods. In L. Dufour-Zavala, D.E. Swayne, J.R. Glisson, J.E. Pearson, W. M. Reed, M. W. Jackwood, & P. R. Woolcock. A Laboratory Manual for the Isolation, Identification, and Characterization of Avian Pathogens 5th edn (pp. 195–203). Madison, WI: The American Association of Avian Pathologists.
- Slaght, S.S., Yang, T.J. & van der Heide, L. (1979). Adaptation of enzyme-linked immunosorbent assay to the avian system. Journal of Clinical Microbiology, 10, 698–702.
- Steer, P.A., O’Rourke, D., Ghorashi, S.A. & Noormohammadi, A. H. (2011). Application of high-resolution melting curve analysis for typing of fowl adenoviruses in field cases of inclusion body hepatitis. Australian Veterinary Journal, 89, 184–192. doi: 10.1111/j.1751-0813.2011.00695.x
- Xie, Z., Luo, S., Fan, Q., Xie, L., Liu, J., Xie, Z., Pang, Y., Deng, X. & Wang, X. (2013). Detection of antibodies specific to the non-structural proteins of fowl adenoviruses in infected chickens but not in vaccinated chickens. Avian Pathology, 42, 491–496. doi: 10.1080/03079457.2013.829553
- Yates, V.J. & Fry, D.E. (1957). Observations on a chicken embryo lethal orphan (CELO) virus. American Journal of Veterinary Research, 18, 657–660.
- Yu, B., Dong, J., Wang, C., Zhan, Y., Zhang, H., Wu, J., Kong, W. & Yu, X. (2013). Characteristics of neutralizing antibodies to adenovirus capsid proteins in human and animal sera. Virology, 437, 118–123. doi: 10.1016/j.virol.2012.12.014
