ABSTRACT
Marek’s disease virus (MDV) and avian leucosis virus (ALV) are known to cause tumours in egg-laying hens. Here, we investigated the aetiology of tumours in a flock of egg-laying hens vaccinated against MDV. We carried out gross pathology and histopathological examinations of the diseased tissues, identified virus antigen and sequenced viral oncogenes to elucidate the cause of death in 21–22-week-old hens. At necropsy, diseased hens had distinctly swollen livers, spleens, and proventriculus, and white tumour nodules in the liver. The spleen and liver had been infiltrated by lymphoid tumour cells, while the proventriculus had been infiltrated by both lymphoid tumour cells and myeloblastic cells. Subtype J ALV (ALV-J) and MDV were widely distributed in the proventricular gland cells, and the lymphoid tumour cells in the liver and the spleen. In addition, positive ALV-J signals were also observed in parts of the reticular cells in the spleen. MDV and ALV-J antigens were observed in the same foci of the proventricular gland cells; however, the two antigens were not observed in the same foci from the spleen and liver. The amino acid sequence of the AN-1 (the representative liver tumour tissue that was positive for both ALV-J and MDV) Meq protein was highly similar to the very virulent MDV QD2014 from China. Compared to the ALV-J HPRS-103 reference strain, 10 amino acids (224-CTTEWNYYAY-233) were deleted from the gp85 protein of AN-1. We concluded that concurrent infection with MDV and ALV-J contributed to the tumorigenicity observed in the flock.
Introduction
Co-infection with immunosuppressive oncogenic viruses, such as avian leucosis virus (ALV), reticuloendotheliosis virus (REV) and Marek’s disease virus (MDV), is widespread, and has become an important epidemiologic factor contributing to the increasing incidence of neoplastic disease (Davidson & Borenstein, Citation1999; Fenton et al., Citation2005; Qin et al., Citation2010). Previous studies have demonstrated some characteristics of co-infection and multi-infection with tumorigenic viruses (Cui et al., Citation2009; Gopal et al., Citation2012); however, it is rare that the histopathological findings from naturally occurring cases of mixed infection are reported.
MDV and subtype J ALV (ALV-J) favour the development of lymphomas and myelocytic series cells, causing Marek’s disease (MD) and myeloid leucosis, respectively (Payne et al., Citation1991a; Schat et al., Citation1991; Nair, Citation2013). Generally, MD can occur at any time, beginning at 3–4 weeks of age or older, with occasional outbreaks in adult chickens reported (Ikezawa et al., Citation2010; Muniyellappa et al., Citation2013; Zhuang et al., Citation2015). Naturally occurring myeloblastosis (myeloblastic myeloid leucosis) usually occurs in adult chickens from broiler breeders, which was first reported in 1988, to layers, Chinese “yellow” chickens and local indigenous breeds (Payne et al., Citation1991b; Sun & Cui, Citation2007; Cui, Citation2016). However, tumour outbreaks may be caused by MDV and ALV-J co-infections, and these outbreaks may be more severe than those caused by a single infection. Vaccines for MD are available in China and have been used for many years to control the spread of the disease. However, some poultry farms experience vaccine failure events, leading to a disease outbreak (Zhang et al., Citation2009; Zhuang et al., Citation2015).
A large number of previous reports have described and compared the histopathological changes in a single virus infection (Pandiri et al., Citation2009; Deng et al., Citation2011; Mete et al., Citation2016). However, to the best of our knowledge, few previous reports have provided a detailed description of lesions from MDV and ALV-J co-infection (Zhang et al., Citation2009). From the morphological point of view, the co-location of virus antigens and the type of tumour that occurs in the same co-infected tumour tissue are unknown. Here, we describe the clinical signs and pathological findings associated with MDV and ALV-J co-infection in a naturally infected flock of egg-laying hens.
Materials and methods
Case history and specimens
In April 2016, a commercial line of local breeder chickens from a breeding company with 9000 egg-laying hens in the Anhui Province of China was reported to be suffering from a tumour outbreak. Farm workers had vaccinated the chickens with the MD CVI988/Rispens vaccine at 1-day old using standard operating procedures. The owner of the chickens gave permission for their birds to be included in this study. The sick hens were 21–22 weeks old. They presented with clinical signs, including: depression, emaciation, white comb and ruffled feathers. The disease had a daily mortality rate ranging from 0.11% to 0.22% of the flock; the overall mortality rate reached 5% at 21–22 weeks old. Diseased hens also laid fewer eggs and produced soft-shelled eggs.
Initial pathogen screening by polymerase chain reaction (PCR) or reverse transcription-polymerase chain reaction (RT-PCR) was conducted to detect the env gene of ALV-A, ALV-B, ALV-J, REV and the meq gene of MDV, according to a previous study (Liu et al., Citation2009). The tumour tissue specimens (liver or spleen) of 11 chickens were randomly collected from 21- to 22 weeks old diseased hens. All 11 chickens were free of ALV-A, ALV-B and REV, and 100% (11/11) were co-infected with MDV and ALV-J based on PCR or RT-PCR results. The liver, spleen and or proventriculus tumour tissue of five chickens co-infected with MDV and ALV-J were used to determine the type of tumour by haematoxylin and eosin (H&E) staining. The liver, spleen and proventriculus tumour tissue of one chicken co-infected with MDV and ALV-J were used to determine the MDV and ALV-J antigens by immunohistochemical staining, and its liver tumour tissue specimen was designated AN-1.
Histopathological examination
Aseptic samples were collected from the liver, spleen and proventriculus and fixed in 10% buffered formalin. The samples were embedded in paraffin, cut into sections, and stained with H&E. Microscopic changes in the tissue were detected by light microscopy.
Immunohistochemistry evaluation
Immunohistochemistry (IHC) was performed using mouse anti-meq monoclonal antibodies (mAbs) meqc specific for MDV Meq protein (kindly provided by Professor Hongjun Chen, Shanghai Veterinary Research Institute, Chinese Academy of Agricultural Sciences) and anti-gp85 mAbs JE9 specific for ALV-J gp85 protein (kindly provided by Professor Aijian Qin, College of Veterinary Medicine, Yangzhou, China) to determine the tissue distribution of MDV and ALV-J. For staining, the paraffin-embedded tissue sections were cut into serial sections; the sections were dried, deparaffined and rehydrated. Endogenous peroxidase was blocked by incubating the sections in an aqueous peroxidase (3%) solution following rehydration. The slides were then incubated overnight at 4°C with specific mAbs against MDV or ALV-J. The next morning, the slides were blocked with bovine serum albumin (Boster, Wuhan, China) for 1 h and then incubated with a biotinylated goat anti-mouse immunoglobulin G at 37°C for 20 min. Following three phosphate-buffered saline washes, the slides were incubated with a Streptavidin–Biotin Complex (Boster, Wuhan, China) at 37°C for 20 min. A 3,3′-diaminobenzidine reagent (Boster) was prepared as per supplier’s guidelines. The sections were then incubated for 5 min in the reagent until a prominent brown colour was visible. Two negative controls were set: the spleen, liver and proventriculus of an uninfected chicken from a different healthy flock were used to confirm specificity of the primary antibody; phosphate-buffered saline was used as another negative control, and the slides were treated as described except for the addition of primary antibody.
Detection of viral oncogenic genes
Tumour tissue samples (liver or spleen) were collected for viral oncogenic gene analyses. Using a DNA extraction kit (Takara Biotechnology, Dalian, China), viral DNA was extracted from the liver tumour tissue, according to the manufacturers’ instructions. The MDV meq and ALV-J gp85 genes were amplified from the tumour tissue using the primers. The primers used for the MDV meq (Zhuang et al., Citation2015) were: 5′-CGCGAATTCTACAGGTGTAAAGAGATG-3′ (forward) and 5′-TAACTCGAGTGCTGAGAGTCACAATGC-3′ (reverse). The primers used for the ALV-J gp85 (Wang et al., Citation2013) were: 5′-GTGGATCCATGGGAGTTCATCTATTGCAACAACCGA-3′ (forward) and 5′-TACTGCAGTTAGCGCCTGCTAACGGTGGTGACC-3′ (reverse). The final PCR reaction was 50 μl consisting of 5 μl of 10× Taq PCR buffer, 2 μl of each primer, 0.5 μl of Taq DNA polymerase, 38.5 μl of ddH2O and 2 μl of the DNA template. The PCR parameters for the meq gene were: 95°C for 5 min, 30 cycles of 94°C for 60 s, 55°C for 60 s, and 72°C for 60 s, and then 72°C for 10 min. The PCR parameters for the gp85 gene were as follows: 95°C for 4 min, 35 cycles of 95°C for 40 s, 57°C for 60 s, and 72°C for 60 s, and then 72°C for 10 min. The PCR products were separated on a 1% agarose gel and then sequenced by Genscript (Shanghai, China). All the sequences were aligned and analysed with the neighbour-joining method based on 1000 replicates in Mega 5 software.
Results
Postmortem examination findings
Necropsies were performed on 11 sick hens. Clinical signs of the birds presented for necropsy were representative of the most common signs observed in the outbreak and included extreme weight loss, prominent sternums, fat loss, a grey abdomen, distinctly swollen spleens and livers, and multifocal white tumour nodules in the liver. The proventriculus from two birds also presented with distinct oedema and erosion.
Histopathological findings
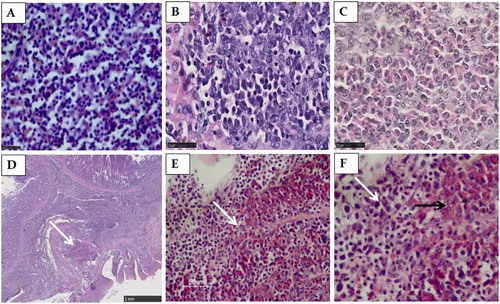
The H&E staining indicated that large areas of the spleen, liver and proventriculus had been infiltrated by lymphoid tumour cells ((A–B, D)); many lymphoid tumour nodules were composed of pleomorphic, neoplastic lymphoid cells scattered in the liver ((B)). The liver from one (one-fifth) bird had been infiltrated by myeloid cells with eosinophilic cytoplasmic granules (myelocytes) ((C)). A large population of pleomorphic and neoplastic lymphoid tumour cells were scattered in the proventricular glands and lamina propria ((D)). It is noteworthy that two types of tumour cells existed in the same foci in the proventricular gland, including both myeloid cells with eosinophilic cytoplasmic granules (myelocytes) and lymphoid tumour cells ((E, F)).
Figure 1. The spleen, liver and proventricular tissues stained by HE. (A) Numerous small lymphoid tumour cells scattered through the spleen. Scale bar = 25 μm. (B) The liver was infiltrated by the pleomorphic, neoplastic lymphoid tumour cells. Scale bar = 25 μm. (C) The liver was infiltrated by myeloid cells with eosinophilic cytoplasmic granules (myelocytes). Scale bar = 25 μm. (D) A large population of pleomorphic, neoplastic lymphoid tumour cells scattered in the proventricular glands and lamina propria of the proventriculus. Scale bar = 1 mm. (E) A higher magnification of the area indicated by an arrow in D. Scale bar = 50 μm. (F) A higher magnification of the area indicated by an arrow in (E). Two types of tumour cells exist in the same foci in the proventriculus, consisting of myeloid cells with eosinophilic cytoplasmic granules (myelocytes) (black arrow) and lymphoid tumour cells (white arrow). Magnification × 1000.
Immunohistochemical findings
ALV-J and MDV immunoreactivity were observed in the lymphoid tumour cells of the spleen and the liver ((A, B and D, E)). In addition, positive ALV-J signals were also observed in parts of the reticular cells in the spleen ((A)). However, ALV-J and MDV immunoreactivity was not detected in the same foci from the spleen and liver.
Figure 2. The spleen, liver and proventricular tissues stained by IHC. (A) ALV-J immunoreactivity in the lymphoid tumour cells and parts of the reticular cells in spleen. Magnification × 400. (B) ALV-J immunoreactivity in tumour cells in the liver. Magnification × 400. (C) ALV-J immunoreactivity in tumour cells in the proventricular gland cells. Magnification × 400. (D) MDV immunoreactivity in the lymphoid tumour cells in the spleen. Magnification × 400. (E) MDV immunoreactivity in the lymphoid tumour cells in the liver. Magnification × 400. (F) MDV immunoreactivity in the proventricular gland cells. Magnification × 400. (G) The spleen of non-infected chicken. Magnification × 400. (H) The liver of non-infected chicken. Magnification × 400. (I) The proventriculus of non-infected chicken. Magnification × 400.
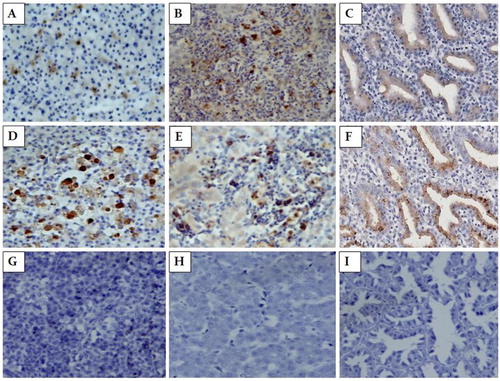
The immunohistochemical staining indicated that ALV-J and MDV were widely distributed in the proventricular gland cells, and some positive signals from two types of antigens were observed in the same foci ((C and F)).
Sequence analysis of the viral oncogenes
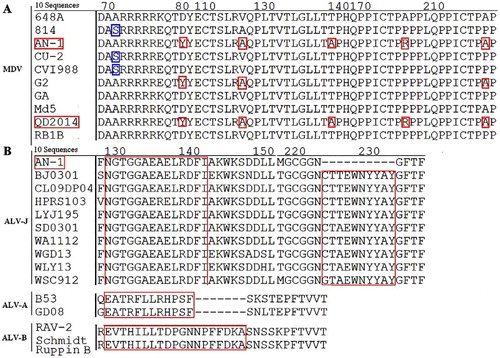
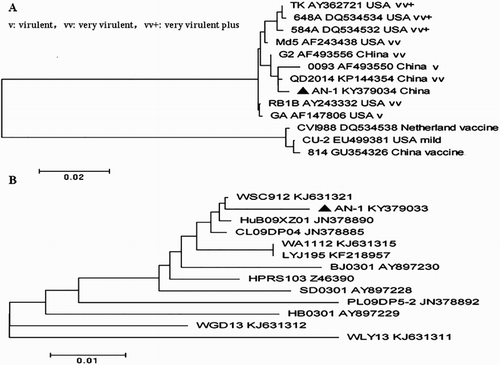
The sequences of the viral oncogenes isolated from the diseased egg-laying hens were edited and aligned using the Mega 5 software. Phylogenetic trees were established based on the meq and gp85 nucleotide sequences. The nucleotide sequence of the meq gene from MDV AN-1 (Genbank: KY379034) shares 82.2–99.6% identity with 12 MDV reference strains, and was similar to the very virulent MDV QD2014 from China (Cai et al., Citation2016) ((A)). Six amino acid mutations at residues 71 (S → A), 80 (D → Y), 115 (V → A), 139 (T → A), 176 (P → R) and 217 (P → A) were found in strain AN-1 ((A)). Similarly, the nucleotide sequence of the gp85 gene from ALV-J AN-1 (Genbank: KY379033) was similar to the pathogenic strain WSC912 ((B)). In comparison to ALV-J HPRS-103 and ALV-A, ALV-B reference strains, the 129 NGTGGAEAELRDFI 142 epitope from AN-1 was highly conserved and consistent with most ALV-J strains. In contrast, the epitope 224 CTTEWNYYAY 233 was deleted from the gp85 protein of AN-1 ((B)).
Figure 3. Phylogenetic tree based on the meq and gp85 gene sequences of MDV and ALV-J AN-1 and their reference strains. Unrooted phylogenetic trees were generated using the distance-based neighbour-joining method with the Mega 5 software. (A) The phylogenetic tree for the meq gene from ALV-J AN-1. (B) The phylogenetic tree for the gp85 gene from ALV-J AN-1.
Discussion
MDV and ALV-J are well known to cause avian tumours. Infection with either virus leads to various illnesses, and in some cases death, and can also reduce egg production, suppress the immune system, and increase disease susceptibility (Sun & Cui, Citation2007; Gopal et al., Citation2012; Muniyellappa et al., Citation2013). MDV infection most often results in lymphomas, while ALV-J often causes myeloblastomas or myelocytomas. ALV-J is also able to cause other types of tumours, including lymphomas (Deng et al., Citation2011).
Here we show that there were two types of tumour cells in the same foci in the proventricular gland. The tumour cells were primarily myeloid cells with eosinophilic cytoplasmic granules (myelocytes) and lymphoid tumour cells. While it was clear that ALV-J infection had caused the myeloid cells with eosinophilic cytoplasmic granules, it was difficult to discern whether the lymphoid tumour cells were the result of MDV or ALV-J infection by H&E staining.
Antigens from MDV and ALV-J were detected by IHC in the proventricular gland cells, but not in lymphoid tumour cells between the proventricular gland or proventricular laminae propria. mAb ALV-J JE9 is specific for ALV-J envelope glycoprotein-gp85 and did not react with subgroups A, B, C, D, E or endogenous ALV (Qin et al., Citation2001). ALV-J was shown to express in the proventricular gland cells, and the result is consistent with the previous report (Wang et al., Citation2017).
MDV is not known to replicate in the proventricular gland cells because the main target of MDV infection is lymphocytes. In fact, MDV was widely distributed in the nucleus of lymphoid tumour cells in the spleen and liver, and this result is consistent with subcellular localization of the Meq protein in the nucleus (Liu et al., Citation1997). We failed to find MDV Meq expression in hepatic cells using anti-meq mAbs; thus, we had set a non-infected chicken control to demonstrate that the stains were not background. The immune antigens for producing anti-meq mAbs specific for MDV were derived from the prokaryotic expression products of the meq gene, and the anti-meq mAbs reacted only to serotype I MDV (unpublished). Thus, this unique tropism might be the meq gene expressed only in tumour cells and transformed cells.
Meq is a transcription factor that directly interacts with p53 and is capable of transforming cells (Qian et al., Citation1995; Liu & Kung, Citation2000). In this study, the MDV Meq protein was expressed in the proventricular gland cells, but no tumour cells were found in glandular epithelium cells. This result indicates that the proventricular gland cells have not been transformed into tumour cells; so Meq expressed in the proventricular gland cells may actually be required for replication and survival of MDV in vivo, just as Meq is expressed in chicken embryo fibroblasts and the insect F9 cell line in vitro (Liu & Kung, Citation2000).
These results showed that Meq is likely to play a dual role as a replication and a transforming protein for MDV (Liu & Kung, Citation2000). However, the mechanism is beyond the scope of this study.
The MDV Meq protein is considered the major factor responsible for the occurrence of tumours (Lee et al., Citation2000; Lupiani et al., Citation2004; Shamblin et al., Citation2004). As demonstrated by the phylogenetic analysis, the MDV AN-1 strain had a distant genetic relationship with the MD vaccine CVI988 from the Netherlands and 814 from China, and the vv and vv+ MDV strains from the USA ((A)). Furthermore, MDV strain AN-1 had six mutations at positions 71, 80, 115, 139, 176 and 217 in the Meq protein ((A)) with the very virulent strain QD2014 from China (Cai et al., Citation2016), suggesting that it might be a new epidemic strain.
gp85 is the main envelope protein in ALV virions and determines the subgroup for each ALV strain (Cui, Citation2016). AN-1 had a highly conserved amino acid sequence at epitope 129 NGTGGAEAELRDFI 142 consistent with the majority of ALV-J strains, but this epitope is markedly different between ALV-A and ALV-B strains (Li et al., Citation2015). The sequence data confirm that AN-1 belongs to the ALV-J subgroup. Compared to other ALV-J strains, such as HPRS-103 and other reference strains, the AN-1 gp85 protein had a deletion at the epitope 224 CTTEWNYYAY 233. These observations suggest that the viruses may be able to mutate key amino acid residues to adapt to changing conditions.
In this case, of the 11 diseased chickens sampled, 100% (11/11) were positive for both MDV and ALV-J. Both virulent MDV and the CVI988 MD vaccine virus carry the meq gene. Although the chickens had been vaccinated with CVI988 at one day of age and this vaccine persists in the birds, the level of CVI988 gradually decreases to a low level as the birds age so; at 21 weeks of age, we can be confident that the positive meq PCR result is due to infection with virulent MDV. The tumours appeared when the hens were 21–22 weeks old, despite being vaccinated against MD at 1-day old. The egg-laying hens were still infected with MDV, indicating that their immune responses may have been suppressed in other ways, or that they may have contracted very virulent MDVs later in life. This may suggest that the concurrence of MDV and ALV-J co-infections may be related to breeding eggs infected with ALV-J. Shang et al. (Citation2005) and Ikezawa et al. (Citation2010) showed that ALV-J could induce lymphocyte apoptosis in immune organs, particularly in young chickens. Lymphocyte death increases susceptibility to other diseases and could be a primary cause of vaccine failure in chickens vaccinated against MD.
The results of the present study may provide novel insights into the co-localization of virus antigens and tumour-type within the same tumour tissue co-infected with MDV and ALV-J, which might help understand the occurrence regularity of natural co-infected viruses in vivo.
Acknowledgements
The authors express their gratitude to Prof. Qin Aijian and Prof. Chen Hongjun for kindly providing anti-ALV-J gp85 and anti-MDV Meq monoclonal antibodies. HL and GW designed the experiments, analysed the data, and wrote the manuscript. YW, QH, CY and LP performed the experiments. HL and KQ performed the histopathological analysis. All authors reviewed the draft of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Cai, Z.H., Zhang, K., Guo, G.L., Yu, H.Q., Wang, L.P., Yu, J.M. & Wang, J.L. (2016). Identification and meq gene analysis of a very virulent Marek’s disease virus strain. Chinese Journal of Veterinary Science, 36, 1501–1506.
- Cui, Z.Z. (2016). Multiplicity of subgroups and viral quasispecies of avian leukosis viruses and its evolution under different selective pressures. Chinese Bulletin of Life Sciences, 28, 283–294.
- Cui, Z., Sun, S., Zhang, Z. & Meng, S. (2009). Simultaneous endemic infections with sub-group J avian leukosis virus and reticuloendotheliosis virus in commercial and local breeds of chickens. Avian Pathology, 38, 443–448. doi: 10.1080/03079450903349188
- Davidson, I. & Borenstein, R. (1999). Multiple infection of chickens and turkeys with avian oncogenic viruses: prevalence and molecular analysis. Acta Virologica, 43, 136–142.
- Deng, H., Wu, Y.F., Lu, Y.K., Wang, Z.F., Yang, H. & Ma, C.Q. (2011). Pathologic research of lymphocytic subgroup J-avian leukosis in Qingyuan local chicken. Acta Veterinaria et Zootechnica Sinica, 42, 1795–1799.
- Fenton, S.P., Reddy, M.R. & Bagust, T.J. (2005). Single and concurrent avian leukosis virus infections with avian leukosis virus-J and avian leukosis virus-A in Australian meat-type chickens. Avian Pathology, 34, 48–54. doi: 10.1080/03079450400025356
- Gopal, S., Manoharan, P., Kathaperumal, K., Chidambaram, B. & Divya, K.C. (2012). Differential detection of avian oncogenic viruses in poultry layer farms and turkeys by use of multiplex PCR. Journal of Clinical Microbiology, 50, 2668–2673. doi: 10.1128/JCM.00457-12
- Ikezawa, M., Goryo, M., Sasaki, J., Haridy, M. & Okada, K. (2010). Late Marek’s disease in adult chickens inoculated with virulent Marek’s disease virus. Journal of Veterinary Medical Science, 72, 1539–1545. doi: 10.1292/jvms.10-0203
- Lee, S., Takagi, M., Ohashi, K., Sugimoto, C. & Onuma, M. (2000). Difference in the meq gene between oncogenic and attenuated strain of Marek’s disease virus serotype 1. Journal of Veterinary Medical Science, 62, 287–292. doi: 10.1292/jvms.62.287
- Li, X.F., Zhu, H.B., Wang, Q., Sun, J.S., Gao, Y.N., Qi, X.L. & Wang, X.M. (2015). Identification of a novel B-cell epitope specific for avian leukosis virus subgroup J gp85 protein. Archives of Virology, 160, 995–1004. doi: 10.1007/s00705-014-2318-6
- Liu, J.L. & Kung, H.J. (2000). Marek’s disease herpesvirus transforming protein MEQ: a c-Jun analogue with an alternative life style. Virus Genes, 21, 51–64. doi: 10.1023/A:1008132313289
- Liu, J.L., Lee, L.F., Ye, Y., Qian, Z. & Kung, H.J. (1997). Nucleolar and nuclear localization properties of a herpesvirus bZIP oncoprotein, MEQ. Journal of Virology, 71, 3188–3196.
- Liu, G.Z., Zhang, H.H., Liu, Q., Qiu, B., Wang, F., Wang, X.W. & Chen, Z.Q. (2009). Isolation and identification of avian leukosis virus-B from layer chickens infected with avian leukosis virus-J. Chinese Journal of Virology, 25, 445–450.
- Lupiani, B., Lee, L.F., Cui, X., Gimeno, I., Anderson, A., Morgan, R.W., Silva R.F., Witter R.L., Kung H.-J. & Reddy, S.M. (2004). Marek’s disease virus-encoded meq gene is involved in transformation of lymphocytes but is dispensable for replication. Proceedings of the National Academy of Sciences of the United States of America, 101, 11815–11820. doi: 10.1073/pnas.0404508101
- Mete, A., Gharpure, R., Pitesky, M.E., Famini, D., Sverlow, K. & Dunn, J. (2016). Marek’s disease in backyard chickens, a study of pathologic findings and viral loads in tumorous and nontumorous birds. Avian Diseases, 60, 826–836. doi: 10.1637/11458-062216-Reg
- Muniyellappa, H.K., Satyanarayana, M.L., Isloor, S. & Gowda, N.K.S. (2013). Marek’s disease outbreak among vaccinated commercial layer flocks in the mining area of Karnataka, India. Veterinary Record, 172, 452.1–452. doi: 10.1136/vr.101203
- Nair, V. (2013). Latency and tumorigenesis in Marek’s disease. Avian Diseases, 57, 360–365. doi: 10.1637/10470-121712-Reg.1
- Pandiri, A.R., Gimeno, I.M., Reed, W.M., Fitzgerald, S.D. & Fadly, A.M. (2009). Subgroup J avian leukosis virus-induced histocytic sarcomatosis occurs only in the persistently viremic but not immunotolerized meat-type chickens. Veterinary Pathology, 46, 282–287. doi: 10.1354/vp.46-2-282
- Payne, L.N., Brown, S.R., Bumstead, N., Howes, K., Frazier, J.A. & Thouless, M.E. (1991a). A novel subgroup of exogenous avian leukosis virus in chickens. Journal of General Virology, 72, 801–807. doi: 10.1099/0022-1317-72-4-801
- Payne, L.N., Gillespie, A.M. & Howes, K. (1991b). Induction of myeloid leukosis and other tumours with the HPRS-103 strain of ALV. Veterinary Record, 129, 447–448. doi: 10.1136/vr.129.20.447
- Qian, Z., Brunovski, P., Rauscher, F., Lee, L. & Kung, H.J. (1995). Transactivation activity of Meq, a Marek’s disease herpesvirus bZIP protein persistently expressed in latently infected transformed T cells. Journal of Virology, 69, 4037–4044.
- Qin, L.T., Gao, Y.L., Pan, W., Deng, X.Y., Sun, F.F., Li, K. & Wang, X.M. (2010). Investigation of co-infection of ALV-J with REV, MDV, CAV in layer chicken flocks in some regions of China. Chinese of Journal of Preventive Veterinary Medicine, 32, 90–93.
- Qin, A.J., Lee, L.F., Fadly, A., Hunt, H. & Cui, Z.Z. (2001). Development and characterization of monoclonal antibodies to subgroup J avian leukosis virus. Avian Diseases, 45, 938–945. doi: 10.2307/1592872
- Schat, K.A., Chen, C.-L.H., Calnek, B.W. & Char, D. (1991). Transformation of T-lymphocyte subsets by Marek’s disease herpesvirus. Journal of Virology, 65, 1408–1413.
- Shamblin, C.E., Greene, N., Arumugaswami, V., Dienglewicz, R.L. & Parcells, M.S. (2004). Comparative analysis of Marek’s disease virus (MDV) glycoprotein-, lytic antigen pp 38- and transformation antigen Meq-encoding genes: association of meq mutations with MDVs of high virulence. Veterinary Microbiology, 102, 147–167. doi: 10.1016/j.vetmic.2004.06.007
- Shang, Y.L., Liu, S.D., Ding, B.J., Xiao, Y.H., Cheng, Z.Z., Sun, X.M. & Chu, Y.L. (2005). Immunosuppressive mechanism of broilers infected with avian leukosis virus subgroup J(ALV-J). Chinese Journal of Veterinary, 25, 573–577.
- Sun, S.H. & Cui, Z.Z. (2007). Epidemiological and pathological studies subgroup J avian leukosis virus infections in Chinese local “yellow” chickens. Avian Pathology, 36, 221–226. doi: 10.1080/03079450701332345
- Wang, B., Liu, F., Wang, H.Q., Li, N., Wei, J.Z., Liu, G.Q. & Wang, G.J. (2013). Construction of infectious clone of avian leukosis virus subgroup J AH-J11 strain. Acta Veterinaria et Zootechnica Sinica, 44, 1976–1981.
- Wang, J.Y., Cheng, M.H., She, R.P., Wu, Q.X., Shi, R.H. & Hu, F.J. (2017). Avian leukosis virus detection and liver pathology observation of slaughter broiler. Chinese Veterinary Science, 47, 114–120.
- Zhang, H.H., Liu, Q., Qiu, B., Liu, G.Z. & Cheng, Z.Q. (2009). Mixed infection of ALV-J and MDV in a flock of Shandong free range chickens. Acta Veterinaria et Zootechnica Sinica, 40, 1215–1221.
- Zhuang, X.Y., Zou, H.T., Shi, H.Y., Shao, H.X., Ye, J.Q., Miao, J., Wu, G. & Qin, A.J. (2015). Outbreak of Marek’s disease in a vaccinated broiler breeding flock during its peak egg-laying period in China. BMC Veterinary Research, 11, 353. doi: 10.1186/s12917-015-0493-7