ABSTRACT
The stand-alone pathogenicity of fowl adenoviruses (FAdVs) had long been disputed, given the ubiquity of the viruses versus sporadic outbreaks, and variation between experimental studies. However, a globally emerging trend of FAdV-associated diseases has marked the past two decades, with hepatitis-hydropericardium syndrome mainly in Asia besides Arabian and Latin American countries, and geographically more disseminated outbreaks of inclusion body hepatitis. Finally, the appearance of FAdV-induced gizzard erosion (AGE) in Asia and Europe completed the range of diseases. Epidemiological studies confirmed serotype FAdV-4 as agent of hepatitis-hydropericardium syndrome, whereas inclusion body hepatitis is related to FAdV-2, -8a, -8b and -11. Members of the biologically more distant serotype FAdV-1 induce AGE. Urged by increasing problems in the field, numerous pathogenicity studies with FAdVs from outbreaks substantiated the primary aetiologic role of particular strains for distinct clinical conditions.
Developments in the poultry industry towards highly specialized genetic breeds and rigorous biosecurity additionally contribute to the growing incidence of FAdV-related diseases. Confirming field observations, recent studies connected a higher susceptibility of broilers with their distinct physiology, implying the choice of bird type as a factor to be considered in infection studies. Furthermore, elevated biosecurity standards have generated immunologically naïve breeding stocks, putting broilers at risk in face of vertical FAdV transmission. Therefore, future prevention strategies should include adequate antibodies in breeders prior to production and – if necessary – vaccination, in order to protect progenies. This review aims to deliver a detailed overview on the current global situation about FAdV-induced diseases, their reproduction in vivo and vaccination strategies.
Introduction
In the middle of the last century, embryonated eggs gained popularity to isolate filterable agents from varied origin but also for vaccine production. In the course of such procedures it was occasionally noticed that a virus could be isolated which was unrelated to the original material but resembled the quail bronchitis virus isolated earlier on (Olson, Citation1950; Yates & Fry, Citation1957). As a consequence, vertical transmission was recognized as one of the first important biological features of fowl adenoviruses (FAdVs). Later on, experimental infections and epidemiological studies were performed in order to determine the role of FAdVs in a newly described disease condition, inclusion body hepatitis (IBH) (Helmboldt & Frazier, Citation1963). The sporadic occurrence of IBH in context with the wide spread of FAdVs in chicken flocks and the importance of immunosuppression to induce IBH initially questioned the primary pathogenicity of FAdVs (Hess, Citation2017).
In later years, the appearance of hepatitis-hydropericardium syndrome (HHS) and adenoviral gizzard erosion (AGE) led towards an increased awareness of FAdVs as primary aetiologic agents, emphasizing the importance of FAdV infections particularly in young chickens, primarily broilers (Hess, Citation2017). This was further supported by more frequent reports of IBH outbreaks in different parts of the world during the last 15 years.
The actual review aims to provide a comprehensive overview about the existing knowledge on field outbreaks and experimental studies on FAdVs on a global scale, in order to underline the changes noticed in recent years. As a consequence of increased mortality and production losses, more efforts are needed in the future to monitor and control FAdV infections, which will be addressed in the final section.
Inclusion body hepatitis
IBH is a disease caused by FAdVs belonging mainly to serotypes FAdV-2, FAdV-11 (species Fowl aviadenovirus D) and FAdV-8a and FAdV-8b (species Fowl aviadenovirus E). FAdV species were established recently to combine closely related serotypes (Harrach et al., Citation2012). Historically, IBH was first reported in chickens by Helmboldt and Frazier (Citation1963), who described an “acute hepatic catastrophe” due to the extent of liver damage in affected birds. In the following years, IBH was perceived as the result from concurrent infections by FAdVs and immunosuppressive agents, such as infectious bursal disease virus (IBDV) and chicken anaemia virus (CAV), in which FAdVs were considered opportunistic agents (Hoffmann et al., Citation1975; Rosenberger et al., Citation1975; Fadly et al., Citation1976). Other reports established an association between IBH and aflatoxins present in the feed (Singh et al., Citation1996). However, reports from Australia, New Zealand, Canada and Japan documented IBH outbreaks in the absence of predisposing factors, strengthening the role of FAdVs as primary pathogens (Reece et al., Citation1986; Christensen & Saifuddin, Citation1989; Gomis et al., Citation2006; Nakamura et al., Citation2011; Steer et al., Citation2011). Moreover, in a recent study, a statistically significant association between flocks being exposed to FAdV and co-infected with CAV or IBDV was not found (Eregae et al., Citation2014). Nonetheless, the importance of FAdVs as primary pathogens is still a matter of debate as strains from the same serotype can vary in their pathogenicity (Cook, Citation1983; Erny et al., Citation1991; Slaine et al., Citation2016; Absalón et al., Citation2017). Furthermore, FAdVs have been isolated from both healthy and sick chickens, a finding also noticed in recent studies (Kaján et al., Citation2013; Niczyporuk, Citation2016).
Field reports
In the last two decades, an increasing number of IBH outbreaks has been reported in different geographic locations stressing the worldwide spread of the disease (; Supplementary table 1). In the field, IBH principally affects broilers up to five weeks of age, but has also been reported sporadically in layers and broiler breeders. Mortality during IBH outbreaks peaks within 3–4 days and can reach 10% and occasionally be as high as 30% (Hess, Citation2013; Schachner et al., Citation2016). Additional clinical signs which may be recorded on affected chickens include poor growth, apathy, prostration, ruffled feathers and huddling behaviour. Clinically, IBH resembles very much HHS, although being less severe. Furthermore, concurrent infections are frequently observed in flocks suffering from IBH, which might explain why certain features of field outbreaks are not experimentally reproducible.
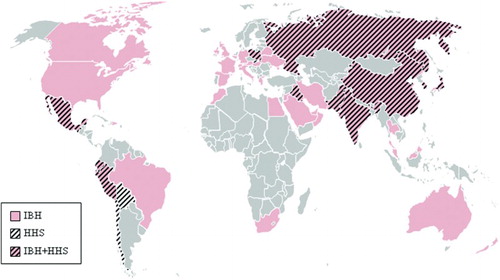
Figure 1. Global distribution map of IBH and HHS according to outbreaks reported in published literature and based on information from the field made available through personal communication, supported by diagnostic investigations. Countries with prevalence of IBH are marked in red, hachures indicate countries from which HHS has been reported.
Experimental studies
In vivo studies have shown that the outcome of experimentally induced IBH is highly dependent on the route of inoculation, viral dose and the age of birds (Supplementary table 2). From early on, the age-related resistance is a marked characteristic of aviadenoviral infections (Clemmer, Citation1972; Cook, Citation1974; Nakamura et al., Citation2000; Lim et al., Citation2011). Typically, when chickens are over one week of age, an invasive route of infection is needed to induce a clinical manifestation of IBH (Hess, Citation2017). However, the mechanisms behind this are not fully understood and, so far, no study has unequivocally clarified this phenomenon. The type of chickens affected by IBH is also a crucial element which influences the outcome of the disease. By using different chicken breeds of specific pathogen-free (SPF) and non-SPF origin, Cook (Citation1974) studied the influence of host, age and route of inoculation on the outcome of an FAdV infection. In that study, SPF Light Sussex chickens were highly susceptible to the infection in comparison to SPF Rhode Island Red chickens, with mortalities of 67% and 33% being recorded, respectively, following intraperitoneal inoculation at day-old. More recently, a mortality rate of 100% was recorded when SPF broilers were orally infected at day-old with an FAdV-8b strain, while in SPF layers inoculated under the same conditions the mortality was 20% (Matos et al., Citation2016a), corroborating the situation reported from the field and a higher susceptibility of broilers.
Following inoculation of chickens by the oral route, it was demonstrated that the virus colonizes the intestinal epithelium at 12 hours post infection and it can be detected in the blood as early as 24 hours post infection (Saifuddin & Wilks, Citation1991a). From 2 and 3 days post infection, the virus is present in its target organs, liver and pancreas. This phase corresponds to the incubation period, in which neither clinical signs nor gross lesions are recorded (Steer et al., Citation2015). The incubation period is succeeded by virus multiplication and a fast viraemia, producing pathological lesions in its target organs, in coincidence with clinical manifestation of the disease (Saifuddin & Wilks, Citation1991a; Steer et al., Citation2015; Matos et al., Citation2016b). Recovery of the birds occurs from 7 to 9 days post infection onwards and is manifested by a reduction in severity of clinical signs, together with cellular regeneration and a decrease of viral load in the target organs (Steer et al., Citation2015; Matos et al., Citation2016b). However, the virus remains latent in the caecal tonsils and it is shed in faeces for long periods. After oral infection at day-old with an FAdV-1 strain, the virus could be recovered from the gut of SPF chickens at 12 weeks post infection (Jones & Georgiou, Citation1984). Moreover, quantity and duration of virus shedding in the faeces do not correlate with the pathogenicity of an individual strain, but rather with the administered viral dose (Cook, Citation1983; Matos et al., Citation2016b; Oliver-Ferrando et al., Citation2017).
Gross pathology
During post-mortem examination, a severe hepatitis in which the liver is swollen, friable, with a marble-like pattern and necrotic foci can be noticed. Additionally, some studies reported necrosis, atrophy, colour change and petechiae in the pancreas of birds suffering from IBH (Winterfield et al., Citation1973; Grimes et al., Citation1978). Moreover, in both experimental and field cases of IBH, swollen and haemorrhagic kidneys were observed (Howell et al., Citation1970; Pettit & Carlson, Citation1972; Young et al., Citation1972; Macpherson et al., Citation1974; Antillón & Lucio, Citation1975; Saifuddin & Wilks, Citation1990; Erny et al., Citation1991; Steer et al., Citation2015; Matos et al., Citation2016b). A detailed histomorphometric evaluation, regarding the glomerular size and cellularity of swollen, pale or mottled kidneys recorded in broilers during an IBH outbreak demonstrated the presence of a glomerulonephritis (Wilson et al., Citation2010). Earlier reports suggested the haemopoietic system as an important target for FAdVs (Pettit & Carlson, Citation1972; Fadly & Winterfield, Citation1973; Rosenberger et al., Citation1974; Hoffmann et al., Citation1975). However, some of the findings described in those studies such as aplastic anaemia were most likley due to co-infections with CAV.
Histology
Histologically, large areas of cellular degeneration and necrosis, lymphoid infiltration and inclusion bodies are typical features noticed in the liver of chickens suffering from IBH. In addition to the hepatitis, aviadenoviral pancreatitis has been described from both field cases and experimental studies, in which necrosis of pancreatic acinar cells, lymphoid infiltration and inclusion bodies were observed (Gallina et al., Citation1973; Grimes et al., Citation1978; Tanimura et al., Citation1993; Goodwin et al., Citation1996; Matos et al., Citation2016a, b). Recent studies demonstrated a correlation between the extension of lesions in target organs and the clinical manifestation of IBH (Steer et al., Citation2015; Matos et al., Citation2016b). Additional lesions in the primary and secondary lymphoid tissues may be observed, such as atrophy of bursa and thymus, and lymphoid depletion in bursa and spleen (Itakura et al., Citation1974; Macpherson et al., Citation1974; Steer et al., Citation2015; Matos et al., Citation2016b). Moreover, focal necrosis, lymphocytic infiltration, nephrosis and, more rarely, inclusion bodies may be found in the kidney (Howell et al., Citation1970; Pettit & Carlson, Citation1972; Itakura et al., Citation1974; Macpherson et al., Citation1974; Antillón & Lucio, Citation1975; Steer et al., Citation2015).
Earlier detailed histological studies on IBH in chickens reported the presence of basophilic and eosinophilic inclusion bodies of variable appearance in the liver, following H&E staining (Itakura et al., Citation1974). Ultrastructurally, the basophilic inclusions consist of oval/hexagonal virus particles, which are around 70 nm in diameter, granular material and sometimes also of lamellae concentrically enclosing the virus core, which are probably related to the process of virus formation. On the other hand, the eosinophilic inclusions contain either moderately electrondense fibrillar-granular material or crystals composed of narrowly spaced thin filaments and may represent degenerated material in cells dying from the effects of the viral infection (Itakura et al., Citation1977). It has been proposed that these inclusions are analogous to human intranuclear aggregates (Strnad et al., Citation2013). Nonetheless, the exact composition and origin of these inclusions still remain to be determined, which may help in elucidating the pathogenesis of IBH.
Metabolic disturbances
Selected clinical chemistry analytes related to the cellular integrity and physiological function of liver and pancreas were recently used as biomarkers for strain pathogenicity and IBH pathogenesis (Matos et al., Citation2016b). As an outcome, disturbances in different enzymatic systems and metabolite concentrations were noticed. Additionally, acute changes in the plasmatic lipase together with severe histopathological lesions indicated the pancreas as an important target organ in IBH pathogenesis. In subsequent experimentally induced IBH, day-old orally infected SPF broiler presented severe clinical signs as a manifestation of hypoglycaemia and severe hepatitis and pancreatitis (Matos et al., Citation2016a). Earlier on, Goodwin et al. (Citation1993) described a flock with spiking mortality, in which broilers suffering from hypoglycaemia had adenoviral inclusion bodies in liver, pancreas and small intestine. Such findings may explain why most IBH outbreaks are reported in broilers, which are physiologically very different from layers (Supplementary table 1). Interestingly, broilers which suffered from hypoglycaemia, metabolic acidosis and hypocalcaemia during an IBH outbreak were effectively treated with glucose together with sodium bicarbonate and calcium, supporting the metabolic component of IBH pathogenesis (Venne & Chorfi, Citation2012).
Hepatitis-hydropericardium syndrome
HHS, also named hydropericardium syndrome (HPS), emerged in Pakistan in the late 1980s, subsequently spreading to other Asian countries, where it became endemic, but also some Arabian countries and parts of Latin America.
Historically, the aetiology of HHS was explained by feed disorders or intoxications (Jaffery, Citation1988; Qureshi, Citation1989), while a viral aetiology of the syndrome was proposed, based upon the observation of basophilic nuclear inclusion bodies in hepatocytes and adenovirus particles in liver homogenates (Cheema et al., Citation1989). In the years following the first emergence of the disease, a gradual recognition of the infectious nature of HHS took place, for which in vivo transmission experiments have played a key role.
Field reports
The first reported cases of the syndrome near Karachi, Pakistan, in 1987 (Anjum et al., Citation1989) were rapidly followed by massive outbreaks across the region, including a further spread to India, where it severely affected the intensive poultry husbandry during the early 1990s (Gowda & Satyanarayana, Citation1994; Kumar et al., Citation1997). In the following two decades, natural outbreaks were documented from various regions, including reports from Middle East (Abdul-Aziz & Al-Attar, Citation1991; Hess et al., Citation1999), Russia (Borisov et al., Citation1997), Slovakia (Jantosovic et al., Citation1991), Central and South America (Mazaheri et al., Citation1998; Toro et al., Citation1999; Rodriguez et al., Citation2014; Vera-Hernández et al., Citation2016), Japan (Abe et al., Citation1998; Mase et al., Citation2010) and Korea (Kim et al., Citation2008; Lim et al., Citation2011; Choi et al., Citation2012).
A substantial amount of epidemiological data collected during this period has strengthened the outstanding role of a single serotype, FAdV-4 of species FAdV-C, in the primary aetiology of HHS (Supplementary table 3).
Particularly during the last decade, a trend towards more epidemic, rather than sporadic, occurrence of outbreaks has been observed in many countries. At present, accumulating reports of severe HHS outbreaks in China provide the latest documentation for the emerging potential of the disease (Zhao et al., Citation2015; Changjing et al., Citation2016; Li et al., Citation2016a, b; Liu et al., Citation2016; Niu et al., Citation2016; Ye et al., Citation2016; Pan et al., Citation2017).
Experimental studies
Prior to the first attempts of disease induction with the isolated agent (Naeem et al., Citation1995), basic transmission experiments were carried out using homogenized tissue samples (Cheema et al., Citation1989). However, conduct of infection experiments in commercial birds, as performed in this and various other studies, requires cautious interpretation, considering the widespread of FAdVs and their respective antibodies.
Concurrent factors and/or agents were still considered mandatory for development of the disease throughout a longer period of time (Afzal et al., Citation1991; Toro et al., Citation1999). This was supported by inconsistent field observations, together with varying results obtained from different infection studies. This notion chiefly prevailed until experimental reproduction of HHS in SPF chickens with plaque purified FAdVs marked a critical milestone to demonstrate the stand-alone virulence of specific virus strains (Mazaheri et al., Citation1998). Parallel to that, experimental evidence led further to the recognition that the outcome of an infection may be primarily determined by the virulence of a particular FAdV strain, rather than additive effects of co-agents (Toro et al., Citation2001).
This is supported by longitudinal comparison of numerous available infection studies (Supplementary table 4), performed with FAdVs belonging to the same serotype, although considerable variances in virulence between strains was noticed (Mazaheri et al., Citation1998; Ahmad, Citation1999; Toro et al., Citation1999). This observation also correlates with the field scenario of FAdV-4 isolation from non-HHS-related specimen (Ojkić et al., Citation2008; Marek et al., Citation2010a; Griffin & Nagy, Citation2011; Niczyporuk, Citation2016).
In the light of contemporary efforts to study virulence markers on a molecular level (Griffin & Nagy, Citation2011; Zhao et al., Citation2015; Vera-Hernández et al., Citation2016), precise assessment of individual differences in clinicopathological properties of FAdV-4 strains is desirable, yet difficult to establish because of apparent variations attributed to host-related susceptibility and efficiency of the infection. Although they may not optimally reflect the natural infection via the oral route, parenteral routes were shown to induce a more severe pathogenicity in various experimental settings (Mazaheri et al., Citation1998; Naeem et al., Citation2001; Ahmad et al., Citation2011; Lim et al., Citation2011; Li et al., Citation2016b). This effect is particularly apparent to induce infection in increasingly older birds; however, potential distortions of the picture may result from the use of commercial chickens with a possible level of maternally derived antibodies, and the use of native vs. cell culture propagated inoculum in various studies.
Altogether, such effects on pathogenicity of a strain are well documented by a plethora of infection studies comparing inoculum dose, infection route, age and genetic background of chickens, whereby the lack of standardization has, at the same time, been a continuous critical remark throughout the relevant literature.
Clinicopathological aspects
The main type of birds affected are broilers, aged 3–6 weeks (Cheema et al., Citation1989; Mittal et al., Citation2014a), with exceptional reports on layers and broiler breeders (Asrani et al., Citation1997), corroborating particular host-related factors, such as age and genetic type of birds, for susceptibility. Besides sporadic reports in other domestic bird species, including pigeons (Naeem & Akram, Citation1995; Hess et al., Citation1998), quails (Karunamoorthy & Manickam, Citation1998), ducks (Chen et al., Citation2015) and ostriches (Changjing et al., Citation2016), a possible carry-over of the disease into wild birds has also been described (Kumar et al., Citation2010).
The course of disease and the clinicopathological signs exhibit certain parallels to IBH (as described in the section “Inclusion body hepatitis”), although being typically more pronounced. Losses are reported within a range of one- to ample two-digit percentages, in which case mortality may be the principal clinical manifestation due to the acute course of the disease. Fluid accumulation in the pericardial sac constitutes the most prominent gross pathological finding which is generally perceived as a pathognomonic feature of HHS. However, there are also reports of the absence of hydropericardium in certain outbreaks (Rodriguez et al., Citation2014). The accompanying hepatic involvement, characterized by swollen, friable, discoloured livers with focal necroses and petechial haemorrhages, bears resemblance to classical IBH; therefore the condition is sometimes also termed IBH-HPS. Histopathological changes, similar to IBH, are principally found in the liver, while inflammatory as well as degenerative processes are also common in other organs, mainly in the heart, kidneys, lungs and intestine (Anjum et al., Citation1989; Cheema et al., Citation1989). Importantly, tropism for lymphoid tissue constitutes an important aspect of the pathogenesis of HHS strains, resulting in degeneration of lymphoid organs alongside with lymphocyte depletion, altogether highlighting the immunosuppressive potential of virulent FAdV-4 strains (Naeem et al., Citation1995a; Shivachandra et al., Citation2003; Schonewille et al., Citation2008).
Adenoviral gizzard erosion
The literature on gizzard erosions in poultry is very limited, although it goes back to the early 1930s when the condition was first reported by nutritionists. Several non-infectious, alimentary factors as well as some infectious causes have been investigated and described to induce macroscopic lesions in the gizzard with FAdV to be considered as one of such (Gjevre et al., Citation2013).
Field reports
Although gizzard erosions have been described already in the context of FAdV infections during IBH and/or HHS outbreaks (Grimes et al., Citation1977; Nakamura et al., Citation2002), detailed investigations in the course of a field outbreak of AGE were conducted by Tanimura et al. (Citation1993). These authors supplied a comprehensive description of AGE concurrent with necrotizing pancreatitis in association with an aviadenovirus infection from a natural outbreak in 60–70 days old pullets with increased mortality using immuno-histochemical and electron microscopic methods. Applying similar methods, adenoviral gizzard lesions were further reported in a retrospective study from pullets and broiler flocks in the USA (Goodwin et al., Citation1993) and from an outbreak with increased mortality in Japanese broilers (Abe et al., Citation2001). However, it was Ono et al. (Citation2001) who, for the first time, characterized the adenovirus as FAdV serotype-1 (FAdV-1) by polymerase chain reaction-restriction fragment length polymorphism as aetiological agent from condemned gizzards in the slaughter house. Although FAdV-8a or -8b were sporadically isolated from affected gizzards (Okuda et al., Citation2004; Mase & Nakamura, Citation2014), the majority of subsequent outbreaks of the disease could be traced back to FAdV-1 strains. Geographically, the disease is mainly described in broilers from Europe and Asia (Supplementary table 5). From such outbreaks various molecular biological approaches have been used in order to differentiate between pathogenic and apathogenic FAdV-1 focusing on the fibre gene (Okuda et al., Citation2006; Marek et al., Citation2010b; Matczuk et al., Citation2017). On the host side, an initial study demonstrated that FAdV-1 interacts with a protein of approximately 200 kDa in the gizzard (Taharaguchi et al., Citation2007). Both, vertical as well as horizontal transmission of FAdV-1, and the subsequent clinical and pathological manifestation of AGE have been described in literature (Ono et al., Citation2007; Grafl et al., Citation2012). In affected broiler flocks, considerable economic losses due to AGE have been documented due to growth retardation, higher mortality rates and/or condemnations of affected gizzards in the slaughterhouse (Supplementary table 5). Several recent reports of AGE outbreaks in layers which coincide with increased mortality altogether indicate an impact of the disease on health and production also in layer-type birds (Lim et al., Citation2012; Totsuka et al., Citation2015; Matczuk et al., Citation2017). Independent of the production type, gross pathology and histology in the course of the disease are alike. Macroscopically, affected birds show gizzard lesions characterized by multiple brown or black areas of erosion of the keratinoid layer as well as inflammation and/or ulceration of the gizzard mucosa underneath (Tanimura et al., Citation1993; Abe et al., Citation2001; Ono et al., Citation2001; Grafl et al., Citation2012; Lim et al., Citation2012; Schade et al., Citation2013; Matczuk et al., Citation2017). In dead birds, sanguineous fluid in the gizzard as well as the proventricular and/or intestinal lumen and gizzard perforation can be observed. In general, no other macroscopic pathological changes are observed in affected birds. Histologically, infiltration with inflammatory cells such as macrophages and lymphocytes are observed in gizzard mucosa, submucosa and/or muscle layer in the affected gizzards (Tanimura et al., Citation1993; Abe et al., Citation2001; Ono et al., Citation2001; Grafl et al., Citation2012; Schade et al., Citation2013; Matczuk et al., Citation2017). Concurrently, erosion and loss of the keratinoid layer as well as degeneration and necrosis of glandular epithelial cells with/without basophilic intranuclear inclusion bodies can be detected.
Experimental studies
Successful experimental reproduction of AGE after infection with FAdV-1 strains isolated from affected gizzards derived from field outbreaks have been reported in SPF layers and broilers as well as in commercial broilers (Supplementary table 6). In one study the development of mild gizzard lesions was reported following infection with FAdV-8 isolated from gizzard lesions of slaughtered broiler chickens (Okuda et al., Citation2004). Characteristic pathomorphological changes of AGE were reproduced by Okuda et al. (Citation2001b) in 5 and 53 days old SPF layers via oral and ocular inoculation. Independent of the type of birds, age and inoculation route, the majority of experimental studies reported no or only mild clinical signs after inoculation with FAdV-1, while characteristic gross lesions were commonly observed in gizzards of infected birds for up to four weeks post infection. Okuda et al. (Citation2001a) documented a slightly delayed onset of pathological changes when using a lower infection dose of FAdV-1. In general, typical histological changes including degeneration and necrosis of the glandular epithelial cells, intranuclear inclusion bodies and lymphatic cell infiltration in the lamina propria and muscle layer were described simultaneous to the detection of gross lesions in the gizzards (Okuda et al., Citation2001b; Ono et al., Citation2003; Grafl et al., Citation2013). However, in these studies the detection of adenoviral intranuclear inclusion bodies in the gizzard mucosa of inoculated birds was only documented up to two weeks post infection. Additionally, birds inoculated parenterally with FAdV-1 showed pathohistological changes also in the pancreas and/or liver (Ono et al., Citation2004; Lim et al., Citation2012). A quite atypical clinical and pathological development of the disease was described by Domanska-Blicharz et al. (Citation2011); while in one-day-old SPF layers infected with FAdV-1 ocular-nasally, mortality of up to 100% and severe gizzard erosions were noticed, 21 days old infected birds did not develop any clinical or pathomorphological signs of AGE. Overall, experimental studies together with field reports indicate that birds hardly develop an age resistance which differentiates AGE from IBH and HHS.
Horizontal transmission of AGE was confirmed in experimental studies reported by Ono et al. (Citation2007) following successful reproduction of AGE not only in orally FAdV-1-infected SPF layers but also in in-contact birds. Furthermore, immunological mechanisms in the context of AGE have been investigated with various experimental models (Okuda et al., Citation2001b; Ono et al., Citation2004; Grafl et al., Citation2013; Grafl et al., Citation2014; for more information see section “Protection and vaccination strategies against FAdV infections”).
Finally, a dietary influence on the outcome of AGE has been excluded for vertically infected broilers, as all SPF broilers experimentally infected with virulent FAdV-1 prior to administration of varying feeding regimes, intended to support gizzard stimulation, developed characteristic gizzard lesions (Grafl et al., Citation2015).
Protection and vaccination strategies against FAdV infections
To this end, understanding of immune responses against adenoviruses derives mainly from investigations in mammals, particularly humans. Although considerable differences in the immune response mechanisms of mammalian and avian species are well acknowledged, similarities in some fundamental structural and biological features between adenovirus genera can help explain certain parallels in the FAdV-directed host response. Adenoviruses are generally known to elicit a strong humoral response, directed mainly against the most abundant capsid protein hexon, constituting the dominant, serotype-specific neutralization epitope (Toogood et al., Citation1992). Antibodies are also commonly generated in response to FAdVs (Calnek et al., Citation1982), although quantity and kinetics of response can vary according to the age of chickens and route of exposure (Maiti & Sarkar, Citation1997; Ojkić & Nagy, Citation2003). The latter may also explain the absence of a neutralizing antibody response, as observed in particular strains, possibly resulting from the failure to establish a systemic infection upon oral infection (Schonewille et al., Citation2010).
Notably, however, neutralizing antibodies seem to be dispensable for clinical protection against FAdVs, as challenge protection studies have independently shown (Schonewille et al., Citation2010; Schachner et al., Citation2014), strengthening the contribution of cell-mediated immunity for combating FAdV infections. The role of cellular immune mechanisms during FAdV infections is also supported by investigations of cytokine expression patterns, indicating a polarization towards the Th1-pathway upon infection with non-pathogenic FAdV-4 and -8b strains (Grgić et al., Citation2013a, b).
In particular with regard to HHS, a situation of high infection pressure has prioritized the development of commercial vaccines for control and prevention of FAdVs, accounting for the topicality of the research conducted in this area, as summarized in . In addition, in certain regions, the practice of immunizing chickens with inactivated vaccines prepared from liver suspensions of diseased flocks has been reported (Chishti et al., Citation1989; Afzal & Ahmad, Citation1990; Anjum, Citation1990; Afzal et al., Citation1991; Kumar et al., Citation1997). Such vaccines have been extensively investigated and are, under the prevailing conditions, perceived as an easy and cheap method to control HHS.
Table 1. Vaccination studies for the control of FAdV-induced disease complexes.
However, various concerns related to safety, efficacy and applicability of this practice limits its use. Incomplete inactivation can lead to a further spread of HHS and other pathogens. Besides this, the respective formulations are poorly characterized, containing undefined amounts of antigen. Varying formaldehyde concentrations and uncertainties as to how individual virus strains differ in their resistance to inactivation further aggravated this lack of standardization, which is also reflected by contradictory findings between studies comparing the efficacy of different formulations (Chishti et al., Citation1989; Hussain et al., Citation1996; Zia et al., Citation2001). Additionally, the need for parenteral application requires extensive handling procedures, and repeated administration might be needed to elicit sufficient protection (Hassan et al., Citation1994; Hussain et al., Citation1999).
Based on the demand for both safer and more efficacious vaccines, alternative systems have been developed. Virus propagation in embryonated eggs or cell culture prior to inactivation enabled a standardized production of antigen (Naeem et al., Citation1995b). Such vaccines are commercially available in some countries for prevention of HHS and IBH, retaining, however, the impediments of relatively cost-intensive production and individual administration. Adaptation of FAdVs to susceptible but atypical culturing systems was shown to attenuate their virulence (Schonewille et al., Citation2010; Mansoor et al., Citation2011), providing the basis for live virus vaccines. These would offer the advantage of application via the oral route, and have in some cases been shown to elicit a stronger antibody response than vaccines based on inactivated antigens (Kaur et al., Citation1997; Mansoor et al., Citation2011).
More recently, efforts towards improved immunoprophylactic strategies are seen in the development of subunit vaccines, generated from recombinant capsid components of the virus. Protection of domestic poultry by recombinant adenoviral proteins has already been demonstrated for certain non-FAdVs, such as egg drop syndrome virus (EDS-V) (Fingerut et al., Citation2003) and haemorrhagic enteritis virus (HEV) (Pitcovski et al., Citation2005), with the EDS-V fiber protein implemented as a commercial vaccine product. For FAdVs the concept of subunit vaccination has been examined using both structural and non-structural proteins of the virus. The structural antigens penton base (Shah et al., Citation2012) and fiber (Schachner et al., Citation2014), derived from FAdV-4, conferred high levels of protection in the face of severe challenge with HHS. The observed level of protection correlated with vaccination-induced antibody levels, although antibodies raised against the fiber did not exhibit any neutralization activity in vitro, while this information is so far unknown for penton base directed antisera. Neutralizing antibodies against recombinant fiber from an FAdV-8b isolate were detected in breeders and progenies following 2× vaccination of breeders (Gupta et al., Citation2017).
On the other hand, hexon-based subunit formulations, derived from FAdV-4 as well as FAdV-8b, were independently reported to elicit weak responses (Schachner et al., Citation2014; Dar et al., Citation2015), the same applying for recombinantly expressed non-structural 100K which conferred no protection against challenge with homologous FAdV-4 (Shah et al., Citation2016).
With regard to the susceptibility of young chickens to clinically manifest FAdV infections, vertical transmission of the virus and antibodies from parental to progeny birds gains high priority to control FAdVs. Both transmission of antigen and a virus-directed response depend on a critical timespan from infection to seroconversion of parental birds, with a more pronounced effect on either virus transmission, or transfer of maternal antibodies to progenies. Based on the presence of FAdVs in eggs of breeders with neutralizing antibodies, earlier works suggest a possible co-existence of viral antigen with neutralizing antibodies (Saifuddin & Wilks, Citation1991b), whereby egg-derived Abs have been incriminated in limiting both duration of virus transmission (Dawson et al., Citation1980) and pathological effects of vertically transmitted FAdVs, resulting in a latent infection (Grgić et al., Citation2006).
Field studies proposing that neutralizing Abs in breeder flocks account for the absence of vertically transmitted FAdVs (Philippe et al., Citation2007) are to some degree contradicted by experimental evidence of vertical transmission despite neutralizing antibodies in breeders (Toro et al., Citation2001; Mazaheri et al., Citation2003). This complies with the finding that neutralizing Abs, elicited by inactivated FAdV-4 vaccine of layer breeders, do not completely protect the respective progenies against intramuscular challenge with HHS (Toro et al., Citation2002), altogether disputing the stand-alone role of a neutralizing humoral response for protection against FAdVs in such settings. In contrast, another study by Kim et al. (Citation2014) reported significant reduction of mortality as well as tissue lesions in progenies derived from FAdV-4 immunized broiler breeders, conferring protection not only against the homologous serotype, but also against challenge with IBH strains of the heterologous serotypes FAdV-8b and -11 (belonging to FAdV-E and FAdV-D, respectively).
This finding is somewhat unanticipated given the phylogenetic and serological distance between immunogenic determinants of heterologous FAdV species, additionally contradicting the frequent observation that chickens are susceptible to co-infections with different FAdV serotypes under field conditions (Gomis et al., Citation2006; Ojkić et al., Citation2008; Kaján et al., Citation2013; Mittal et al., Citation2014b; Li et al., Citation2016b). On the other hand, it cannot be outruled that formaldehyde treatment alters structural components of the virion (Akhtar et al., Citation2000), which might explain the broader antigenicity of such a formulation.
In Australia, where IBH is controlled by a commercial live vaccine based on FAdV-8b, disease outbreaks related to FAdV-8b and FAdV-11 were reported (Steer et al., Citation2011). This not only demonstrates limited efficacy of this method since various breeder flocks had failed to generate antibodies in response to vaccination, it also underlines a poor cross-protectivity between the relevant serotypes which is also reported from Canada with autogenous vaccines (Venne, Citation2013). The heterogeneity of IBH-causing FAdV types, belonging to two different FAdV species, argues for the use of dual-serotype vaccines against this disease, such as implemented by an inactivated, autogenous product combining serotypes from FAdV-D and FAdV-E, which has been shown to elicit adequate progeny protection against various challenge strains (Alvarado et al., Citation2007). With updated FAdV nomenclature in place, it is particularly interesting to note that the respective vaccine apparently contains serotype FAdV-8a (El-Attrache & Villegas, Citation2001; Alvarado et al., Citation2007; Schachner et al., Citation2016), while at the same time providing adequate protection against challenge with the phylogenetically and serologically distinct serotype FAdV-8b (hitherto designated as FAdV-9). Efficacy of potential new approaches to killed IBH vaccines, using in ovo administered formulations containing inactivated serotype FAdV-8b together with next-generation adjuvants (Sarfraz et al., Citation2017), has been proposed on basis of immune response evaluation, without providing challenge protection studies.
In contrast to HHS and IBH, which share similarities in pathogenesis and protective mechanisms, marked differences are observed in the protection against AGE. Both SPF and commercial broilers with homologous maternal antibodies developed characteristic clinical signs as well as pathomorphological gizzard lesions post ocular and/or oral infection with a virulent FAdV-1, indicating that vertically transmitted humoral immunity does not play a role in protection against AGE (Okuda et al., Citation2001b; Grafl et al., Citation2013). However, a resistance to re-infection with the same pathogenic FAdV-1 has been described previously (Ono et al., Citation2004) and after live vaccination of day-old birds with the non-pathogenic FAdV-1 reference strain CELO, complete protection against AGE could be demonstrated (Grafl et al., Citation2014). This indicates that the development of a local immunity seems to be critical in the development of the disease. Independent of this, experimental suppression of the immune system due to IBDV infection or cyclophosphamide treatment prior to inoculation with FAdV-1 resulted in higher gizzard lesion scores compared to those in non-immunosuppressed control birds (Muroga et al., Citation2006).
Outlook and perspectives
On a global scale, an increase of FAdV-induced disease outbreaks has been recorded, particularly within the last 10 to 15 years. To date, at least one, but more often a combination of more than one FAdV-associated condition, has been reported from every continent with the limitation that documented outbreaks still remain underrepresented in face of the actual epidemiologic situation (). The lack of documentation may partly be explained by a certain reluctance to publish singular cases, particularly from regions with already existing reports available. On the other hand, the metabolic component of FAdV infections, often resulting in reduced performance without obvious signs and mortalities, may easily conceal outbreaks. In this context, it remains to be clarified how recently developed parameters such as clinical chemistry analytes can be applied to assess subclinical FAdV manifestations.
The increasing incidence of FAdV-related diseases is substantially linked to the contemporary trends in poultry production, husbandry and rearing, following rigorously elevated standards in biosecurity and environmental conditions. Importantly, this development has not only led to the desired prevention of pathogen introduction into breeder stocks, but has also deprived parental birds of the possibility to generate a natural immune response against FAdVs prior to production, failing to transfer maternal antibodies to the progeny. The interference of an FAdV infection with birds’ metabolism seems to be a key factor in the development of natural IBH and HHS outbreaks, as increasingly observed in very young birds. Furthermore, it can be hypothesized that the lack of immune competency at an early stage of life, at which age resistance mechanism against FAdV infection are not effective, contributes to the development of FAdV-induced diseases.
Future strategies to control IBH and HHS should aim to address the harmonization of protection strategies alongside with monitoring of the status of flocks, which are ideally realized through combined serological monitoring and vaccination. On both ends, such systems are to date still not fully commercially established and/or need to be adapted to the requirements in the field. For serological monitoring, detection methods for rapid and large-scale application, covering the relevant clinical types and their respective vaccine products, would be highly desirable. Given the variety of FAdV serotypes, often co-existing in the field and inducing varying degrees of serological cross-reactions, the use of existing assays such as enzyme-linked immunosorbent assays (ELISAs) based on whole virus particles is currently a compromise at the expense of specificity and sensitivity of the test system, whereas the adequately type-specific and sensitive serum neutralization test imposes severe limitations on large-scale sample processing. Addressing these criteria, recently developed ELISAs based on recombinant proteins hold promise for a next-generation test system which allows for differentiation of FAdVs along with applicability for mass screening. Additionally, this approach may pave the way towards a strategy for differentiating between infected and vaccinated animals, possibly in context with certain viral subunits that have been shown as protective immunogens.
Considering the significance of both vertical transmission and an early horizontal infection with FAdVs for induction of IBH and HHS, there is an increasing need for immunization strategies targeting maternal antibody production with transfer to progenies. The use of recombinant FAdV proteins, so far at an experimental stage, holds promise for the development of respective vaccination strategies, being both efficacious and safe without the risk of pathogen dissemination, while potential use of such vaccines in context of the discussed settings remains to be evaluated.
Paradoxically, with the extensive use of adenoviruses as vectors mainly in human medicine, and recognition of FAdVs as an alternative to mastadenovirus vectors, FAdVs are no longer confined to their role as pathogen, but have also emerged in the context of serving as backbone for the construction of analogous vaccines. However, it remains to be investigated how this approach fits into an attainable vaccination strategy, given a possible interference with maternally derived Abs in a scenario where young chickens are immunized, and, in the case of breeder vaccination, concerns about insufficient antibody induction, together with the previously reported inefficacy of live virus vaccination to protect progenies. While important principles of pathogenesis and protective mechanisms are shared between IBH and HHS, implicating that many of the above-discussed aspects for control apply to both diseases alike, AGE takes a quite unique position among the FAdV-induced conditions, which will require the development of somewhat different approaches for its control.
Supplemental data
Download Zip (235.3 KB)Acknowledgements
The authors wish to thank their cooperation partners in the field for providing sample material and contributing valuable information to the relevant cases.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Abdul-Aziz, T.A. & Al-Attar, M.A. (1991). New syndrome in Iraqi chicks. The Veterinary Record, 129, 272. doi: 10.1136/vr.129.12.272
- Abe, T., Nakamura, K., Tojo, H., Mase, M., Shibahara, T., Yamaguchi, S. & Yuasa, N. (1998). Histology, immunohistochemistry, and ultrastructure of hydropericardium syndrome in adult broiler breeders and broiler chicks. Avian Diseases, 42, 606–612. doi: 10.2307/1592690
- Abe, T., Nakamura, K., Tojo, T. & Yuasa, N. (2001). Gizzard erosion in broiler chicks by group I avian adenovirus. Avian Diseases, 45, 234–239. doi: 10.2307/1593034
- Absalón, A.E., Morales-Garzón, A., Vera-Hernández, P.F., Cortés-Espinosa, D. V., Uribe-Ochoa, S.M., García, L.J. & Lucio-Decanini, E. (2017). Complete genome sequence of a non-pathogenic strain of Fowl Adenovirus serotype 11: minimal genomic differences between pathogenic and non-pathogenic viruses. Virology, 501, 63–69. doi: 10.1016/j.virol.2016.11.006
- Afzal, M. & Ahmad, I. (1990). Efficacy of an inactivated vaccine against hydropericardium syndrome in broilers. The Veterinary Record, 126, 59–60.
- Afzal, M., Muneer, R. & Stein, G. (1991). Studies on the aetiology of hydropericardium syndrome (Angara disease) in broilers. The Veterinary Record, 128, 591–593. doi: 10.1136/vr.128.25.591
- Ahmad, K. (1999). In vivo pathogenicity of hydropericardium-hepatitis syndrome (Angara disease) and efficacy of vaccines. Pakistan Veterinary Journal, 19, 200–203.
- Ahmad, M.D., Zaman, S., Mushtaq, M.H., Anjum, A.A. & Akram, M. (2011). Comparative pathogenicity of liver homogenate and cell culture propagated hydropericardium syndrome virus in broiler birds. Pakistan Veterinary Journal, 31, 321–326.
- Akhtar, M., Ahmed, R., Hayat, C.S., Hussain, I. & Ashfaque, M. (2000). Comparative immune response of formalin inactivated and binary ethyleneimine inactivated Angara disease vaccines. Pakistan Journal of Biological Sciences, 3, 1313–1314. doi: 10.3923/pjbs.2000.1313.1314
- Alvarado, I.R., Villegas, P., El-Attrache, J., Jensen, E., Rosales, G., Perozo, F. & Purvis, L.B. (2007). Genetic characterization, pathogenicity, and protection studies with an avian adenovirus isolate associated with inclusion body hepatitis. Avian Diseases, 51, 27–32. doi: 10.1637/0005-2086(2007)051[0027:GCPAPS]2.0.CO;2
- Anjum, A.D. (1990). Experimental transmission of hydropericardium syndrome and protection against it in commercial broiler chickens. Avian Pathology, 19, 655–660. doi: 10.1080/03079459008418721
- Anjum, A.D., Sabri, M.A. & Iqbal, Z. (1989). Hydropericarditis syndrome in broiler chickens in Pakistan. The Veterinary Record, 124, 247–248. doi: 10.1136/vr.124.10.247
- Antillón, A. & Lucio, B. (1975). Case report: inclusion body hepatitis in Mexico. Avian Diseases, 19, 195–197. doi: 10.2307/1588969
- Asrani, R.K., Gupta, B.K., Sharma, S.K., Singh, S.P. & Katoch, R.C. (1997). Hydropericardium-hepatopathy syndrome in Asian poultry. The Veterinary Record, 141, 271–273. doi: 10.1136/vr.141.11.271
- Borisov, V.V., Borisov, A.V. & Gusev, A.A. (1997). Hydropericardium syndrome in chickens in Russia. Proceedings of the Tenth International Congress of Veterinary Poultry Association (p. 258), Budapest, Hungary.
- Calnek, B.W., Shek, W.R., Menendez, N.A. & Stiube, P. (1982). Serological cross-reactivity of avian adenovirus serotypes in an enzyme-linked immunosorbent assay. Avian Diseases, 26, 897–906. doi: 10.2307/1589878
- Changjing, L., Haiying, L., Dongdong, W., Jingjing, W., Youming, W., Shouchun, W., Jida, L., Ping, L., Jianlin, W., Shouzhen, X., Shangjin, C., Yi, Z. & Yanbo, Y. (2016). Characterization of fowl adenoviruses isolated between 2007 and 2014 in China. Veterinary Microbiology, 197, 62–67. doi: 10.1016/j.vetmic.2016.11.005
- Cheema, A.H., Ahmad, J. & Afzal, M. (1989). An adenovirus infection of poultry in Pakistan. Revue Scientifique et Technique de ĺOffice International des Epizooties, 8, 789–795. doi: 10.20506/rst.8.3.420
- Chen, H., Dou, Y., Zheng, X., Tang, Y., Zhang, M., Zhang, Y., Wang, Z. & Diao, Y. (2015). Hydropericardium hepatitis syndrome emerged in Cherry Valley ducks in China. Transboundary and Emerging Diseases, 64, 1262–1267. doi: 10.1111/tbed.12500
- Chishti, M.A., Afzal, M. & Cheema, A.H. (1989). Preliminary studies on the development of vaccine against the “hydropericardium syndrome” of poultry. Revue Scientifique et Technique de ĺOffice International des Epizooties, 8, 797–801. doi: 10.20506/rst.8.3.432
- Choi, K.S, Kye, S.J., Kim, J.Y., Jeon, W.J., Lee, E.K., Park, K.Y. & Sung, H.W. (2012). Epidemiological investigation of outbreaks of fowl adenovirus infection in commercial chickens in Korea. Poultry Science, 91, 2502–2506. doi: 10.3382/ps.2012-02296
- Christensen, N.H. & Saifuddin, M. (1989). A primary epidemic of inclusion body hepatitis in broilers. Avian Diseases, 33, 622–630. doi: 10.2307/1591135
- Clemmer, D.I. (1972). Age-associated changes in fecal excretion patterns of strain 93 chick embryo lethal orphan virus in chicks. Infection and Immunity, 5, 60–64.
- Cook, J.K. (1974). Pathogenicity of Avian Adenoviruses for day-old chicks. Journal of Comparative Pathology, 84, 505–515. doi: 10.1016/0021-9975(74)90043-7
- Cook, J.K. (1983). Fowl adenoviruses: studies on aspects of the pathogenicity of six strains for 1-day-old chicks. Avian Pathology, 12, 35–43. doi: 10.1080/03079458308436147
- Dar, A., Tipu, M., Townsend, H., Potter, A., Gerdts, V. & Tikoo, S. (2015). Administration of poly[di(sodium carboxylatoethylphenoxy)phosphazene] (PCEP) and avian beta defensin as adjuvants in inactivated inclusion body hepatitis virus and its hexon protein-based experimental vaccine formulations in chickens. Avian Diseases, 59, 518–524. doi: 10.1637/11202-052815-Reg.1
- Dawson, G.J., Chang, P.W., Yates, V.J. & Fry, D.E. (1980). Neutralizing antibodies to CELO and avian adenovirus-associated viruses in the albumen of chicken eggs. Avian Diseases, 24, 890–895. doi: 10.2307/1589964
- Domanska-Blicharz, K., Tomczyk, G., Smietanka, K., Kozaczynski, W. & Minta, Z. (2011). Molecular characterization of fowl adenoviruses isolated from chickens with gizzard erosions. Poultry Science, 90, 983–989. doi: 10.3382/ps.2010-01214
- El-Attrache, J. & Villegas, P. (2001). Genomic identification and characterization of avian adenoviruses associated with inclusion body hepatitis. Avian Diseases, 45, 780–787. doi: 10.2307/1592857
- Eregae, M.E., Dewey, C.E., McEwen, S.A., Ouckama, R., Ojkić, D. & Guerin, M.T. (2014). Flock prevalence of exposure to avian adeno-associated virus, chicken anemia virus, fowl adenovirus, and infectious bursal disease virus among Ontario broiler chicken flocks. Avian Diseases, 58, 71–77. doi: 10.1637/10612-071113-Reg.1
- Erny, K.M., Barr, D.A. & Fahey, K.J. (1991). Molecular characterization of highly virulent fowl adenoviruses associated with outbreaks of inclusion body hepatitis. Avian Pathology, 20, 597–606. doi: 10.1080/03079459108418799
- Fadly, A.M. & Winterfield, R.W. (1973). Isolation and some characteristics of an agent associated with inclusion body hepatitis, hemorrhages, and aplastic anemia in chickens. Avian Diseases, 17, 182–193. doi: 10.2307/1588936
- Fadly, A.M., Winterfield, R.W. & Olander, H.J. (1976). Role of the bursa of Fabricius in the pathogenicity of inclusion body hepatitis and infectious bursal disease viruses. Avian Diseases, 20, 467–477. doi: 10.2307/1589379
- Fingerut, E., Gutter, B., Gallili, G., Michael, A. & Pitcovski, J. (2003). A subunit vaccine against the adenovirus egg-drop syndrome using part of its fiber protein. Vaccine, 21, 2761–2766. doi: 10.1016/S0264-410X(03)00117-8
- Gallina, A.M., Winterfield, R.W. & Fadly, A.M. (1973). Adenovirus infection and disease. II. Histopathology of natural and experimental disease. Avian Diseases, 17, 343–353. doi: 10.2307/1589218
- Gjevre, A.-G., Kaldhusdal, M. & Eriksen, G.S. (2013). Gizzard erosion and ulceration syndrome in chickens and turkeys: a review of causal or predisposing factors. Avian Pathology, 42, 297–303. doi: 10.1080/03079457.2013.817665
- Gomis, S., Goodhope, R., Ojkic, D. & Willson, P. (2006). Inclusion body hepatitis as a primary disease in broilers in Saskatchewan, Canada. Avian Diseases, 50, 550–555. doi: 10.1637/7577-040106R.1
- Goodwin, M.A., Hill, D.L., Dekich, M.A. & Putnam, M.R. (1993). Multisystemic adenovirus infection in broiler chicks with hypoglycemia and spiking mortality. Avian Diseases, 37, 625–627. doi: 10.2307/1591701
- Goodwin, M.A., Latimer, K.S., Resurreccion, R.S., Miller, P.G. & Campagnoli, R.P. (1996). DNA in situ hybridization for the rapid diagnosis of massive necrotizing avian adenovirus hepatitis and pancreatitis in chicks. Avian Diseases, 40, 828–831. doi: 10.2307/1592305
- Gowda, R.N.S. & Satyanarayana, M.L. (1994). Hydropericardium syndrome in poultry. Indian Journal of Veterinary Pathology, 18, 159–161.
- Grafl, B., Aigner, F., Liebhart, D., Marek, A., Prokofieva, I., Bachmeier, J. & Hess, M. (2012). Vertical transmission and clinical signs in broiler breeders and broilers experiencing adenoviral gizzard erosion. Avian Pathology, 41, 599–604. doi: 10.1080/03079457.2012.740614
- Grafl, B., Liebhart, D., Günes, A., Wernsdorf, P., Aigner, F., Bachmeier, J. & Hess, M. (2013). Quantity of virulent fowl adenovirus serotype 1 correlates with clinical signs, macroscopical and pathohistological lesions in gizzards following experimental induction of gizzard erosion in broilers. Veterinary Research, 44, 38. doi: 10.1186/1297-9716-44-38
- Grafl, B., Prokofieva, I., Wernsdorf, P., Dublecz, K. & Hess, M. (2015). Clinical signs and progression of lesions in the gizzard are not influenced by inclusion of ground oats or whole wheat in the diet following experimental infection with pathogenic fowl adenovirus serotype 1. Avian Pathology, 44, 230–236. doi: 10.1080/03079457.2015.1028886
- Grafl, B., Prokofieva, I., Wernsdorf, P., Steinborn, R. & Hess, M. (2014). Infection with an apathogenic fowl adenovirus serotype-1 strain (CELO) prevents adenoviral gizzard erosion in broilers. Veterinary Microbiology, 172, 177–185. doi: 10.1016/j.vetmic.2014.05.020
- Grgić, H., Philippe, C., Ojkić, D. & Nagy, É. (2006). Study of vertical transmission of fowl adenoviruses. The Canadian Journal of Veterinary Research, 70, 230–233.
- Grgić, H., Poljak, Z., Sharif, S. & Nagy, É. (2013a). Pathogenicity and cytokine gene expression pattern of a serotype 4 fowl adenovirus isolate. PloS ONE, 8, e77601. doi: 10.1371/journal.pone.0077601
- Grgić, H., Sharif, S., Haghighi, H.R. & Nagy, É. (2013b). Cytokine patterns associated with a serotype 8 fowl adenovirus infection. Viral Immunology, 26, 143–149. doi: 10.1089/vim.2012.0078
- Griffin, B.D. & Nagy, É. (2011). Coding potential and transcript analysis of fowl adenovirus 4: insight into upstream ORFs as common sequence features in adenoviral transcripts. Journal of General Virology, 92, 1260–1272. doi: 10.1099/vir.0.030064-0
- Grimes, T.M., Fletcher, O.J. & Munnell, J.F. (1978). Comparative study of experimental inclusion body hepatitis of chickens caused by two serotypes of avian adenovirus. Veterinary Pathology, 15, 249–263. doi: 10.1177/030098587801500211
- Grimes, T.M., King, D.J., Kleven, S.H. & Fletcher, O.J. (1977). Involvement of a type-8 avian adenovirus in the etiology of inclusion body hepatitis. Avian Diseases, 21, 26–38. doi: 10.2307/1589361
- Gupta, A., Ahmed, K.A., Ayalew, L.E., Popowich, S., Kurukulasuriya, S., Goonewardene, K., Gunawardana, T., Karunarathna, R., Ojkic, D., Tikoo, S.K., Willson, P. & Gomis, S. (2017). Immunogenicity and protective efficacy of virus-like particles and recombinant fiber proteins in broiler-breeder vaccination against fowl adenovirus (FAdV)-8b. Vaccine, 35, 2716–2722. doi: 10.1016/j.vaccine.2017.03.075
- Harrach, B., Benkö, M., Both, G.W., Brown, M., Davison, A.J., Echavarria, M., Hess, M., Jones, M.S., Kajon, A., Lehmkuhl, H.D., Mautner, V., Mittal, S.K. & Wadell, G. (2012). Family Adenoviridae. In A.M.Q. King, M.J. Adams, E.B. Carstens & E.J. Lefkowitz (Eds.), Virus taxonomy: Ninth Report of the International Committtee on Taxonomy of Viruses, 9th edn (pp. 125–141). New York, NY: Elsevier Academic Press.
- Hassan, N.U., Afzal, M., Hameed, A. & Khan, A.R. (1994). Immune response to inactivated hydropericardium syndrome vaccine in broilers. Pakistan Veterinary Journal, 14, 5–10.
- Helmboldt, C.F. & Frazier, M.N. (1963). Avian hepatic inclusion bodies of unknown significance. Avian Diseases, 7, 446–450. doi: 10.2307/1587881
- Hess, M. (2013). Avidenovirus infections. In D.E. Swayne, J.R. Glisson, L.R. McDougald, L.K. Nolan, D.L. Suarez & V. Nair (Eds.), Diseases of poultry, 13th edn (pp. 290–300). Ames: Wiley-Blackwell.
- Hess, M. (2017). Commensal or pathogen – a challenge to fulfil Koch’s postulates. British Poultry Science, 58, 1–12. doi: 10.1080/00071668.2016.1245849
- Hess, M., Raue, R. & Prusas, C. (1999). Epidemiological studies on fowl adenoviruses isolated from cases of infectious hydropericardium. Avian Pathology, 28, 433–439. doi: 10.1080/03079459994443
- Hess, M., Prusas, C., Vereecken, M. & De Herdt, P. (1998). Isolation of fowl adenoviruses serotype 4 from pigeons with hepatic necrosis. Berliner und Münchener Tierärztliche Wochenschrift, 111, 140–142.
- Hoffmann, R., Wessling, E., Dorn, P. & Dangschat, H. (1975). Lesions in chickens with spontaneous or experimental infectious hepato-myelopoietic disease (inclusion body hepatitis) in Germany. Avian Diseases, 19, 224–236. doi: 10.2307/1588976
- Howell, J., MacDonald, D.W. & Christian, R.G. (1970). Inclusion body hepatitis. Canadian Veterinary Journal, 11, 99–101.
- Hussain, I., Anjum, A.A. & Khan, I.U.H. (1996). An oil emulsion vaccine against hydropericardium syndrome in broiler chickens. Pakistan Veterinary Journal, 16, 125–127.
- Hussain, I., Munir, R., Akhtar, M. & Ahmad, R. (1999). Evaluation and comparison of hydropericardium syndrome vaccines in broiler chicks. Pakistan Veterinary Journal, 19, 88–90.
- Itakura, C., Matsushita, S. & Goto, M. (1977). Fine structure of inclusion bodies in hepatic cells of chickens naturally affected with inclusion body hepatitis. Avian Pathology, 6, 19–32. doi: 10.1080/03079457708418209
- Itakura, C., Yasuba, M. & Goto, M. (1974). Histopathological studies on inclusion body hepatitis in broiler chickens. Japanese Journal of Veterinary Science, 36, 329–340. doi: 10.1292/jvms1939.36.329
- Jaffery, M.S. (1988). A treatise on Angara disease (hydropericardium-pulmonary oedema-hepatonephritis syndrome). Journal of the Pakistan Veterinary Medical Association, 34, 1–33.
- Jantosovic, J., Konard, J., Saly, J., Skardova, I., Kusev, J. & Beninghausova, K. (1991). Hydropericardium syndrome in chicks. Veterinastvi, 41, 261–263.
- Jones, R.C. & Georgiou, K. (1984). Experimental infection of chickens with adenoviruses isolated from tenosynovitis. Avian Pathology, 13, 13–23. doi: 10.1080/03079458408418504
- Junnu, S., Lertwatcharasarakul, P., Jala, S., Phattanakulanan, S., Monkong, A., Kulprasertsri, S., Thivalai, C., Chakritbudsabong, W., Chaichoun, K. & Songserm, T. (2015). An inactivated vaccine for prevention and control of inclusion body hepatitis in broiler breeders. The Thai Journal of Veterinary Medicine, 45, 55–62.
- Kaján, G.L., Kecskeméti, S., Harrach, B. & Benkő, M. (2013). Molecular typing of fowl adenoviruses, isolated in Hungary recently, reveals high diversity. Veterinary Microbiology, 167, 357–363. doi: 10.1016/j.vetmic.2013.09.025
- Kaur, A., Oberoi, M.S. & Singh, A. (1997). Neutralising antibody and challenge response to live and inactivated avian adenovirus-1 in broilers. Tropical Animal Health And Production, 29, 141–146. doi: 10.1007/BF02633009
- Karunamoorthy, G. & Manickam, R. (1998). Hydropericardium syndrome in quails. Poultry Times of India, 2, 1–31.
- Kim, J.N., Byun, S.H., Kim, M.J., Kim, J.j., Sung, H.W. & Mo, I.P. (2008). Outbreaks of hydropericardium syndrome and molecular characterization of Korean fowl adenoviral isolates. Avian Diseases, 52, 526–530. doi: 10.1637/8178-112207-Case
- Kim, M.-S., Lim, T.-H., Lee, D.-H., Youn, H.-N., Yuk, S.-S., Kim, B.-Y., Choi, S.-W., Jung, C.-H., Han, J.-H. & Song, C.-S. (2014). An inactivated oil-emulsion fowl Adenovirus serotype 4 vaccine provides broad cross-protection against various serotypes of fowl Adenovirus. Vaccine, 32, 3564–3568. doi: 10.1016/j.vaccine.2014.03.015
- Kumar, R., Chandra, R., Shukla, S.K., Agrawal, D.K. & Kumar, M. (1997). Hydropericardium syndrome (HPS) in India: a preliminary study on the causative agent and control of the disease by inactivated autogenous vaccine. Tropical Animal Health and Production, 29, 158–164. doi: 10.1007/BF02633014
- Kumar, R., Kumar, V., Asthana, M., Shukla, S.K. & Chandra, R. (2010). Isolation and identification of a fowl adenovirus from wild black kites (Milvus migrans). Journal of Wildlife Diseases, 46, 272–276. doi: 10.7589/0090-3558-46.1.272
- Li, L., Luo, L., Luo, Q., Zhang, T., Zhao, K., Wang, H., Zhang, R., Lu, Q., Pan, Z., Shao, H., Zhang, W. & Wen, G. (2016a). Genome sequence of a fowl adenovirus serotype 4 strain lethal to chickens, isolated from China. Genome Announcements, 4(2), pii: e00140-16. doi: 10.1128/genomeA.00140-16
- Li, H., Wang, J., Qiu, L., Han, Z. & Liu, S. (2016b). Fowl adenovirus species C serotype 4 is attributed to the emergence of hepatitis-hydropericardium syndrome in chickens in China. Infection, Genetics and Evolution, 45, 230–241. doi: 10.1016/j.meegid.2016.09.006
- Lim, T.H., Kim, B.Y., Kim, M.S., Jang, J.H., Lee, D.H., Kwon, Y.K., Lee, J.B., Park, S.Y., Choi, I.S. & Song, C.S. (2012). Outbreak of gizzard erosion associated with fowl adenovirus infection in Korea. Poultry Science, 91, 1113–1117. doi: 10.3382/ps.2011-02050
- Lim, T.-H., Lee, H.-J., Lee, D.-H., Lee, Y.-N., Park, J.-K., Youn, H.-N., Kim, M.-S., Youn, H.-S., Lee, J.-B., Park, S.-Y., Choiand, I.-S. & Song, C.-S. (2011). Identification and virulence characterization of fowl adenoviruses in Korea. Avian Diseases, 55, 554–560. doi: 10.1637/9730-032011-Reg.1
- Liu, Y., Wan, W., Gao, D., Li, Y., Yang, X., Liu, H., Yao, H., Chen, L., Wang, C. & Zhao, J. (2016). Genetic characterization of novel fowl aviadenovirus 4 isolates from outbreaks of hepatitis-hydropericardium syndrome in broiler chickens in China. Emerging Microbes and Infections, 5, e117. doi: 10.1038/emi.2016.115
- Macpherson, I., McDougall, J.S. & Laursen-Jones, A.P. (1974). Inclusion body hepatitis in a broiler integration. The Veterinary Record, 95, 286–289. doi: 10.1136/vr.95.13.286
- Maiti, N.K. & Sarkar, P. (1997). Humoral immune response of chicks to different clinical isolates of avian adenovirus type-1. Comparative Immunology, Microbiology and Infectious Diseases, 20, 59–62. doi: 10.1016/S0147-9571(96)00027-6
- Mansoor, M.K., Hussain, I., Arshad, M. & Muhammad, G. (2011). Preparation and evaluation of chicken embryo-adapted fowl adenovirus serotype 4 vaccine in broiler chickens. Tropical Animal Health and Production, 43, 331–338. doi: 10.1007/s11250-010-9694-z
- Marek, A., Günes, A., Schulz, E. & Hess, M. (2010a). Classification of fowl adenoviruses by use of phylogenetic analysis and high-resolution melting-curve analysis of the hexon L1 gene region. Journal of Virological Methods, 170, 147–154. doi: 10.1016/j.jviromet.2010.09.019
- Marek, A., Schulz, E., Hess, C. & Hess, M. (2010b). Comparison of the fibers of fowl adenovirus a serotype 1 isolates from chickens with gizzard erosions in Europe and apathogenic reference strains. Journal of Veterinary Diagnostic Investigation, 22, 937–941. doi: 10.1177/104063871002200613
- Mase, M. & Nakamura, K. (2014). Phylogenetic analysis of fowl adenoviruses isolated from chickens with gizzard erosion in Japan. Journal of Veterinary Medical Science, 76, 1535–1538. doi: 10.1292/jvms.14-0312
- Mase, M., Nakamura, K. & Imada, T. (2010). Characterization of Fowl adenovirus serotype 4 isolated from chickens with hydropericardium syndrome based on analysis of the short fiber protein gene. Journal of Veterinary Diagnostic Investigation, 22, 218–223. doi: 10.1177/104063871002200207
- Matczuk, A.K., Niczyporuk, J.S., Kuczkowski, M., Woźniakowski, G., Nowak, M. & Wieliczko, A. (2017). Whole genome sequencing of Fowl aviadenovirus A – a causative agent of gizzard erosion and ulceration, in adult laying hens. Infection, Genetics and Evolution, 48, 47–53. doi: 10.1016/j.meegid.2016.12.008
- Matos, M., Grafl, B., Liebhart, D. & Hess, M. (2016a). The outcome of experimentally induced inclusion body hepatitis (IBH) by fowl aviadenoviruses (FAdVs) is crucially influenced by the genetic background of the host. Veterinary Research, 47, 69. doi: 10.1186/s13567-016-0350-0
- Matos, M., Grafl, B., Liebhart, D., Schwendenwein, I. & Hess, M. (2016b). Selected clinical chemistry analytes correlate with the pathogenesis of inclusion body hepatitis experimentally induced by fowl aviadenoviruses. Avian Pathology, 45, 520–529. doi: 10.1080/03079457.2016.1168513
- Mazaheri, A., Prusas, C., Voß, M. & Hess, M. (1998). Some strains of serotype 4 fowl adenoviruses cause inclusion body hepatitis and hydropericardium syndrome in chickens. Avian Pathology, 27, 269–276. doi: 10.1080/03079459808419335
- Mazaheri, A., Prusas, C., Voß, M. & Hess, M. (2003). Vertical transmission of fowl Adenovirus serotype 4 investigated in specified pathogen-free birds after experimental infection. Archiv für Geflügelkunde, 67, 6–10.
- Mittal, D., Jindal, N. & Khokhar, R.S. (2014a). Epidemiological studies on inclusion body hepatitis-hydropericardium syndrome in broiler chickens in Haryana state. The Haryana Veterinarian, 53, 34–38.
- Mittal, D., Jindal, N., Tiwari, A.K. & Khokhar, R.S. (2014b). Characterization of fowl adenoviruses associated with hydropericardium syndrome and inclusion body hepatitis in broiler chickens. Virus Diseases, 25, 114–119. doi: 10.1007/s13337-013-0183-7
- Muroga, N., Taharaguchi, S., Ohta, H., Yamazaki, K. & Takase, K. (2006). Pathogenicity of fowl adenovirus isolated from gizzard erosions to immuno-suppressed chickens. Journal of Veterinary Medical Science, 68, 289–291. doi: 10.1292/jvms.68.289
- Naeem, K. & Akram, H.S. (1995). Hydropericardium syndrome outbreak in a pigeon flock. The Veterinary Record, 136, 296–297. doi: 10.1136/vr.136.12.296
- Naeem, K., Niazi, T., Malik, S.A. & Cheema, A.H. (1995a). Immunosuppressive potential and pathogenicity of an avian adenovirus isolate involved in hydropericardium syndrome in broilers. Avian Diseases, 39, 723–728. doi: 10.2307/1592408
- Naeem, K., Rabbani, M., Hussain, M. & Cheema, A.H. (1995b). Development of cell culture vaccine against hydropericardium syndrome in poultry. Pakistan Veterinary Journal, 15, 150–151.
- Naeem, K., Rahim, A. & Majeed, I.U. (2001). Post infection dissemination pattern of avian-adenovirus involved in hydropericardium syndrome. Pakistan Veterinary Journal, 21, 152–156.
- Nakamura, K., Mase, M., Yamaguchi, S. & Yuasa, N. (2000). Induction of hydropericardium in one-day-old specific-pathogen-free chicks by adenoviruses from inclusion body hepatitis. Avian Diseases, 44, 192–196. doi: 10.2307/1592524
- Nakamura, K., Mase, M., Yamamoto, Y., Takizawa, K., Kabeya, M., Wakuda, T., Matsuda, M., Chikuba, T., Yamamoto, Y., Ohyama, T., Takahashi, K., Sato, N., Akiyama, N., Honma, H. & Imai, K. (2011). Inclusion body hepatitis caused by fowl adenovirus in broiler chickens in Japan, 2009–2010. Avian Diseases, 55, 719–723. doi: 10.1637/9813-052511-Case.1
- Nakamura, K., Tanaka, H., Mase, M., Imada, T. & Yamada, M. (2002). Pancreatic necrosis and ventricular erosion in adenovirus-associated hydropericardium syndrome of broilers. Veterinary Pathology, 39, 403–406. doi: 10.1354/vp.39-3-403
- Niczyporuk, J.S. (2016). Phylogenetic and geographic analysis of fowl adenovirus field strains isolated from poultry in Poland. Archives of Virology, 161, 33–42. doi: 10.1007/s00705-015-2635-4
- Niu, Y.-J., Sun, W., Zhang, G.-H., Qu, Y.-J., Wang, P.-F., Sun, H.-L., Xiao, Y.-H. & Liu, S.-D. (2016). Hydropericardium syndrome outbreak caused by fowl adenovirus serotype 4 in China in 2015. Journal of General Virology, 97, 2684–2690. doi: 10.1099/jgv.0.000567
- Ojkić, D., Martin, E., Swinton, J., Vaillancourt, J-P., Boulianne, M. & Gomis, S. (2008). Genotyping of Canadian isolates of fowl adenoviruses. Avian Pathology, 37, 95–100. doi: 10.1080/03079450701805324
- Ojkić, D. & Nagy, É. (2003). Antibody response and virus tissue distribution in chickens inoculated with wild-type and recombinant fowl adenoviruses. Vaccine, 22, 42–48. doi: 10.1016/S0264-410X(03)00544-9
- Okuda, Y., Ono, M., Shibata, I. & Sato, S. (2004). Pathogenicity of serotype 8 fowl adenovirus isolated from gizzard erosions of slaughtered broiler chickens. Journal of Veterinary Medical Science, 66, 1561–1566. doi: 10.1292/jvms.66.1561
- Okuda, Y., Ono, M., Shibata, I., Sato, S. & Akashi, H. (2006). Comparison of the polymerase chain reaction-restriction fragment length polymorphism pattern of the fiber gene and pathogenicity of serotype-1 fowl adenovirus isolates from gizzard erosions and from feces of clinically healthy chickens in Japan. Journal of Veterinary Diagnostic Investigation, 18, 162–167. doi: 10.1177/104063870601800204
- Okuda, Y., Ono, M., Yazawa, S., Imai, Y., Shibata, I. & Sato, S. (2001a). Pathogenicity of serotype 1 fowl adenovirus in commercial broiler chickens. Avian Diseases, 45, 819–827. doi: 10.2307/1592862
- Okuda, Y., Ono, M., Yazawa, S., Shibata, I. & Sato, S. (2001b). Experimental infection of specific-pathogen-free chickens with serotype-1 fowl adenovirus isolated from a broiler chicken with gizzard erosions. Avian Diseases, 45, 19–25. doi: 10.2307/1593007
- Oliver-Ferrando, S., Dolz, R., Calderón, C., Valle, R., Rivas, R., Pérez, M., Biarnés, M., Blanco, A., Bertran, K., Ramis, A., Busquets, N. & Majó, N. (2017). Epidemiological and pathological investigation of fowl aviadenovirus serotypes 8b and 11 isolated from chickens with inclusion body hepatitis in Spain (2011–2013). Avian Pathology, 46, 157–165. doi: 10.1080/03079457.2016.1232477
- Olson, N.O. (1950). A respiratory disease (bronchitis) of quail caused by a virus. Proceedings of the 54th Annual Meeting of the United States Livestock Sanitary Association (pp. 171–174). Phoenix, AZ.
- Ono, M., Okuda, Y., Shibata, I., Sato, S. & Okada, K. (2007). Reproduction of adenoviral gizzard erosion by the horizontal transmission of fowl adenovirus serotype 1. Journal of Veterinary Medical Science, 69, 1005–1008. doi: 10.1292/jvms.69.1005
- Ono, M., Okuda, Y., Shibata, I., Sato, S. & Okada, K. (2004). Pathogenicity by parenteral injection of fowl adenovirus isolated from gizzard erosion and resistance to reinfection in adenoviral gizzard erosion in chickens. Veterinary Pathology, 41, 483–489. doi: 10.1354/vp.41-5-483
- Ono, M., Okuda, Y., Yazawa, S., Imai, Y., Shibata, I., Sato, S. & Okada, K. (2003). Adenoviral gizzard erosion in commercial broiler chickens. Veterinary Pathology, 40, 294–303. doi: 10.1354/vp.40-3-294
- Ono, M., Okuda, Y., Yazawa, S., Shibata, I., Tanimura, N., Kimura, K., Haritani, M., Mase, M. & Sato, S. (2001). Epizootic outbreaks of gizzard erosion associated with adenovirus infection in chickens. Avian Diseases, 45, 268–275. doi: 10.2307/1593040
- Pan, Q., Liu, L., Gao, Y., Liu, C., Qi, X., Zhang, Y., Wang, Y., Li, K., Gao, L., Wang, X. & Cui, H. (2017). Characterization of a hypervirulent fowl adenovirus 4 with the novel genotype newly prevalent in China and establishment of reproduction infection model of hydropericardium syndrome in chickens. Poultry Science, 96, 1581–1588. doi: 10.3382/ps/pew431
- Pettit, J.R. & Carlson, H.C. (1972). Inclusion-body hepatitis in broiler chickens. Avian Diseases, 16, 858–863. doi: 10.2307/1588767
- Philippe, C., Grgić, H., Ojkić, D. & Nagy, É. (2007). Serologic monitoring of a broiler breeder flock previously affected by inclusion body hepatitis and testing of the progeny for vertical transmission of fowl adenoviruses. The Canadian Journal of Veterinary Research, 71, 98–102.
- Pitcovski, J., Fingerut, E., Gallili, G., Eliahu, D., Finger, A. & Gutter B. (2005). A subunit vaccine against hemorrhagic enteritis adenovirus. Vaccine, 23, 4697–4702. doi: 10.1016/j.vaccine.2005.03.049
- Qureshi, A.A. (1989). Hydropericardium and ascitis. Poultry International, 28, 44–48.
- Rani, V., Kumar, R., Upadhyay, A.K. & Kumar, M. (2012). Therapeutic efficacy of chicken egg yolk immunoglobulins against hydropericardium syndrome in broiler chickens. Indian Journal of Animal Sciences, 82, 552–556.
- Reece, R.L., Grix, D.C. & Barr, D.A. (1986). An unusual case of inclusion body hepatitis in a cockerel. Avian Diseases, 30, 224–227. doi: 10.2307/1590640
- Rosenberger, J.K., Eckroade, R.J., Klopp, S. & Krauss, W.C. (1974). Characterization of several viruses isolated from chickens with inclusion body hepatitis and aplastic anemia. Avian Diseases, 18, 399–409. doi: 10.2307/1589107
- Rosenberger, J.K., Klopp, S., Eckroade, R.J. & Krauss, W.C. (1975). The role of the infectious bursal agent and several avian adenoviruses in the hemorrhagic-aplastic-anemia syndrome and gangrenous dermatitis. Avian Diseases, 19, 717–729. doi: 10.2307/1589185
- Rodriguez, J., Koga, Y., Alvarado, A. & Tinoco, R. (2014). Molecular characterization of Peruvian fowl adenovirus (FAdV) isolates. Advances in Microbiology, 4, 595–603. doi: 10.4236/aim.2014.410065
- Saifuddin, M. & Wilks, C.R. (1990). Reproduction of inclusion body hepatitis in conventionally raised chickens inoculated with a New Zealand isolate of avian adenovirus. New Zealand Veterinary Journal, 38, 62–65. doi: 10.1080/00480169.1990.35618
- Saifuddin, M. & Wilks, C.R. (1991a). Pathogenesis of an acute viral hepatitis: inclusion body hepatitis in the chicken. Archives of Virology, 116, 33–43. doi: 10.1007/BF01319229
- Saifuddin, M. & Wilks, C.R. (1991b). Vertical transmission of avian adenovirus associated with inclusion body hepatitis. New Zealand Veterinary Journal, 39, 50–52. doi: 10.1080/00480169.1991.35659
- Sarfraz, M., Suleman, M., Tikoo, S.K., Wheler, C., Potter, A.A., Gerdts, V. & Dar, A. (2017). Immune responses to in ovo vaccine formulations containing inactivated fowl adenovirus 8b with poly[di(sodium carboxylatoethylphenoxy)]phosphazene (PCEP) and avian beta defensin as adjuvants in chickens. Vaccine, 35, 981–986. doi: 10.1016/j.vaccine.2016.12.023
- Schachner, A., Marek, A., Grafl, B. & Hess, M. (2016). Detailed molecular analyses of the hexon loop-1 and fibers of fowl aviadenoviruses reveal new insights into the antigenic relationship and confirm that specific genotypes are involved in field outbreaks of inclusion body hepatitis. Veterinary Microbiology, 186, 13–20. doi: 10.1016/j.vetmic.2016.02.008
- Schachner, A., Marek, A., Jaskulska, B., Bilic, I. & Hess, M. (2014). Recombinant FAdV-4 fiber-2 protein protects chickens against hepatitis-hydropericardium syndrome (HHS). Vaccine, 32, 1086–1092. doi: 10.1016/j.vaccine.2013.12.056
- Schade, B., Schmitt, F., Böhm, B., Alex, M., Fux, R., Cattoli, G., Terregino, C., Monne, I., Currie, R.J.W. & Olias, P. (2013). Adenoviral gizzard erosion in broiler chickens in Germany. Avian Diseases, 57, 159–163. doi: 10.1637/10330-082312-Case.1
- Schonewille, E., Jaspers, R., Paul, G. & Hess, M. (2010). Specific-pathogen-free chickens vaccinated with a live FAdV-4 vaccine are fully protected against a severe challenge even in the absence of neutralizing antibodies. Avian Diseases, 54, 905–910. doi: 10.1637/8999-072309-Reg.1
- Schonewille, E., Singh, A., Göbel, T.W., Gerner, W., Saalmüller, A. & Hess, M. (2008). Fowl adenovirus (FAdV) serotype 4 causes depletion of B and T cells in lymphoid organs in specific pathogen-free chickens following experimental infection. Veterinary Immunology and Immunopathology, 121, 130–139. doi: 10.1016/j.vetimm.2007.09.017
- Shah, M.S., Ashraf, A., Khan, M.I., Rahman, M., Habib, M. & Qureshi, J.A. (2016). Molecular cloning, expression and characterization of 100 K gene of fowl adenovirus-4 for prevention and control of hydropericardium syndrome. Biologicals, 44, 19–23. doi: 10.1016/j.biologicals.2015.10.002
- Shah, M.S., Ashraf, A., Rahman, M., Khan, M.I. & Qureshi, J.A. (2012). A subunit vaccine against hydropericardium syndrome using adenovirus penton capsid protein. Vaccine, 30, 7153–7156. doi: 10.1016/j.vaccine.2012.10.013
- Shivachandra, S.B., Sah, R.L., Singh, S.D., Kataria, J.M. & Manimaran, K. (2003). Immunosuppression in broiler chicks fed aflatoxin and inoculated with fowl adenovirus serotype-4 (FAV-4) associated with hydropericardium syndrome. Veterinary Research Communications, 27, 39–51. doi: 10.1023/A:1022058623634
- Singh, A., Oberoi, M.S., Jand, S.K. & Singh, B. (1996). Epidemiology of inclusion body hepatitis in poultry in northern India from 1990 to 1994. Revue Scientifique et Technique de ĺOffice International des Epizooties, 15, 1053–1060. doi: 10.20506/rst.15.3.976
- Slaine, P.D., Ackford, J.G., Kropinski, A.M., Kozak, R.A., Krell, P.J. & Nagy, É. (2016). Molecular characterization of pathogenic and non-pathogenic fowl aviadenovirus serotype 11 isolates. Canadian Journal of Microbiology, 62, 993–1002. doi: 10.1139/cjm-2016-0297
- Steer, P.A., O’Rourke, D., Ghorashi, S.A. & Noormohammadi, A.H. (2011). Application of high-resolution melting curve analysis for typing of fowl adenoviruses in field cases of inclusion body hepatitis. Australian Veterinary Journal, 89, 184–192. doi: 10.1111/j.1751-0813.2011.00695.x
- Steer, P.A., Sandy, J.R., O’Rourke, D., Scott, P.C., Browning, G.F. & Noormohammadi, A.H. (2015). Chronological analysis of gross and histological lesions induced by field strains of fowl adenovirus serotypes 1, 8b and 11 in one-day-old chickens. Avian Pathology, 44, 106–113. doi: 10.1080/03079457.2015.1007919
- Strnad, P., Nuraldeen, R., Guldiken, N., Hartmann, D., Mahajan, V., Denk, H. & Haybaeck, J. (2013). Broad spectrum of hepatocyte inclusions in humans, animals, and experimental models. Comprehensive Physiology, 3, 1393–1436. doi: 10.1002/cphy.c120032
- Taharaguchi, A., Kono, Y., Ohta, H. & Takase, K. (2007). Putative host cell receptor for Fowl Adenovirus detected in gizzard. Journal of Veterinary Medical Science, 69, 1203–1205. doi: 10.1292/jvms.69.1203
- Tanimura, N., Nakamura, K., Imai, K., Maeda, M., Gobo, T., Nitta, S., Ishihara, T. & Amano, H. (1993). Necrotizing pancreatitis and gizzard erosion associated with adenovirus infection in chickens. Avian Diseases, 37, 606–611. doi: 10.2307/1591697
- Toogood, C.I., Crompton, J. & Hay, R.T. (1992). Antipeptide antisera define neutralizing epitopes on the adenovirus hexon. Journal of General Virology, 73, 1429–1435. doi: 10.1099/0022-1317-73-6-1429
- Toro, H., González, C., Cerda, L., Morales, M.A., Dooner, P. & Salamero, M. (2002). Prevention of inclusion body hepatitis/hydropericardium syndrome in progeny chickens by vaccination of breeders with fowl adenovirus and chicken anemia virus. Avian Diseases, 46, 547–554. doi: 10.1637/0005-2086(2002)046[0547:POIBHH]2.0.CO;2
- Toro, H., González, O., Escobar, C., Cerda, L., Morales, M.A. & Gonzalez, C. (2001). Vertical induction of the inclusion body hepatitis/hydropericardium syndrome with fowl adenovirus and chicken anemia virus. Avian Diseases, 45, 215–222. doi: 10.2307/1593031
- Toro, H., Prusas, C., Raue, R., Cerda, L., Geisse, C., González, C. & Hess, M. (1999). Characterization of fowl adenoviruses from outbreaks of inclusion body hepatitis/hydropericardium syndrome in Chile. Avian Diseases, 43, 262–270. doi: 10.2307/1592616
- Totsuka, M., Goda, M., Isogai, K., Yamamoto, Y., Sukigara, S. & Suzuki, T. (2015). Gizzard erosion of brown layer chicken with fowl adenovirus infection. Journal of the Japanese Society on Poultry Diseases, 51, 11–12.
- Vera-Hernández, P.F., Morales-Garzón, A., Cortés-Espinosa, D.V., Galiote-Flores, A., García-Barrera, L.J., Rodríguez-Galindo, E.T., Toscano-Contreras, A., Lucio-Decanini, E. & Absalón, A.E. (2016). Clinicopathological characterization and genomic sequence differences observed in a highly virulent fowl aviadenovirus serotype 4. Avian Pathology, 45, 73–81. doi: 10.1080/03079457.2015.1125443
- Venne, D. (2013). Field data of the changing clinical picture over time of inclusion body hepatitis in Canada with an emphasis on diagnosis, prevention and trials on supportive treatments. Proceedings of the 24th Annual Australian Poultry Science Symposium (pp. 222–229), Sydney, New South Wales.
- Venne, D. & Chorfi, Y. (2012). Blood biochemical changes in birds with inclusion body hepatitis and the effect of supportive treatments during outbreaks. In D. Frame (Ed.). Proceedings of the 61st Western Poultry Disease Conference (pp. 73–75). Madison, WI: Omnipress.
- Wilson, F.D., Wills, R.W., Senties-Cue, C.G., Maslin, W.R., Stayer, P.A. & Magee, D.L. (2010). High incidence of glomerulonephritis associated with inclusion body hepatitis in broiler chickens: routine histopathology and histomorphometric studies. Avian Diseases, 54, 975–980. doi: 10.1637/9050-090709-Reg.1
- Winterfield, R.W., Fadly, A.M. & Gallina, A.M. (1973). Adenovirus infection and disease. I. Some characteristics of an isolate from chickens in Indiana. Avian Diseases, 17, 334–342. doi: 10.2307/1589217
- Yates, V.J. & Fry, D.E. (1957). Observations on a chicken embryo lethal orphan (CELO) virus. The American Journal of Veterinary Research, 18, 657–660.
- Ye, J., Liang, G., Zhang, J., Wang, W., Song, N., Wang, P., Zheng, W., Xie, Q., Shao, H., Wan, Z., Wang, C., Chen, H., Gao, W. & Qin, A. (2016). Outbreaks of serotype 4 fowl adenovirus with novel genotype, China. Emerging Microbes and Infections, 5, e50. doi: 10.1038/emi.2016.50
- Young, J.A., Purcell, D.A. & Kavanagh, P.J. (1972). Inclusion body hepatitis outbreak in broiler flocks. The Veterinary Record, 90, 7.
- Zia, M.R., Rahman, S.U., Siddique, M. & Sandhu, M.A. (2001). Comparative efficiency of two oil emulsion-hydropericardium/hepatitis syndrome vaccines. International Journal of Agricultural Biology, 3, 436–438.
- Zhao, J., Zhong, Q., Zhao, Y., Hu, Y.-X. & Zhang, G.-Z. (2015). Pathogenicity and complete genome characterization of fowl adenoviruses isolated from chickens associated with inclusion body hepatitis and hydropericardium syndrome in China. PloS ONE, 10(7), e0133073. doi: 10.1371/journal.pone.0133073
