ABSTRACT
The detection of avian coronaviruses (AvCoV) in wild birds and the emergence of new AvCoV have increased in the past few years. In the present study, the pathogenicity of three AvCoV isolates was investigated in day-old chicks. One AvCoV isolated from a pigeon, which clustered with the Massachusetts vaccine serotype, and two AvCoV isolated from chickens, which grouped with a Brazilian genotype lineage, were used. Clinical signs, gross lesions, histopathological changes, ciliary activity, viral RNA detection, and serology were evaluated during 42 days post infection. All AvCoV isolates induced clinical signs, gross lesions in the trachea, moderate histopathological changes in the respiratory tract, and mild changes in other tissues. AvCoV isolated from the pigeon sample caused complete tracheal ciliostasis over a longer time span. Specific viral RNA was detected in all tissues, but the highest RNA loads were detected in the digestive tract (cloacal swabs and ileum). The highest antibody levels were also detected in the group infected with an isolate from the pigeon. These results confirm the pathogenicity of Brazilian variants, which can cause disease and induce gross lesions and histopathological changes in chickens. Our results suggest that non-Galliformes birds can also play a role in the ecology of AvCoV.
Research Highlights
The two strains isolated from chickens induced lesions mainly in the kidney.
The pigeon strain mainly caused respiratory signs and histopathological changes.
High RNA loads were detected in cloaca and ileum up to 42 dpi.
The highest antibody levels were detected in chicks infected with a pigeon strain.
Introduction
AvCoV, which include infectious bronchitis virus and other bird coronaviruses, belong to the genus Gammacoronavirus, subfamily Coronavirinae, family Coronaviridae (ICTV, Citation2014). AvCoV can induce diseases in Galliformes birds, such as chickens, turkeys, pheasants, peafowl, partridge, guinea fowl, and quails (Guy et al., Citation1997; Cavanagh et al., Citation2002; Jackwood & Wit, Citation2013; Torres et al., Citation2013; Ducatez et al., Citation2015). Most of the reported clinical signs are associated with respiratory illness and sometimes kidney disease, egg drop and loss in egg quality, and mortality in young birds, depending on the affected species.
The detection of AvCoV in wild birds has increased in the past few years in a wide range of bird species belonging to diverse orders such as Charadriiformes, Anseriformes, Columbiformes, Galliformes, Pelecaniformes, and Psittaciformes (Jonassen et al., Citation2005; Hughes et al., Citation2009; Tarnagda et al., Citation2011; Domanska-Blicharz et al., Citation2014; Kim & Oem, Citation2014). Strains genetically very close to vaccine strains (such as the Massachusetts (Mass) strain of infectious bronchitis virus) and more genetically distant strains have been detected in various wild and feral birds (Ferreira et al., Citation2007; Woo et al., Citation2009; Felippe et al., Citation2010). Nevertheless, only two studies have reported clinical signs in non-Galliformes birds. An AvCoV was isolated from racing pigeons that had ruffled feathers, dyspnoea, and mucus at the beak (Barr et al., Citation1988). Another study described an AvCoV isolated from a teal that could cause nephritis (Liu et al., Citation2005).
Wild birds are reservoirs of other viruses, such as avian influenza and avian avulavirus (Olsen et al., Citation2006; Kim et al., Citation2008). The prevalence of viruses in wild aquatic birds seems to vary according to bird sampling and location (Jordan et al., Citation2015; Wille et al., Citation2016). Therefore, the importance of wild birds that could act as a vector of AvCoV to the poultry industry (Cavanagh, Citation2005) remains unclear. Our group detected AvCoV from pigeon and chicken samples that were classified into the two most common phylogenetic groups circulating in Brazil: Massachusetts and Brazilian variant strains (Felippe et al., Citation2010). In the present study, the AvCoV were isolated from pigeon and chicken samples and inoculated into day-old chicks. The infection outcomes of each strain were evaluated and compared, covering the possible sites of virus strain replication. These results may offer a deeper understanding of the pathogenicity of the Brazilian variants and provide new insights into the role of pigeons in the eco-epidemiology of AvCoV.
Materials and methods
Chickens
One hundred and ninety-nine one-day-old, specific-pathogen-free (SPF) White Leghorn chicks (provided by the Biovet Laboratory, Vargem Grande Paulista, Brazil) were maintained in isolators at the research facility of Biovet Laboratory at a controlled temperature and were supplied with feed and water ad libitum. All experimental procedures were performed according to the Brazilian Ethics Community guidelines and approved by the State University of Campinas Animal Ethics Committee (CEUA Protocol n°005/13).
Viruses
Three avian coronavirus strains previously isolated by the Laboratory of Animal Virology at IB-UNICAMP were used (Felippe et al., Citation2010): (1) an avian coronavirus strain designated pigeon/Brazil/UNICAMP67 T/2007 (pigeon-67T strain) that was isolated from a pigeon (Columba livia) and classified as the Massachusetts group (GenBank accession number HM561882); (2) an avian coronavirus strain chicken/Brazil/UNICAMP801//2007 (chicken-801 strain) that was isolated from broiler breeders (Gallus gallus domesticus) located in southern Brazil (GenBank accession number HM561889); and (3) an avian coronavirus strain chicken/Brazil/UNICAMP810/2007 (chicken-810 strain) that was isolated from broilers (G. gallus domesticus) located in south-eastern Brazil (GenBank accession number HM561891). Both chicken strains were isolated from chickens with respiratory signs, and these strains were classified into the Brazilian variant group based on partial S1 gene sequencing. Viruses were grown in 9-day-old embryonated chicken eggs and titred according to the Reed and Muench method (Reed & Muench, Citation1938).
Experimental design
One hundred and sixty-five one-day-old chicks were split into three groups and infected with the AvCoV strains. Groups were infected with the AvCoV pigeon-67T strain, chicken-801 strain, or chicken-810 strain by intraocular and intranasal routes with 0.2 ml containing 105.5 embryo infectious dose (EID50) per chicken. These groups were designated pigeon-67T, chicken-801, and chicken-810, respectively. A fourth group, with 34 one-day-old chicks, was maintained as a control group. Chickens were monitored daily by visual observation for clinical signs (Elbers et al., Citation2005) and for survival during the 42 days of the experiment. Five birds from each infected group were randomly selected and euthanized at 2, 4, 5, 7, 9, 11, 14, 21, 28, 35, or 42 days post infection (dpi). Samples of the cloaca, blood, and organs (sinus, trachea, lung, caecal tonsil, kidney, and testis) were collected on these days. However, at 2 dpi, the caecal tonsils were not collected, and at 2 and 4 dpi, blood was not collected. In the control group, two birds per day were selected and euthanized. All selected organs were used for viral RNA quantification by real-time RT-PCR specific to AvCoV, and the organs collected at 2, 5, and 7 dpi were tested for histopathological changes. Trachea samples collected at 4, 7, 9, 11, 14, and 21 dpi were also used for measuring and evaluating ciliary activity in the trachea. Blood was collected for measurement of the humoral immune response by the indirect ELISA test.
Tracheal ciliostasis
The entire trachea was removed immediately after the birds were euthanized at 4, 7, 9, 11, 14, and 21 dpi, and three thin tracheal rings (one from the top, one from the bottom, and one from the middle of the trachea) were selected from each bird and then cut and placed in Minimal Essential Medium (MEM with Hank’s salt) in a 24-well microplate. The tracheal rings were observed for ciliary activity using a microscope and were scored for the inhibition of ciliary activity. The corresponding scores were: 0: all cilia beating; 1: 75% beating; 2: 50% beating, 3: 25% beating; 4: all cilia stopped (Cook et al., Citation1999). The average of the ciliary activity scores from each group per day was then calculated.
Detection and quantification of viral RNA in organs
Tissue samples were pooled per interval, group, and organ. Pooled samples were homogenized in 400 µl of ice-cold Dulbecco MEM (Life Technologies, Carlsbad, CA, USA). Tissue homogenates were centrifuged at 4°C for 10 min at 1000×g, and supernatants were collected. Viral RNA from tissue suspensions was purified using the QIAmp Viral RNA mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Real-time RT-PCR targeting the untranslated region (UTR) of AvCoV was used according to a previous study (Callison et al., Citation2006), with slight modifications. Briefly, the Quantitec Probe RT-PCR kit (Hilden, Germany) was used with final concentrations of 500 nM of each primer and 100 nM of the Taqman probe labelled with FAM fluorescence, in a total individual reaction volume of 25 μl containing 5 μl of RNA (0.2–20 ng). After an initial reverse transcription step (50°C for 30 min) and an initial denaturation step at 95°C for 15 min, 45 cycles (95°C for 15 s then 60°C for 1 min) were performed with fluorescence detection at the end of the annealing-extension step. Amplification and fluorescence detection were conducted in an Applied Biosystems 7500 Real-time PCR cycler (Foster City, USA). An external standard curve was created using spectrophotometrically determined copy number standards of purified PCR product for the UTR gene. Threshold cycles (Ct) were converted to copy numbers as described previously (Ferreira et al., Citation2007). Ct values above 37.09 were considered negative.
Histopathology
For histopathological evaluation, organs from inoculated and control groups were collected and fixed in 4% paraformaldehyde for 12 h at room temperature. The specimens were subjected to diaphanization with xylene, dehydrated in graded ethanol, embedded in paraffin, cut in 5-μm-thick sections, and processed by standard histological procedures (Bancroft & Gamble, Citation2008) Histopathological changes were evaluated on sections stained with haematoxylin and eosin (H&E). One sample for each collected organ was examined from each animal. All the sections were examined by light microscopy. The analysis was performed blindly by three independent investigators who evaluated the slides, each one containing three parts.
Detection of specific antibodies against AvCoV
The humoral response in chickens was evaluated by an indirect ELISA using ID Screen®IBV Indirect (ID vet Innovative Diagnostic, France) according to the manufacturer’s instructions. The microplate was read at 450 nm and evaluated for the presence of antibodies.
Statistical analysis
One-way analysis of variance (ANOVA) and Student’s t-test were performed to compare the quantity of viral RNA detected in the infected groups with the control group. Differences among target tissues of all infected groups were also evaluated. The differences were considered significant at P < 0.05.
Results
Clinical signs and gross findings
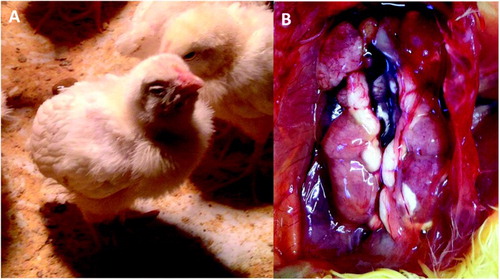
No mortality was observed in any infected group. No clinical signs or mortality were observed in the control group. All infected groups showed clinical signs from 2 to 9 dpi. At 2 dpi, chickens from all infected groups appeared depressed, with signs of listlessness, ruffled feathers, and lowered wings. The peak of morbidity was at 5 dpi, with 70% for chicken-801 and -810 groups and 80% for the pigeon-67T group.
Respiratory problems, such as dyspnea and coughing, were also observed at 2 dpi in the pigeon-67T group. At 5 dpi, birds from all groups had tracheal rales and mild signs of anorexia, watery faeces, and lack of coordination ((A)). After 9 dpi, birds gradually recovered until 14 dpi, when no clinical signs were observed. At necropsy, congestion and catarrhal exudates were observed in the tracheal lumen among 4 and 9 dpi chicks in all infected groups. Urate deposition in the kidney was observed in chickens infected with the chicken-810 and chicken-801 strains at 5 dpi ((B)). Gross lesions were not observed in any other organs examined after infection with any strain.
Figure 1. Clinical signs/gross lesions were observed after infection with AvCoV strains. (A) Chicks infected with the pigeon-67T strain at 5 dpi presenting clinical signs such as depression and prostration. (B) Gross lesions observed in the chicken-810 group at 4 dpi with swollen and pale kidneys and ureters distended with white urates.
Inhibition of ciliary activity
Complete ciliostasis (0% active cilia) was observed at 4, 7, and 9 dpi in the pigeon-67T group (). In the chicken-801 group, complete ciliostasis was found a few days later at 9 and 11 dpi. The chicken-810 group showed ciliostasis gradually increasing until 11 dpi, but this group did not have a complete absence of ciliary activity. For all infected groups, a recovery of 50% of ciliary activity was observed at 14 dpi, and full recovery was observed at 21 dpi. As expected, no ciliostasis was observed in the control group.
Table 1. Average ciliary activity scores observed from 4 dpi till 21 dpi after infection using different AvCoV isolates in experimental groups.
Virus RNA detected after infection
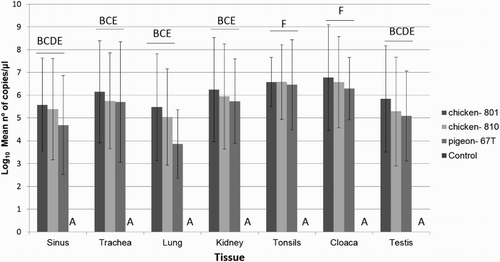
Viral RNA specific to AvCoV was detected in all infected groups in the sinus, trachea, lung, caecal tonsils, cloaca, kidney, and testis after virus inoculation and at different days post infection (). No viral RNA was detected in the control group. No significant difference was found among the average RNA quantity measured by real-time RT-PCR (P > 0.05) in the infected groups. The viral RNA loads were significantly higher in the cloacal swabs and caecal tonsils compared to other tissues (P < 0.05) for all infected groups. Viruses were detected in many tissues until the end of the experiment at 42 dpi, except in the testis of all infected groups.
Figure 2. Viral RNA measurement by real-time RT-PCR in sinus, trachea, lung, kidney, caecal tonsils, cloaca and testis of non-infected chickens, and chickens infected with pigeon-67T, chicken-801, or chicken-810 strains. The means ± standard deviation with no common letters differ significantly (P < 0.05).
Histopathology
Moderate congestion, mild degeneration of pseudostratified ciliated cells, and lack of cilia were observed in the sinuses after inoculation with pigeon-67T strain at 2 dpi and chicken-801 and chicken-810 strains at 5 dpi. Discrete mononuclear cell infiltrates, oedema in lamina propria, and a moderate hyperkeratosis were also observed in the sinuses of chicks infected with the pigeon-67T strain at 5 dpi. All infected groups also had a severe lack of cilia in pseudostratified ciliated cells and moderate congestion in the trachea at 5 and 7 dpi. Severe diffuse congestion was observed in lungs of the chicken-801 group at 7 and at 5 dpi in the pigeon-67T and chicken-810 groups. Lungs of birds infected with the pigeon-67T strain had moderate erythrocyte infiltration in the parabronchi and atrium, mild lymphocytic interstitial pneumonia, and moderate oedema. Additionally, multiple demineralization areas were observed in the parabronchi and atrium, and multiple granulomas (characterized by central necrosis surrounded by multinucleated giant cells, epithelia histiocytes, and heterophils) were seen in the lungs in the pigeon-67 T group at 5 dpi.
Renal lesions, such as mixed inflammatory infiltration in the renal pelvis, moderate oedema, and a discrete presence of hyaline cylinders in the tubules, were observed in the chicken-801 and chicken-810 groups at 5 dpi. Interstitial nephritis was observed in chickens from the pigeon-67 T group at 2 and 5 dpi. Oedema in lamina propria was found in ileum samples of birds infected with the chicken-810 strain, but only a very discrete oedema was observed in birds infected with the 801 strain at 7 dpi; no characteristic lesions were observed in the ileum in chicks infected with the 67T strain. No characteristic lesions were found in the testis and caecal tonsils of infected groups nor in any organ of the control group.
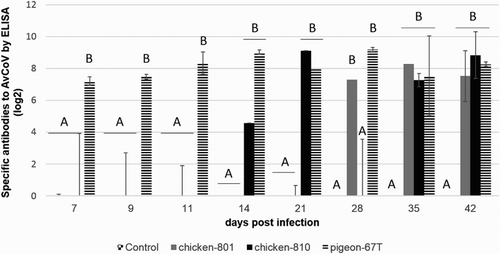
Antibody response
Specific antibodies to avian coronavirus were detected in the chickens inoculated with the chicken-801, chicken-810, and pigeon-67T strains (). The pigeon-67T strain induced the highest detectable immune response at 7 dpi until the end of the experiment. The chicken-801 and -810 groups had detectable antibody titres at 28 and 14 dpi, respectively, and a long-lasting immune response was observed until the end of the experiment in all inoculated groups. As expected, no specific antibody against AvCoV was detected in the control group.
Discussion
AvCoV have been isolated from many wild birds (Jonassen et al., Citation2005; Hughes et al., Citation2009; Tarnagda et al., Citation2011; Domanska-Blicharz et al., Citation2014; Kim & Oem, Citation2014). Nonetheless, few reports describe the pathogenicity of AvCoV isolates from other species in chickens. In 1985, an AvCoV was isolated from racing pigeons which had respiratory signs and mortality (Barr et al., Citation1988). The AvCoV isolate was inoculated into 4-week-old SPF chickens and induced marked respiratory rales. An AvCoV isolate from teal caused listlessness, huddling, ruffled feathers, dark shrunken combs, and 80% mortality in 15-day-old SPF chickens (Liu et al., Citation2005). Infected birds gradually recovered after 20 dpi. An AvCoV isolate from peafowl induced similar clinical signs in chickens from 5–14 dpi, but all birds survived (Sun et al., Citation2007). In our study, chicks infected with the pigeon-67T strain developed severe clinical signs, mainly respiratory signs, compared to the chicken strains (chicken-801 and chicken-810). All birds showed clinical signs and morbidity up to 9 dpi similar to those previously reported for different AvCoV strains (Otsuki et al., Citation1990). The AvCoV isolate from teal caused severe gross lesions in the kidney (Liu et al., Citation2005). In our study, the pigeon isolate did not cause lesions in the kidney. As discussed in an earlier report (De Wit et al., Citation2015), the Brazilian variants from chickens did induce gross lesions in the kidney.
Ciliostasis at different levels is induced by AvCoV from chicken samples after experimental infection in chickens (Raj & Jones, Citation1997; Benyeda et al., Citation2009; De Wit et al., Citation2015). Our AvCoV isolated from a pigeon was able to induce the complete absence of ciliary activity, earlier and for a longer period than the chicken-801 and chicken-810 strains. Our results using a Mass-like isolate from a pigeon are in accordance with a previous study (Otsuki et al., Citation1990) that reported ciliostasis induced by the M41 strain in White Leghorn chickens up to 9 dpi. Similarly, following infection of chicken tracheal organ culture with M41, a strain that mainly causes respiratory lesions, higher apoptosis was observed compared to the predominantly nephropathogenic strains (Chhabra et al., Citation2016).
Histopathological changes in the lung and kidney were reported after infection with a pheasant isolate (Sun et al., Citation2007). In the present study, histopathological changes in the respiratory tract of chickens infected with the pigeon-67T strain were more severe than in chickens infected with the chicken-801 and chicken-810 strains. The pigeon strain also induced moderate interstitial pneumonia, oedema, and mononuclear infiltration in mice at 3 dpi (Martini et al., Citation2015).
Avian coronavirus is able to replicate in epithelial cells of different organs, but the trachea is most commonly indicated as the primary site of replication in Galliformes birds using isolates from those birds (Otsuki et al., Citation1990; Raj & Jones, Citation1997; Fan et al., Citation2012). In our study, the pigeon strain as well as the chicken strains replicated in a wide variety of tissues, since RNA was identified in all eight investigated tissues. High viral RNA levels were detected in the sinus, trachea, and lungs, suggesting virus replication is occurring in those tissues. Additionally, high amounts of viral RNA were also detected in a broad range of tissues, especially in the digestive tract but also in the testis and kidney. Interestingly, no significant histopathological change in the gastrointestinal tract could be observed, despite long-term shedding of high viral RNA loads as previously noted (Ambali & Jones, Citation1990; Raj & Jones, Citation1997; Benyeda et al., Citation2009; Benyeda et al., Citation2010).
Similarly to a previous study (Barr et al., Citation1988), our pigeon-67T strain induced a higher humoral immune response compared to the chicken-801, chicken-810, and control groups. On the other hand, the low antibody levels detected in the chicken-801 and chicken-810 groups might be due to a different cross-reaction with the antigen used in the ELISA. A previous study also showed a low antigenic relationship by virus neutralization due to 25% divergence of the Mass and Brazilian variant lineages in their S1 amino acid sequences (Chacon et al., Citation2011).
The use of live vaccines can result in reversion of virulence (Jackwood & Lee, Citation2017) and reports indicate the circulation of vaccine strains in wild birds (Sun et al., Citation2007; Domanska-Blicharz et al., Citation2014). The AvCoV strain isolated from a pigeon was able to cause clinical signs, gross lesions in the trachea, ciliostasis, and histopathological changes. Similar findings, except for additional kidney gross lesions, were observed in chickens infected with Brazilian variants as expected. RT-PCR detected viral RNA in eight different tissues after infection with the three AvCoV isolates. Our results suggest that non-Galliformes birds can also play an essential role in the ecology of AvCoV, mainly due to the high genetic diversity of AvCoV.
Ethical approval
All applicable international, national, and institutional guidelines for the care and use of birds were followed (CEUA number Biovet, n°005/13 – June 24th, 2013).
Acknowledgements
We are especially grateful to Biovet Laboratory, Brazil, for providing the chickens and allowing us the use of the animal facilities.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ambali, A.G. & Jones, R.C. (1990). Early pathogenesis in chicks of infection with an enterotropic strain of infectious bronchitis virus. Avian Diseases, 34, 809–817. doi: 10.2307/1591367
- Bancroft, J.D. & Gamble, M. (2008). Theory and Practice of Histological Techniques 6th ed. Amsterdam: Elsevier Health Sciences.
- Barr, D.A., Reece, R.L., O’Rourke, D., Button, C. & Faragher, J.T. (1988). Isolation of infectious bronchitis virus from a flock of racing pigeons. Australian Veterinary Journal, 65, 228. doi: 10.1111/j.1751-0813.1988.tb14468.x
- Benyeda, Z., Mato, T., Suveges, T., Szabo, E., Kardi, V., Abonyi-Toth, Z., Rusvai, M. & Palya, V. (2009). Comparison of the pathogenicity of QX-like, M41 and 793/B infectious bronchitis strains from different pathological conditions. Avian Pathology, 38, 449–456. doi: 10.1080/03079450903349196
- Benyeda, Z., Szeredi, L., Mato, T., Suveges, T., Balka, G., Abonyi-Toth, Z., Rusvai, M. & Palya, V. (2010). Comparative histopathology and immunohistochemistry of QX-like, Massachusetts and 793/B serotypes of infectious bronchitis virus infection in chickens. Journal of Comparative Pathology, 143, 276–283. doi: 10.1016/j.jcpa.2010.04.007
- Callison, S.A., Hilt, D.A., Boynton, T.O., Sample, B.F., Robison, R., Swayne, D.E. & Jackwood, M.W. (2006). Development and evaluation of a real-time Taqman RT-PCR assay for the detection of infectious bronchitis virus from infected chickens. Journal of Virological Methods, 138, 60–65. doi: 10.1016/j.jviromet.2006.07.018
- Cavanagh, D. (2005). Coronaviruses in poultry and other birds. Avian Pathology, 34, 439–448. doi: 10.1080/03079450500367682
- Cavanagh, D., Mawditt, K., Welchman Dde, B., Britton, P. & Gough, R.E. (2002). Coronaviruses from pheasants (Phasianus colchicus) are genetically closely related to coronaviruses of domestic fowl (infectious bronchitis virus) and turkeys. Avian Pathology, 31, 81–93. doi: 10.1080/03079450120106651
- Chacon, J.L., Rodrigues, J.N., Assayag Junior, M.S., Peloso, C., Pedroso, A.C. & Ferreira, A.J. (2011). Epidemiological survey and molecular characterization of avian infectious bronchitis virus in Brazil between 2003 and 2009. Avian Pathology, 40, 153–162. doi: 10.1080/03079457.2010.544641
- Chhabra, R., Kuchipudi, S.V., Chantrey, J. & Ganapathy, K. (2016). Pathogenicity and tissue tropism of infectious bronchitis virus is associated with elevated apoptosis and innate immune response. Virology, 488, 232–241. doi: 10.1016/j.virol.2015.11.011
- Cook, J.K., Orbell, S.J., Woods, M.A. & Huggins, M.B. (1999). Breadth of protection of the respiratory tract provided by different live-attenuated infectious bronchitis vaccines against challenge with infectious bronchitis viruses of heterologous serotypes. Avian Pathology, 28, 477–485. doi: 10.1080/03079459994506
- De Wit, J.J., Brandao, P., Torres, C.A., Koopman, R. & Villarreal, L.Y. (2015). Increased level of protection of respiratory tract and kidney by combining different infectious bronchitis virus vaccines against challenge with nephropathogenic Brazilian genotype subcluster 4 strains. Avian Pathology, 44, 352–357. doi: 10.1080/03079457.2015.1058916
- Domanska-Blicharz, K., Jacukowicz, A., Lisowska, A., Wyrostek, K. & Minta, Z. (2014). Detection and molecular characterization of infectious bronchitis-like viruses in wild bird populations. Avian Pathology, 43, 406–413. doi: 10.1080/03079457.2014.949619
- Ducatez, M.F., Liais, E., Croville, G. & Guerin, J.L. (2015). Full genome sequence of Guinea fowl coronavirus associated with fulminating disease. Virus Genes, 50, 514–517. doi: 10.1007/s11262-015-1183-z
- Elbers, A.R., Koch, G. & Bouma, A. (2005). Performance of clinical signs in poultry for the detection of outbreaks during the avian influenza A (H7N7) epidemic in The Netherlands in 2003. Avian Pathology, 34, 181–187. doi: 10.1080/03079450500096497
- Fan, W.Q., Wang, H.N., Zhang, Y., Guan, Z.B., Wang, T., Xu, C.W., Zhang, A.Y. & Yang, X. (2012). Comparative dynamic distribution of avian infectious bronchitis virus M41, H120, and SAIBK strains by quantitative real-time RT-PCR in SPF chickens. Bioscience, Biotechnology, and Biochemistry, 76, 2255–2260. doi: 10.1271/bbb.120521
- Felippe, P.A., da Silva, L.H., Santos, M.M., Spilki, F.R. & Arns, C.W. (2010). Genetic diversity of avian infectious bronchitis virus isolated from domestic chicken flocks and coronaviruses from feral pigeons in Brazil between 2003 and 2009. Avian Diseases, 54, 1191–1196. doi: 10.1637/9371-041510-Reg.1
- Ferreira, H.L., Spilki, F.R., de Almeida, R.S., Santos, M.M. & Arns, C.W. (2007). Inhibition of avian metapneumovirus (AMPV) replication by RNA interference targeting nucleoprotein gene (N) in cultured cells. Antiviral Research, 74, 77–81. doi: 10.1016/j.antiviral.2006.12.002
- Guy, J.S., Barnes, H.J., Smith, L.G. & Breslin, J. (1997). Antigenic characterization of a turkey coronavirus identified in poult enteritis- and mortality syndrome-affected turkeys. Avian Diseases, 41, 583–590. doi: 10.2307/1592148
- Hughes, L.A., Savage, C., Naylor, C., Bennett, M., Chantrey, J. & Jones, R. (2009). Genetically diverse coronaviruses in wild bird populations of northern England. Emerging Infectious Diseases, 15, 1091–1094. doi: 10.3201/eid1507.090067
- ICTV. (2014). Virus Taxonomy: Classification and Nomenclature of Viruses: Ninth Report of the International Committee on Taxonomy of Viruses. San Diego: Elsevier Academic Press.
- Jackwood, M. & Wit, S. (2013). Infectious Bronchitis. In D. Swayne. Diseases of Poultry 13th edn (pp. 139–160). Ames, IA: John Wiley & Sons.
- Jackwood, M.W. & Lee, D.H. (2017). Different evolutionary trajectories of vaccine-controlled and non-controlled avian infectious bronchitis viruses in commercial poultry. PLoS One, 12, e0176709. doi: 10.1371/journal.pone.0176709
- Jonassen, C.M., Kofstad, T., Larsen, I.L., Lovland, A., Handeland, K., Follestad, A. & Lillehaug, A. (2005). Molecular identification and characterization of novel coronaviruses infecting graylag geese (Anser anser), feral pigeons (Columbia livia) and mallards (Anas platyrhynchos). Journal of General Virology, 86, 1597–1607. doi: 10.1099/vir.0.80927-0
- Jordan, B.J., Hilt, D.A., Poulson, R., Stallknecht, D.E. & Jackwood, M.W. (2015). Identification of avian coronavirus in wild aquatic birds of the central and eastern USA. Journal of Wildlife Diseases, 51, 218–221. doi: 10.7589/2014-03-070
- Kim, H.R. & Oem, J.K. (2014). Surveillance of avian coronaviruses in wild bird populations of Korea. Journal of Wildlife Diseases, 50, 964–968. doi: 10.7589/2013-11-298
- Kim, L.M., King, D.J., Guzman, H., Tesh, R.B., Travassos da Rosa, A.P., Bueno, R., Jr., Dennett, J.A. & Afonso, C.L. (2008). Biological and phylogenetic characterization of pigeon paramyxovirus serotype 1 circulating in wild North American pigeons and doves. Journal of Clinical Microbiology, 46, 3303–3310. doi: 10.1128/JCM.00644-08
- Liu, S., Chen, J., Chen, J., Kong, X., Shao, Y., Han, Z., Feng, L., Cai, X., Gu, S. & Liu, M. (2005). Isolation of avian infectious bronchitis coronavirus from domestic peafowl (Pavo cristatus) and teal (Anas). Journal of General Virology, 86, 719–725. doi: 10.1099/vir.0.80546-0
- Martini, M.C., Gameiro, J., Cardoso, T.C., Caserta, L.C., Gualberto, A.C., Keid, L.B., Oliveira, T.M., dos Santos, M.M., Arns, C.W. & Ferreira, H.L. (2015). Experimental infection of inbred BALB/c and A/J mice with Massachusetts and Brazilian strains of infectious bronchitis virus (IBV). Archives of Virology, 160, 1785–1790. doi: 10.1007/s00705-015-2443-x
- Olsen, B., Munster, V.J., Wallensten, A., Waldenstrom, J., Osterhaus, A.D. & Fouchier, R.A. (2006). Global patterns of influenza a virus in wild birds. Science, 312, 384–388. doi: 10.1126/science.1122438
- Otsuki, K., Huggins, M.B. & Cook, J.K. (1990). Comparison of the susceptibility to avian infectious bronchitis virus infection of two inbred lines of white leghorn chickens. Avian Pathology, 19, 467–475. doi: 10.1080/03079459008418700
- Raj, G.D. & Jones, R.C. (1997). Infectious bronchitis virus: immunopathogenesis of infection in the chicken. Avian Pathology, 26, 677–706. doi: 10.1080/03079459708419246
- Reed, L.J. & Muench, H. (1938). A simple method of estimating fifty per cent endpoints. American Journal of Epidemiology, 27, 493–497. doi: 10.1093/oxfordjournals.aje.a118408
- Sun, L., Zhang, G.H., Jiang, J.W., Fu, J.D., Ren, T., Cao, W.S., Xin, C.A., Liao, M. & Liu, W.J. (2007). A Massachusetts prototype like coronavirus isolated from wild peafowls is pathogenic to chickens. Virus Research, 130, 121–128. doi: 10.1016/j.virusres.2007.06.003
- Tarnagda, Z., Yougbare, I., Kam, A., Tahita, M.C. & Ouedraogo, J.B. (2011). Prevalence of infectious bronchitis and Newcastle disease virus among domestic and wild birds in H5N1 outbreaks areas. The Journal of Infection in Developing Countries, 5, 565–570. doi: 10.3855/jidc.1441
- Torres, C.A., Villarreal, L.Y., Ayres, G.R., Richtzenhain, L.J. & Brandao, P.E. (2013). An avian coronavirus in quail with respiratory and reproductive signs. Avian Diseases, 57, 295–299. doi: 10.1637/10412-100412-Reg.1
- Wille, M., Muradrasoli, S., Nilsson, A. & Jarhult, J.D. (2016). High prevalence and putative lineage maintenance of avian coronaviruses in Scandinavian waterfowl. PLoS One, 11, e0150198. doi: 10.1371/journal.pone.0150198
- Woo, P.C., Lau, S.K., Lam, C.S., Lai, K.K., Huang, Y., Lee, P., Luk, G.S., Dyrting, K.C., Chan, K.H. & Yuen, K.Y. (2009). Comparative analysis of complete genome sequences of three avian coronaviruses reveals a novel group 3c coronavirus. Jounal of Virology, 83, 908–917. doi: 10.1128/JVI.01977-08