ABSTRACT
This article reports nine cases of neurological disease in brown layer pullets that occured in various European countries between 2015 and 2018. In all cases, the onset of neurological clinical signs was at 4–8 weeks of age and they lasted up to 22 weeks of age. Enlargement of peripheral nerves was the main lesion observed in all cases. Histopathological evaluation of nerves revealed oedema with moderate to severe infiltration of plasma cells. Marek’s disease (MD) was ruled out by real-time PCR as none of the evaluated tissues had a high load of oncogenic MD virus (MDV) DNA, characteristics of MD. Based on the epidemiological data (layers with clinical signs starting at 5–8 weeks of age), gross lesions (peripheral nerve enlargement with a lack of tumours in other organs), histopathological lesions (oedema and infiltration of plasma cells), and no evidence of high load of MDV DNA, we concluded that those cases were due to peripheral neuropathy (PN). PN is an autoimmune disease easily misdiagnosed as MD, leading to a costly enforcement of the vaccination protocol. Additional vaccination against MD does not protect against PN and could worsen the clinical signs by over-stimulating the immune system. Differential diagnosis between PN and MD should always be considered in cases of neurological disease with enlargement of peripheral nerves as the only gross lesion. This case report shows for the first time how real-time PCR to detect oncogenic MDV is a very valuable tool in the differential diagnosis of PN and MD.
Introduction
Marek’s disease (MD) is a lymphoproliferative disease of chickens induced by an alpha-herpesvirus, Marek’s disease virus (MDV), of the genus Mardivirus that was first isolated in 1968 (Churchill, Citation1968). MD, initially described by Joseph Marek (Citation1907), is characterized by the development of lymphoma and enlargement of peripheral nerves and is by far the most common cause of paralysis associated with enlarged nerves in chickens. Enlargement of nerves, however, is not a pathognomonic lesion of MD as it can appear in other diseases. Lymphoid infiltration of the nerves that could be confused with MD lesions occurs also in non-bursal reticuloendotheliosis, induced by reticuloendotheliosis virus under certain laboratory conditions (Witter et al., Citation1970; Witter et al., Citation2010). Furthermore, peripheral neuropathy (PN) syndrome, first described in SPF chickens (Biggs et al., Citation1982) and later reported in commercial flocks (Julian, Citation1992; Witter & Bacon, Citation1995; Bacon et al., Citation2001), is characterized by lesions in the nerves that are almost indistinguishable, both grossly and histologically, from those lesions induced by MDV. Because of such similarities, it is not uncommon that PN cases get misdiagnosed as MD.
PN is an autoimmune disease that affects pullets between 6 and 9 weeks of age (Julian, Citation1992; Witter & Bacon, Citation1995; Bacon et al., Citation2001). The first descriptions of PN were in an SPF flock of Rhode Island Red chickens (Biggs et al., Citation1982) but all PN cases reported in commercial flocks until now occurred in White Leghorns (Julian, Citation1992; Witter & Bacon, Citation1995; Bacon et al., Citation2001). Development of PN is associated with certain B-haplotypes, with the B*19 haplotype being the most susceptible, and multiple factors (including common vaccination with MD, infectious bursal disease, Newcastle disease, and infectious bronchitis vaccines (Bacon et al., Citation2001) or infection with Campylobacter jejuni (Bader et al., Citation2010)) can elicit an outbreak in susceptible chickens.
Bacon et al. (Citation2001) established the criteria for the diagnosis of PN. They considered the clinical history of the chickens (pullets of 6–9 weeks of age) with neurological clinical signs and gross lesions of the nerves in the absence of tumours as the most relevant criteria to consider in the diagnosis of PN. Histopathology of the peripheral nerves is commonly conducted to confirm the diagnosis. However, PN lesions in the nerves consist of oedema and infiltration of plasma cells, which can also occur after infection with MDV. Therefore, unless other criteria (clinical history, age, and gross lesions) are taken into consideration, it is likely that many cases of PN get diagnosed as MD by only histopathological evaluation of the nerves. It is therefore desirable to use other methods that can rule out MD in the diagnosis of PN. Evaluation of load of oncogenic MDV in tumours, feather pulp, spleen, or blood by real-time PCR has been reported to be a very useful criterion in the diagnosis of MD (Gimeno et al., Citation2005a; Gimeno et al., Citation2008; Cortes et al., Citation2011).
Differential diagnosis of PN and MD, although it can be challenging, is very relevant to implement proper methods of control. In case of suspect MD, vaccination programmes of MD are commonly strengthened by increasing vaccine dose, adding strains of other serotypes or adding a second dose of the vaccine. These practices will not only help in the control of PN but they might worsen the extent of the problem as they will over-activate the immune system (Bacon et al., Citation2001)
In this article, we investigated nine cases of neurological disease in brown layers that occurred in Europe from 2015 to 2018. In addition to evaluating the clinical history and the lesions, both grossly and histologically, we have used real-time PCR to rule out MD.
Materials and methods
Case history
Nine clinical cases of neurological disease in brown laying hens that occurred between 2015 and 2018 in Hungary, Austria and Bulgaria are reported. All cases occurred in commercial flocks of brown layers in either floor or cage systems. Flocks were vaccinated against MD using CVI988 strain (Rispens) or a combination of CVI988 plus either the herpesvirus of turkey (HVT) or a recombinant HVT. Cases are summarized in . Briefly, cases 1 and 2 occurred in Hungary in December 2015. In case 1, neurological clinical signs started around 7–8 weeks of age and lasted until 22 weeks. The transfer of the pullets from the brooder house to the production farm occurred at 16 weeks of age. There were 11,700 birds in the flock and the mortality rate was 3.06%. In case 2, neurological clinical signs were observed between 6 and 14 weeks of age. There were 75,740 birds in the flock and the mortality rate was 0.66%. Cases 3–5 happened in Austria in May 2017 (cases 3 and 4) and in June 2017 (case 5). In case 3, a flock of 21,000 pullets was affected with a mortality rate of 2.24%. Pullets showed neurological signs between 6 and 12 weeks of age. In case 4, a flock of 45,000 pullets was affected with a mortality rate of 0.7%. Neurological clinical signs were shown between 7 and 13 weeks. In case 5, a flock of 71,000 pullets was affected with a mortality rate of 0.7%. Pullets showed neurological signs between 4 and 7 weeks of age. Case 6 occurred in Bulgaria in April 2017. A flock of 71,000 pullets was affected with a mortality rate of 0.7%. Neurological clinical signs were shown between 4 and 7 weeks of age. Cases 7 and 8 occurred in Hungary in November 2017. In case 7, a flock of 20,000 pullets was affected and the mortality rate was 2%. Neurological clinical signs were observed between 5 and 10 weeks. In case 8, a flock of 20,000 pullets was affected and the mortality rate was 3%. Neurological clinical signs were observed between 5 and 10 weeks. Case 9 occurred in Hungary in February 2018 in a farm that housed 5100 brown pullets and 9180 white pullets. Interestingly only the brown pullets developed neurological clinical signs between 5 and 14 weeks of age with a mortality rate of 8.76%. White pullets did not develop neurological disease and the mortality rate was 0.7%.
Table 1. Summary of clinical cases.
Necropsy and sample collection
Birds that were showing neurological signs were humanely euthanized. At necropsy, all chickens were examined for gross lesions in brain, lymphoid organs (bursa of Fabricius, thymus, and spleen), peripheral nerves, viscera, and skin. In addition, evaluation of muscle, joints, and vertebrae was conducted to rule out a musculoskeletal problem.
Tissues for histopathology were collected in cases 2 (brain and sciatic nerves), 3 (sciatic nerve), 6 (kidney, liver proventriculus, and sciatic nerve), 7 (sciatic nerves), and 8 (sciatic nerves). Case 2 was submitted to the laboratory of National Food Chain Safety Office Animal Health Diagnostic Directorate (Hungary), case 3 was submitted to the University of Veterinary Medicine in Vienna (Austria), case 6 was submitted to ORBIO laboratory (Lyon, France), and cases 2, 7, and 8 were submitted to our lab at NCSU. In addition, tissue imprints were collected in Flinders Technology Associates cards for real-time PCR in cases 1 (feather pulp), 2 (proventriculus, spleen, feather pulp, and sciatic nerves), 6 (kidney, spleen, liver, and bursa), 8 (feather pulp and spleen), and 9 (spleen, liver, sciatic nerves, and feather pulp). Our laboratory at NCSU processed all of the samples for real-time PCR.
Histopathology
Tissues were immersed in 10% buffered formalin before they were dehydrated in graded ethanol solutions, embedded in a low melting point (53–55°C) paraffin wax, sectioned at 5 µm, mounted on glass slides and stained with Haematoxylin and Eosin (H&E).
Real-time PCR
DNA was extracted from tissue imprints collected in Flinders Technology Associates® cards as reported (Cortes et al., Citation2009). We used ArchivePure DNA tissue kit (5-Prime Inc, Gaithersburg, MD, USA), following the manufacturer’s recommendations. Samples were amplified with primers specific for the chicken GAPDH, a 62 bp fragment that lies between ORFs HVT072 and HVT073 of the HVT genome, and with MAMA primers that are specific for the pp38 gene of CVI988 strains (CVI988 primers) and for the oncogenic strains of serotype 1 MDV (oncogenic primers) (Gimeno et al., Citation2014). shows the sequence for the respective forward and reverse primers. PCR amplifications were done using the MX3005P® Stratagene (Stratagene, La Jolla, CA) in a 25 μl PCR containing 50 ng of DNA, 0.2 μM of each primer, and SYBR® Green Based Master Mix (Brilliant II Fast SYBR Green qPCR Master Mix) that contains the appropriate buffers, nucleotides and hot-start Taq-polymerase (Agilent Technologies, New Castle, DE). The reaction was cycled 40 times at 95°C denaturation for 15 s and a 60°C combined annealing/extension for 60 s. Fluorescence was acquired at the end of the annealing/extension phase. The melting curves were obtained at the end of amplification by cooling the sample at 2°C/s to 60°C and then increasing the temperature to 95°C at 0.1°C/s. The parameter Ct (Threshold cycle) was calculated for each PCR by establishing a fixed threshold. Ct is the fractional cycle number at which the fluorescence passes the fixed threshold. Relative quantification of the load of MDV DNA was evaluated by the comparative Ct method (Delta delta Ct [DDCT]) as reported earlier (Gimeno et al., Citation2008). Chickens were divided into three categories based on the load of oncogenic MDV DNA present in feather pulp at 21 days: negative, latency levels, and tumour levels as reported (Gimeno et al., Citation2014).
Table 2. Oligonucleotides used for real-time PCR.
Results
Clinical signs
Neurological signs occurred in the nine presented cases and are summarized in . Briefly, clinical signs described included ataxia and paralysis (9 out of 9 cases) (), and curled toes (3 out of 9 cases). The onset of clinical signs started at 4–8 weeks of age (average 6.7 weeks) and lasted until 7–22 weeks of age (average 15 weeks). Mortality due to starvation or culling ranged from 0.66% to 8.76%. Egg production was not affected in any of the cases described in this report.
Gross lesions
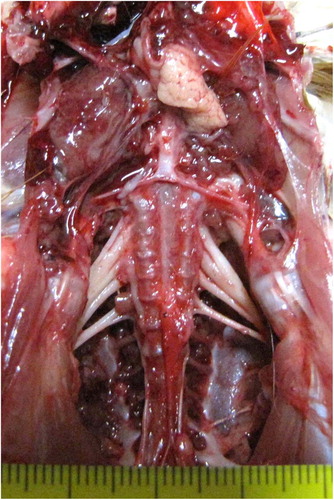
displays the necropsy findings for all reported cases. Hypertrophy of peripheral nerves was the main lesion described in all reported cases (). In addition, tibial rotation was described in case 2. Neither brain lesions nor visceral tumours were found in any of the reported flocks.
Histopathology
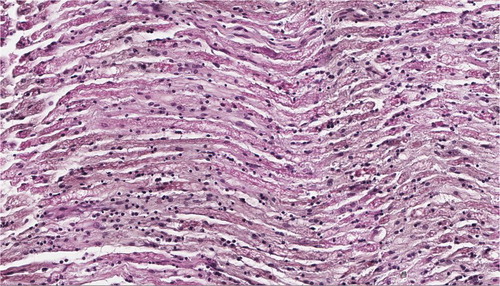
Histopathological evaluation was done in cases 2, 3, 6, 7, and 8. In all cases, lesions were restricted to peripheral nerves and no lesions were found in brain, liver, proventriculus, or kidney. In all evaluated cases, peripheral nerves showed oedema and moderate to severe infiltration of lymphocytes and plasma cells ((A,B)). Other findings included demyelination in case 2 and fibrosis, Schwann cell proliferation, and axon fragmentation in case 6. Interpretation of the histopathological results differed among institutions. Case 2 was submitted to the laboratory of National Food Chain Safety Office Animal Health Diagnostic Directorate (Hungary) and to us. The Hungarian laboratory diagnosed MD based on the histopathological findings but we found the lesions to be consistent with PN and suggested running further a diagnostic test (real-time PCR) to rule out MD. Our interpretation was the same for the other two cases that we received for histopathological analysis (cases 7 and 8). Identical nerve lesions to those reported in case 2 were found in case 3 and they were interpreted by the University of Veterinary Medicine of Vienna as MD. Diagnosis of case 6 by the ORBIO laboratory (Lyon, France) was inconclusive citing vitamin deficiency, intoxication, idiopathic polyneuritis, or an inflammatory form of MD as possible diagnosis.
Diagnosis of MD by real-time PCR
Load of oncogenic MDV DNA was evaluated in various tissues of cases 1, 2, 6, 8, and 9 and results are presented in . Oncogenic MDV DNA was found in 16 tissues (out of 83 tissues submitted) of cases 2, 6, 8, and 9. In all positive tissues, load of MDV DNA was very low compatible with tissues latently infected with MDV. None of the submitted tissues had high MDV DNA load compatible with tumours induced by MDV. Real-time PCR specific for the MD vaccine strains allowed us to confirm that vaccination against MD was properly conducted (data not shown).
Table 3. Real-time PCR results for Marek’s disease diagnosis.
Discussion
In this report, we describe nine cases of neurological disease that occurred in three European countries between 2015 and 2018. In all cases, brown laying hens of 4–8 weeks of age developed neurological clinical signs (ataxia and paralysis) that were associated with enlargement of peripheral nerves in the absence of other lesions. Based on the clinical history, histopathological evaluation of the nerves and real-time PCR we concluded that those cases were caused by PN.
PN was first described as an idiopathic polyneuritis occurring in SPF chickens by Biggs et al. (Citation1982). Later Julian (Citation1992), Witter & Bacon (Citation1995), and Bacon et al. (Citation2001) reported a similar syndrome in commercial white layers characterized by the onset of paralysis at 6 to 8 weeks of age with mortality usually lower than 3%, associated with starvation or culling. Enlargement of nerves was the only gross lesion found and histologically oedema and infiltration of plasma cells in peripheral nerves were the main lesions (Julian, Citation1992; Witter & Bacon, Citation1995; Bacon et al., Citation2001). Bacon et al. (Citation2001) reproduced the syndrome under laboratory conditions by transferring whole blood or buffy coat cells from affected chickens into chickens of various B-haplotypes. Blood sonication did not affect transmission but PN did not occur when plasma was transferred. The authors demonstrated that PN was clearly associated with specific B-haplotypes, the B*19 haplotype being the most susceptible, and they postulated that PN was an autoimmune disease that could be exacerbated by a variety of antigens such as vaccination against MD, infectious bursal disease, Newcastle disease, and infectious bronchitis. Later, Bader et al. (Citation2010) demonstrated that C. jejuni could also exacerbate the development of PN in brown leghorn layers. Bacon et al. (Citation2001) established the criteria for the diagnosis of PN including age at the onset of clinical signs (about 6 weeks of age), gross lesions (enlargement of peripheral nerves in absence of visceral lymphoma), and histopathological findings of oedema and plasma cell infiltration of the nerves. The nine cases that we reported in this manuscript match all the diagnostic criteria for PN. The average onset of the clinical signs was 6–7 weeks of age (although it could happen as early as 4 weeks of age), only peripheral nerves were affected, and they had oedema and infiltration of plasma cells. Mortality observed in the reported cases ranged from 0.7% to 8.76%. Previous reports described mortality of up to 3% (Julian, Citation1992; Witter & Bacon, Citation1995) but Bacon et al. (Citation2001) described mortality of up to 9% in commercial chickens of B*19 haplotype after various vaccinations. In the present study, we confirmed further the diagnosis of PN by ruling out MD using real-time PCR.
PN is easily confused with MD as clinical signs and gross lesions in the nerves are identical. Furthermore, oedema and infiltration of plasma cells in nerves, which are characteristics of PN, could also be induced by MDV. Nerve lesions induced by MDV have been classified into three types named A, B, and C (Payne & Biggs, Citation1967; Lawn & Payne, Citation1979). Type A lesions are characterized by neoplastic infiltration of lymphocytes in the nerves; type B lesions are inflammatory lesions identical to those described in PN; and type C lesions are minor inflammatory lesions (Payne & Biggs, Citation1967). It was postulated that B-type lesions occur as an immune response to the A lesions (Lawn & Payne, Citation1979). However, B-type lesions have been described in the absence of A-type lesions after inoculation of nononcogenic SB-1 strain into chicken embryos at 8 days of embryonation (Calnek et al., Citation1980), after inoculation of MDV in which either the phosphoprotein pp38 gene (Gimeno et al., Citation2005b) or the oncogene meq (Lupiani et al., Citation2004) had been deleted, and after inoculation of old chickens that had never been exposed to MDV with highly virulent MDV (Witter & Gimeno, Citation2006). It is likely that B-type lesions are immune mediated, thus the similarities with the lesions described in PN. The misdiagnosis of PN as MD is not uncommon and certainly occurred in two of the cases that we included in this study (cases 2 and 3). Even trained pathologists could misdiagnose PN as MD if they are not aware of this syndrome.
The incidence of PN in commercial flocks is unknown and probably it is higher than expected due to the confusion with MD. In this study, we reported nine cases that occurred in a short span of time (2 years) in a restricted geographical area (Hungary, Austria, and Bulgaria). Similar cases of neurological disease in brown laying hens had seen before in the same area but the diagnosis was either MD or inconclusive (Kőrösi, unpublished). It is very likely that cases of PN also occur in other areas but are misdiagnosed as MD or not reported. All previous reports of PN in commercial flocks affected white layers (Julian, Citation1992; Witter & Bacon, Citation1995; Bacon et al., Citation2001). The nine cases reported in this study affected brown layers instead. Interestingly in case 9, both white and brown layers were housed in the same farm but only the brown layers developed PN. Since PN is related to the B-haplotype, it is likely that all affected genetic lines share the susceptible B-haplotypes. Bacon et al. (Citation2001) suggested that removing those susceptible haplotypes from the genetic lines will control PN.
In the nine cases of PN reported in this article, the mortality rate ranged from 0.7 to 8.76%. The cost invested in each of these birds is high as they are 6 week old pullets or even young layers. Misdiagnosing PN as MD increases the economic losses since MD vaccination tends to be reinforced by increasing vaccine dose, adding vaccines of other MD serotypes, or implementing revaccination protocols. The reinforcement of vaccination instead of controlling the problem might exacerbate it by over-stimulating the immune responses and certainly will increase the cost of vaccination (Bacon et al. Citation2001). Hence, an accurate diagnosis of PN is important to minimize the economic loses.
As suggested by Bacon et al. (Citation2001), the genetics of the affected chickens (PN has been only reported so far in layers), the onset of neurological clinical signs at about 6 weeks of age, and enlargement of peripheral nerves in the absence of visceral tumours are all criteria to consider in the diagnosis of PN. In addition, histopathology will aid in the diagnosis by revealing the presence of oedema and infiltration of plasma cells. However, even a trained pathologist can confuse MDV-induced B-type lesions with PN. Ruling out MD by real-time PCR is an important step in the diagnosis of PN. Real-time PCR to measure the load of oncogenic MDV DNA is a very useful technique in the diagnosis of MD because it allows differentiation between tissues latently infected with MDV (most commercial chickens) and tissues that have tumours induced by MDV (Gimeno et al., Citation2005a; Witter, et al., Citation2010; Gimeno & Wakenell, Citation2016). To the knowledge of the authors, this is the first time real-time PCR has been used to rule out MD in cases of PN. Two of the submitted cases had been misdiagnosed as MD and only by ruling out MD using real-time PCR, could we confirm the diagnoses of PN in those cases.
This report thus shows that PN is still a problem in the field and the incidence might be higher than expected due to misdiagnosis with MD. We have also shown that, in addition to the clinical history (age, neurological clinical signs, and enlargement of gross nerves without visceral tumours) and histopathology, real-time PCR to rule out MD is a very valid tool in the investigation of PN cases. Any case of neurological disease in young pullets presenting nerve enlargement without tumours in other viscera should be further investigated to rule out MD.
Acknowledgments
The authors thank Dr Fletcher and Dr Pandiri for assistance with the histopathological images and for helpful discussion on the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Bacon, L.D., Witter, R.L. & Silva, R.F. (2001). Characterization and experimental reproduction of peripheral neuropathy in White Leghorn chickens. Avian Pathology, 30, 487–499. doi: 10.1080/03079450120078680
- Bader, S.R., Kothlow, S., Trapp, S., Schwarz, S.C., Philipp, H.C., Weigend, S., Sharifi, A.R., Preisinger, R., Schmahl, W., Kaspers, B., Matiasek, K. (2010). Acute paretic syndrome in juvenile White Leghorn chickens resembles late stages of acute inflammatory demyelinating polyneuropathies in humans. Journal of Neuroinflammation, 7, 7. doi: 10.1186/1742-2094-7-7
- Biggs, P.M., Shilleto, R.F.W., Lawn, A.M. & Cooper, D.M. (1982). Idiopathic polyneuritis in SPF chickens. Avian Pathology, 11, 163–178. doi: 10.1080/03079458208436090
- Calnek, B.W., Schat, K.A. & Fabricant, J. (1980). Modification of Marek’s disease pathogenesis by in ovo infection or prior vaccination. In M. Essex, G. Todaro, & H. zur Hausen (Eds.), Viruses in naturally occurring cancers, Cold Spring Harbor conferences on cell proliferation 7 ed. (pp. 185–197). New York: Cold Spring Harbor Laboratory.
- Churchill, A.E. (1968). Herpes-type virus isolated in cell culture from tumors of chickens with Marek’s disease. I. Studies in cell culture. Journal of the National Cancer Institute, 41, 939–950.
- Cortes, A.L., Montiel, E.R. & Gimeno, I.M. (2009). Validation of Marek’s disease diagnosis and monitoring of Marek’s disease vaccines from samples collected in FTA® cards. Avian Diseases, 53, 510–516. doi: 10.1637/8871-041009-Reg.1
- Cortes, A.L., Montiel, E.R., Lemiere, S. & Gimeno, I.M. (2011). Comparison of blood and feather pulp samples for the diagnosis of Marek’s disease and for monitoring Marek’s disease vaccination by real time PCR. Avian Diseases, 55, 302–310. doi: 10.1637/9578-101510-ResNote.1
- Gimeno, I.M., Cortes, A.L. & Silva, R.F. (2008). Load of challenge Marek’s disease virus DNA in blood as a criterion for early diagnosis of Marek’s disease tumors. Avian Diseases, 52, 203–208. doi: 10.1637/8089-081407-Reg.1
- Gimeno, I.M., Dunn, J., Cortes, A.L., El-Gohari, A.E. & Silva, R.F. (2014). Detection and differentiation of CVI988 (Rispens vaccine) from other serotype 1 Marek’s disease viruses. Avian Diseases, 58(2), 232–243. doi: 10.1637/10666-091713-Reg.1
- Gimeno, I.M. & Wakenell, P.S. (2016). Marek’s disease. In S.M. Williams, L. Dufour-Zavala, M.W. Jackwood, M.D. Lee, B. Lupiani, & W.M. Reed (Eds.), A laboratory manual for the isolation, identification and characterization of avian pathogens (pp. 249–258). Jacksonville, FL: American Association of Avian Pathologists.
- Gimeno, I.M., Witter, R.L., Fadly, A.M. & Silva, R.F. (2005a). Novel criteria for the diagnosis of Marek’s disease virus-induced lymphomas. Avian Pathology, 34, 332–340. doi: 10.1080/03079450500179715
- Gimeno, I.M., Witter, R.L., Hunt, H.D., Reddy, S.M., Lee, L.F. & Silva, R.F. (2005b). The pp38 gene of Marek’s disease virus (MDV) is necessary for cytolytic infection of B cells and maintenance of the transformed state but not for cytolytic infection of the feather follicle epithelium and horizontal spread of MDV. Journal of Virology, 79, 4545–4549. doi: 10.1128/JVI.79.7.4545-4549.2005
- Julian, R.J. (1992). Peripheral neuropathy causing ‘range paralysis’ in Leghorn pullets. Proceedings of the 129th Annual Meeting of the American Veterinary Medical Association, 130–130.
- Lawn, A.M. & Payne, L.N. (1979). Chronological study of ultrastructural changes in the peripheral nerves in Marek’s disease. Neuropathology and Applied Neurobiology, 5, 485–497. doi: 10.1111/j.1365-2990.1979.tb00645.x
- Lupiani, B., Lee, L.F., Cui, X., Gimeno, I.M., Anderson, A., Morgan, R.W., Silva, R.F., Witter, R.L., Kung, H.-J., Reddy, S.M. (2004). Marek’s disease virus-encoded Meq gene is involved in transformation of lymphocytes but is dispensable for replication. Proceedings of the National Academy of Sciences of the United States, 101, 11815–11820. doi: 10.1073/pnas.0404508101
- Marek, J. (1907). Multiple Nervenentzündung (Polyneuritis) bei Hühnern. Deutsche Tierarztliche Wochenschrift, 15, 417–421.
- Payne, L.N. & Biggs, P.M. (1967). Studies on Marek’s disease. II. Pathogenesis. Journal of the National Cancer Institute, 39, 281–302.
- Witter, R.L. & Bacon, L.D. (1995). A naturally occurring neuropathy of chickens not associated with Marek’s disease. Proceedings of the Annual Meeting of the 132th American Veterinary Medical Association (pp. 140–140).
- Witter, R.L. & Gimeno, I.M. (2006). Susceptibility of adult chickens, with and without prior vaccination, to challenge with Marek’s disease virus. Avian Diseases, 50, 354–365. doi: 10.1637/7498-010306R.1
- Witter, R.L., Gimeno, I.M., Pandiri, A.K. & Fadly, A.M. (2010). Tumor diagnosis manual: The differential diagnosis of lymphoid and myeloid Tumors in the chicken. Jacksonville: Florida The American Association of Avian Pathologists.
- Witter, R.L., Purchase, H.G. & Burgoyne, G.H. (1970). Peripheral nerve lesions similar to those of Marek’s disease in chickens inoculated with reticuloendotheliosis virus. Journal of the National Cancer Institute, 45, 567–577.