ABSTRACT
Riemerella anatipestifer (RA) is a widely distributed bacterial pathogen of birds responsible for remarkable losses to poultry production, especially among waterfowl. We characterized the genomic diversity of 166 field isolates of RA, collected from geese and ducks, using enterobacterial repetitive intergenic consensus (ERIC)-polymerase chain reaction (PCR). The field strains and five reference strains showed 17 distinct patterns consisting of five to 12 bands ranging from approximately 150–1800bp. The majority of the strains belonged to two closely related ERIC-PCR types (A and B), while the other types contained only a few isolates each. There was no association between ERIC-PCR type and host species, place, or year of isolation; however the ERIC-PCR pattern was correlated with serotype for most isolates. The majority of serotype 1 strains (101/107) belonged to ERIC-PCR type A while the remaining six strains represented five different ERIC-PCR types (D, G, L, M, and O). Serotypes 1,7 and 7 corresponded to ERIC-PCR types B and C, respectively. Serotypes 2, 4, and 10 could be subdivided by ERIC-PCR revealing two to four patterns within each serotype. These results indicate that ERIC-PCR may be a suitable technique for the molecular identification of RA serotypes, and the detection of subtypes within certain serotypes may aid further epidemiological investigations.
RESEARCH HIGHLIGHTS
ERIC-PCR analysis of field R. anatipestifer strains revealed 17 distinct patterns
Most strains belonged to two closely related ERIC-PCR types
Serotype 1 was the most prevalent serotype representing 64.5% of the strains
ERIC-PCR may be suitable for molecular identification of R. anatipestifer serotypes
Introduction
Riemerella anatipestifer (RA) is a Gram-negative rod-shaped bacterium that belongs to the family Flavobacteriaceae (Segers et al., Citation1993; Subramaniam et al., Citation1997). RA is the aetiological agent of anatipestifer syndrome, a contagious disease primarily of waterfowl but which can affect a variety of other birds, including turkeys and chickens, causing remarkable losses to the poultry production industry (Ruiz & Sandhu, Citation2013).
Serotyping is currently the most widely practiced method for classifying RA isolates using agglutination or agar gel precipitin (AGP) tests (Harry, Citation1969; Bisgaard, Citation1982; Sandhu & Leister, Citation1991), with 21 serotypes distinguished to date (Ruiz & Sandhu, Citation2013). Immunity induced by RA vaccines appears to be serotype-specific with no cross-protection (Sandhu, Citation1991; Pathanasophon et al., Citation1996), and accurate determination of serotypes thus has great practical significance in controlling RA infections. However, classical serotyping has several disadvantages, including the need for a set of specific antisera, the occurrence of false reactions, and non-typeable isolates.
A wide range of molecular techniques has been tested for subtyping R. anatipestifer isolates, including pulsed-field gel electrophoresis (PFGE) of SmaI-digested bacterial DNA (Yu et al., Citation2008), amplification followed by restriction digestion analysis of the ompA gene (Yu et al., Citation2008), sequencing of the 16S rRNA and ompA genes (Tsai et al., Citation2005), and matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry analysis (Philipp et al., Citation2013). Huang et al. (Citation1999) used repetitive element polymerase chain reaction fingerprinting (rep-PCR) to differentiate RA isolates from ducks in Singapore, and Yu et al. (Citation2008) found that rep-PCR and PFGE revealed high genotypic variations among RA isolates originated from ducks in Taiwan. However, only ompA sequencing has yet demonstrated a partial correlation between genotypes and serotypes in RA (Chen et al., Citation2015).
Enterobacterial repetitive intergenic consensus (ERIC)-PCR is a type of rep-PCR that provides a rapid, easy to perform, and cost-effective method for fingerprinting bacterial genomes, and has been utilized successfully to develop a PCR assay for the identification of RA (Kardos et al., Citation2007), although its specificity has been questioned recently arguing for additional confirmation with other methods (Christensen & Bisgaard, Citation2010; Rubbenstroth et al., Citation2013).
In the present study we analysed the genetic diversity of RA using ERIC-PCR and determined its possible correlation with serotype in the investigated isolates.
Materials and methods
Bacterial isolates
One hundred and sixty-six RA field isolates were collected from ducks and geese in Hungary from 2000 to 2017. The isolates from geese originated from 85 farms at 42 locations, while the isolates from ducks represented 22 farms at 18 locations (Supplementary Table 1). Some of the strains were isolated at the same farms from various time points but always from different individuals. Samples taken from various organs (brain, liver, pericardium, lung, oviduct, trachea, air sac, or conjunctiva) were cultivated on Columbia agar (Biolab Zrt, Hungary), supplemented with 5% sheep blood, at 37 °C for 24 h under microaerophilic conditions. The identity of RA-suspect isolates was confirmed by species-specific PCR (Rubbenstroth et al., Citation2013). Type strains of RA were kindly supplied by Prof. Joachim Frey (Institute of Veterinary Bacteriology, University of Bern, Switzerland). The following serotype reference strains were used in this study: serotype 1 (DRL 24105, Duck Research Laboratory, New York, USA), serotype 2 (HPRS 2527, Houghton Poultry Research Station, Houghton, United Kingdom), serotype 4 (HPRS 2565), serotype 7 (DRL 27179), and serotype 10 (HPRS 2199) (Huang et al., Citation1999; Pathanasophon et al., Citation2002).
Serotyping
The serotypes of the respective RA isolates were determined by AGP according to Brogden et al. (Citation1982). Antisera against serotypes 1, 2, 4, 7, and 10 were raised in 16-week-old specific-pathogen-free roosters. The RA strains were grown on Columbia agar, enriched with 5% sheep blood, at 37 °C for 24 h under microaerophilic conditions. The bacteria were harvested with phosphate-buffered saline and the turbidity of the suspension was adjusted to 3 McFarland standards. Heat-stable antigens were prepared as described previously (Heddleston et al., Citation1972) and used as antigens in the AGP. The AGP test was incubated in a humid chamber and evaluated after 48 h. Strains non-typeable with the five reference antisera produced for this study were serotyped by Ripac-Labor GmbH (Potsdam, Germany) as described by Rubbenstroth et al. (Citation2013).
DNA extraction
One loopful of bacterial sample from a single colony was re-suspended in 50 µl of PCR-grade water (VWR, Radnor, PA, USA) in a microcentrifuge tube. The tube was placed in a boiling water bath for 20 min and then centrifuged at 12,000 × g for 5 min. The supernatant containing microbial DNA was used as the PCR template. The DNA concentration of the supernatant was measured using a NanoDrop spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA) and the final concentration was adjusted to 50 ng/µl by dilution with PCR-grade water.
ERIC-PCR
The PCR was performed in 25-μl volumes containing 1 × DreamTaq buffer supplemented with MgCl2 (25 mM) (Thermo Fisher Scientific Inc., Asheville, NC, USA), 0.2 mM dNTPs (Thermo Fisher Scientific Inc.), 15 pmol of each primer (ERICR1 5′-ATGTAAGCTCCTGGGGATTCAC-3′ and ERIC2 5′-AAGTAAGTGACTGGGGTGAGCG-3′; Versalovic et al., Citation1991), and 2 U of DreamTaq DNA Polymerase (Thermo Fisher Scientific Inc.). Bacterial DNA (50 ng) was used as the template. PCR amplification was performed using a C1000 Thermal Cycler (Bio-Rad Laboratories, Inc., Hercules, CA, USA) using the following cycles: initial denaturation at 95 °C for 5 min, followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 40 °C for 2 min, and extension at 72 °C for 2 min, followed by a final extension at 72 °C for 7 min. An 8-μl aliquot of amplified PCR product was separated by gel electrophoresis on 1.5% (w/v) agarose gels (TopVision agarose; Thermo Fisher Scientific Inc.) in 1 × AccuGENE TAE buffer (Lonza, Verviers, Belgium) for 4 h at 120 V, stained with GelRed™ Nucleic Acid Gel Stain (Biotium Inc., Hayward, CA, USA), and photographed under UV transillumination with the Kodak Gel Logic 212 Imaging System (Rochester, NY, USA). Product sizes were estimated using HyperLadder 50 bp (Bioline, London, UK) and GeneRuler DNA Ladder Mix (Thermo Fisher Scientific Inc.) as molecular size markers. ERIC-PCR fragment sizes were analysed using PyElph (version 1.4; Pavel & Vasile, Citation2012). Patterns of each isolate were converted into a binary matrix based on the presence (1) or absence (0) of the DNA bands. To construct the dendrogram, levels of similarity between the profiles were calculated using the Dice coefficient, and cluster analysis of similarity matrices was calculated by the unweighted-pair group method with arithmetic averages.
Results
Identification
All isolates involved in this study proved to be RA in the species-specific PCR.
Serotyping
The precipitation lines seen in the AGP test were clear and specific, and we found no non-typeable strains. Each strain gave reaction with only one specific antiserum except for one group of strains that reacted with both serotype 1- and serotype 7-specific antisera. Eight different serotypes were identified among the 166 RA field strains examined in this study (). Serotype 1 was the most prevalent serotype representing 64.5% of the strains, followed by serotypes 1,7 (16.9%), 2 (7.2%), 7 (4.8%), and 4 (3.6%). The other serotypes (10, 13, 17, and 18) occurred very rarely and were only represented by one or two strains. Serotype 1,7 showed a higher incidence in ducks than in geese, serotypes 4, 7, 10, and 17 were found only in geese, while serotypes 13 and 18 were detected only in ducks.
Table 1. Distribution of R. anatipestifer serotypes and ERIC-PCR types among strains from geese and ducks in Hungary.
ERIC-PCR
The ERIC-PCR discriminated the RA isolates into 17 types (). The 166 field isolates and five reference strains showed reproducible patterns consisting of five to 12 bands ranging from approximately 150–1800bp. There were differences in the concentrations of some amplified fragments, as well as in the occurrence of numerous polymorphic bands. The same patterns were observed when ERIC-PCR was repeated at least three times, proving the reproducibility of the method. The majority of the strains belonged to two closely related ERIC-PCR types (A and C), while the other types contained only a few isolates each.
Figure 1. Genetic relatedness of R. anatipestifer representative isolates. Genetic relatedness was assessed from the ERIC-PCR amplicon patterns using the unweighted-pair group method with arithmetic mean generated by PyElph (version 1.4; Pavel & Vasile, Citation2012) software. The genetic distances are shown above the branches.
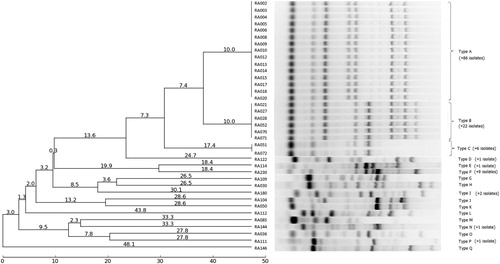
There was no association between ERIC-PCR types and the host species, or place or year of isolation. However, we did detect a correlation between ERIC-PCR pattern and serotype (). All but one ERIC-PCR type belonged just to one serotype, with ERIC-PCR type I being the only exception, representing three rare serotypes (13, 17, and 18). ERIC-PCR types B and C corresponded to serotypes 1,7 and 7, respectively. The vast majority of serotype 1 strains (101/107) belonged to ERIC-PCR type A, whereas the remaining six strains represented five different ERIC-PCR types (D, G, L, M, and O). Serotypes 2, 4, and 10 could also be subdivided by ERIC-PCR showing two to four patterns within each of these serotypes.
Discussion
The first crucial step in diagnosis is the proper identification of the pathogen which is a prerequisite for subsequent typing of the strains. Recently, a species-specific PCR (Rubbenstroth et al., Citation2013) and the matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF) (Hess et al., Citation2013) have proved to be the most appropriate methods for this. We applied the former to confirm the identity of all RA suspect isolates used in this study.
The prevalence of serotypes seems to vary among countries and over time. An earlier study found that serotypes 1, 2, and 5 were the most common serotypes in the USA (Sandhu & Leister, Citation1991), while serotypes 1, 15, 10, and 7 were frequently related to outbreaks of anatipestifer disease in duck farms in Singapore (Loh et al., Citation1992), and serotype 7 was the most prevalent serotype in Thailand, followed by serotypes 5, 10, 21, and 1 (Pathanasophon et al., Citation2002). Ryll et al. (Citation2001) reported the dominance of serotype 4 in clinically healthy Pekin ducks in Denmark, a serotype only found in geese in the current study, while most isolates collected from ducks in Taiwan during 2006–2008 belonged to serotypes 2, 6, 8, and 21 (Chen et al., Citation2015). The current results suggested that serotype 1 was the most prevalent serotype in Hungary. However, the high incidence of serotype 1,7, a type with multiple antigenic factors, deserves attention. This seems to represent a new antigenic combination, and its potential to cause disease or induce protection needs further clarification. Pathanasophon et al. (Citation2002) hypothesized that a vaccine produced from a strain with multiple antigenic determinants might protect against infections with all serotypes that carry the homologous antigens.
Classical AGP serotyping is not widely feasible because of the high cost of the antisera, difficulties in interpreting the results, and the need for technical expertise. In addition, in many cases, isolates cannot be serotyped or produce multiple cross-reactions in serological tests (Rimler & Nordholm, Citation1998). Various techniques for rapid genotyping have therefore been tried for differentiating among RA isolates. Rimler & Nordholm (Citation1998) used DNA fingerprinting to subtype the two most common serotypes (1 and 2) and showed that serotype 1 contained 17 different profiles and serotype 2 contained five different profiles, but no relationship between fingerprint profile and serotype could be established. Philipp et al. (Citation2013) applied PFGE for subtyping serotype 14 isolates and detected nine distinct band patterns using SmaI restriction endonuclease; however, the occurrence of these subtypes has not been studied for other serotypes. Using cluster analysis, they showed a correlation between band pattern, geographical origin, and time of isolation, with the four serotype 14 strains from Hungary forming a separate cluster distinct from isolates from Germany. Interestingly, however, no serotype 14 strains were identified in our RA collection. Yu et al. (Citation2008) investigated the genomic diversity of RA isolates from disease outbreaks in goose and duck farms in Taiwan and found no correlation between serotypes and any of the molecular methods applied (PCR-restriction fragment length polymorphism, repetitive sequence analysis, and PFGE). Chen et al. (Citation2015) compared four techniques for molecular typing of RA isolates and reported some correlation between ompA sequencing and serotypes, though most ompA clusters contained more than one serotype and vice versa. Rep-PCR analysis of RA isolates from ducks in Singapore identified nine different patterns (Huang et al., Citation1999), but the relationships between serotypes and types obtained by the DNA-based method were unclear, with some serotypes (1, 7, 11, 13, 15 and 19) consisting of more than one rep-PCR pattern, and strains from different serotypes (2 and 9) showing the same rep-PCR profiles.
Our results suggest that ERIC-PCR may be a feasible technique for the molecular determination of the serotypes of RA isolates that is highly important because of the serotype-specific nature of protection. Moreover, the detection of subtypes within certain serotypes may be useful during epidemiological investigations. Information on the transmission, carry-over, or persistence of a strain in a flock may help to determine the measures necessary to prevent the introduction or spread of the pathogen. The further characterization of RA within serotypes might also increase the possibility of selecting more efficacious autogenous vaccine candidates.
On the other hand, neither ERIC-PCR types nor serotypes showed correlation with the origin of our RA isolates. Strains collected form the same location at different time points represented various types indicating the simultaneous presence of multiple serotypes at the same place as well as the change of dominant serotypes over time. Therefore, there remains a great need for more refined methods appropriate to examine the epidemiological traits of RA infections. Zhu et al. (Citation2015) analysed the sequences of seven housekeeping genes of RA strains from ducks in China with the multilocus sequence typing (MLST) method and established a database that they found suitable for epidemiological examinations. Looking at the recent tendencies, single-nucleotide polymorphism analysis or whole genome sequencing most likely will provide the tools for exploring epidemiological contexts as well as better understanding of the pathogenesis of the disease (Wang et al., Citation2014).
Supplemental Material
Download MS Word (30.3 KB)Acknowledgements
The outstanding technical assistance of É. Hegedűs and K. Oryszcsák is highly appreciated. We also thank Susan Furness, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bisgaard, M. (1982). Antigenic studies on Pasteurella anatipestifer, species incertae sedis, using slide and tube agglutination. Avian Pathology, 11, 341–350. doi: 10.1080/03079458208436109
- Brogden, K.A., Rhoades, K.R. & Rimler, R.B. (1982). Serologic types and physiological characteristics of 46 avian cultures. Avian Diseases, 26, 891–896. doi: 10.2307/1589877
- Chen, C.-L., Wang, S.-T., Chu, C. & Wang, S.-H. (2015). Comparison of four molecular typing methods for Riemerella anatipestifer. Taiwan Veterinary Journal, 41, 177–185. doi: 10.1142/S1682648515500080
- Christensen, H. & Bisgaard, M. (2010). Phylogenetic relationships of Riemerella anatipestifer serovars and related taxa and an evaluation of specific PCR tests reported for R. anatipestifer. Journal of Applied Microbiology, 108, 1612–1619. doi: 10.1111/j.1365-2672.2009.04558.x
- Harry, E.G. (1969). Pasteurella (Pfeifferella) anatipestifer serotypes isolated from cases of anatipestifer septicaemia in ducks. Veterinary Record, 84, 673. doi: 10.1136/vr.84.26.673
- Heddleston, K.L., Gallagher, J.E. & Rebers, P.A. (1972). Gel diffusion precipitin test for serotyping Pasteurella multocida from avian species. Avian Diseases, 16, 925–936. doi: 10.2307/1588773
- Hess, C., Enichlmayr, H., Jandreski-Cvetkovic, D., Liebhart, D., Bilic, I. & Hess, M. (2013). Riemerella anatipestifer outbreaks in commercial goose flocks and identification of isolates by MALDI-TOF mass spectrometry. Avian Pathology, 42, 151–156. doi: 10.1080/03079457.2013.775401
- Huang, B., Subramaniam, S., Chua, K.L., Kwang, J., Loh, H., Frey, J. & Tan, H.M. (1999). Molecular fingerprinting of Riemerella anatipestifer by repetitive sequence PCR. Veterinary Microbiology, 67, 213–219. doi: 10.1016/S0378-1135(99)00032-2
- Kardos, G., Nagy, J., Antal, M., Bistyák, A., Tenk, M. & Kiss, I. (2007). Development of a novel PCR assay specific for Riemerella anatipestifer. Letters in Applied Microbiology, 44, 145–148. doi: 10.1111/j.1472-765X.2006.02053.x
- Loh, H., Teo, T.P. & Tan, H.C. (1992). Serotypes of ‘‘Pasteurella’’ anatipestifer isolates from ducks in Singapore: A proposal of new serotypes. Avian Pathology, 21, 453–459. doi: 10.1080/03079459208418863
- Pathanasophon, P., Phuektes, P., Tanticharoenyos, T., Narongsak, W. & Sawada, T. (2002). A potential new serotype of Riemerella anatipestifer isolated from ducks in Thailand. Avian Pathology, 31, 267–270. doi: 10.1080/03079450220136576
- Pathanasophon, P., Sawada, T., Pramoolsinsap, T. & Tanticharoenyos, T. (1996). Immunogenicity of Riemerella anatipestifer broth culture bacterin and cell-free culture filtrate in ducks. Avian Pathology, 25, 705–719. doi: 10.1080/03079459608419176
- Pavel, A.B. & Vasile, C.I. (2012). PyElph – a software tool for gel images analysis and phylogenetics. BMC Bioinformatics, 13, 9. doi: 10.1186/1471-2105-13-9
- Philipp, H.-C., Taras, D., Liman, M., Grosse-Herrenthey, A., Ronchen, S. & Behr, P. (2013). Molecular typing of Riemerella anatipestifer serotype 14, an emerging pathogen for ducks. Archiv für Geflügelkunde, 77, 218–225.
- Rimler, R.B. & Nordholm, G.E. (1998). DNA fingerprinting of Riemerella anatipestifer. Avian Diseases, 42, 101–105. doi: 10.2307/1592581
- Rubbenstroth, D., Ryll, M., Knobloch, J.K., Köhler, B. & Rautenschlein, S. (2013). Evaluation of different diagnostic tools for the detection and identification of Riemerella anatipestifer. Avian Pathology, 42, 17–26. doi: 10.1080/03079457.2012.752066
- Ruiz, J. & Sandhu, T.S. (2013). Riemerella anatipestifer infection. In D.E. Swayne, J.R. Glisson, L.R. McDougald, L.K. Nolan, D.L. Suarez & V.L. Nair (Eds.), Diseases of Poultry (13th edn). (pp. 823–828) Ames: Wiley-Blackwell.
- Ryll, M., Christensen, H., Bisgaard, M., Christensen, J.P., Hinz, K.H. & Kohler, B. (2001). Studies on the prevalence of Riemerella anatipestifer in the upper respiratory tract of clinically healthy ducklings and characterization of untypable strains. Journal of Veterinary Medicine B, 48, 537–546. doi: 10.1046/j.1439-0450.2001.00471.x
- Sandhu, T. (1991). Immunogenicity and safety of a live Pasteurella anatipestifer vaccine in White Pekin ducklings: Laboratory and field trials. Avian Pathology, 20, 423–432. doi: 10.1080/03079459108418780
- Sandhu, T.S. & Leister, M.L. (1991). Serotypes of ‘Pasteurella’ anatipestifer isolates from poultry in different countries. Avian Pathology, 20, 233–239. doi: 10.1080/03079459108418760
- Segers, P., Mannheim, W., Vancanneyt, M., De Brandt, K., Hinz, K.H., Kersters, K. & Vandamme, P. (1993). Riemerella anatipestifer gen. nov., comb. nov., the causative agent of septicemia anserum exsudativa, and its phylogenetic affiliation within the Flavobacterium-Cytophaga rRNA homology group. International Journal of Systematic Bacteriology, 43, 768–776. doi: 10.1099/00207713-43-4-768
- Subramaniam, S., Chua, K.-L., Tan, H.-M., Loh, H., Kuhnert, P. & Frey, J. (1997). Phylogenetic position of Riemerella anatipestifer based on 16s rRNA gene sequences. International Journal of Systematic Bacteriology, 47, 562–565. doi: 10.1099/00207713-47-2-562
- Tsai, H.J., Liu, Y.T., Tseng, C.S. & Pan, M.J. (2005). Genetic variation of the ompA and 16S rRNA genes of Riemerella anatipestifer. Avian Pathology, 34, 55–64. doi: 10.1080/03079450400025471
- Versalovic, J., Koeuth, T. & Lupski, J.R. (1991). Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Research, 19, 6823–6831. doi: 10.1093/nar/19.24.6823
- Wang, X., Liu, W., Zhu, D., Yang, L., Liu, M., Yin, S., Wang, M., Jia, R., Chen, S., Sun, K., Cheng, A. & Chen, X. (2014). Comparative genomics of Riemerella anatipestifer reveals genetic diversity. BMC Genomics, 15, 479.
- Yu, C.Y., Liu, Y.W., Chou, S.J., Chao, M.R., Weng, B.C., Tsay, J.G., Chiu, C.H., Wu, C.C., Lin, T.L., Chang, C.C. & Chu, C. (2008). Genomic diversity and molecular differentiation of Riemerella anatipestifer associated with eight outbreaks in five farms. Avian Pathology, 37, 273–279. doi: 10.1080/03079450802056546
- Zhu, B., Chao, M., Yang, X. & Zhou, D. (2015). Multilocus sequence typing of the Guangdong isolates of Riemerella anatipestifer from ducks in China. Open Journal of Animal Sciences, 5, 332–342. doi: 10.4236/ojas.2015.53037
